Fatty liver disease is the commonest liver condition globally [1]. Traditionally, the term "non-alcoholic fatty liver disease (NAFLD)" is used to describe a diagnosis of exclusion and absence of significant alcohol use. NAFLD is estimated to affect 25-33% of the global population [2, 3] and is a major cause of complications including but not limited to cardiovascular disease (CVD) [4], hepatocellular carcinoma [5] and extrahepatic malignancy. However, an international call was recently made to propose “metabolic associated fatty liver disease” (MAFLD) as a replacement definition for NAFLD [6]. Briefly, MAFLD describes a diagnosis of inclusion when hepatic steatosis occurs in (1) overweight or (2) Type 2 Diabetes Mellitus (T2DM) or (3) ≥2 factors of metabolic dysregulation [6]. As such, the newer criteria allow any individual with T2DM and hepatic steatosis to be included in MAFLD, while NAFLD can only be made with the exclusion of other chronic liver diseases and the absence of significant alcohol use. As a result, MAFLD is significantly more prevalent than NAFLD, and a recent meta-analysis estimates up to 36% of the global population has MAFLD [3]. A recent meta-analysis of 12,620,736 individuals found a significant association of MAFLD with systemic commodities and mortality [7]. While initially thought to serve as a direct replacement for the older definition, the term MAFLD has since sparked international debates due to the different clinical characteristics and outcomes of NAFLD.
Amongst the risk factors of fatty liver, T2DM is a known common factor affecting both diseases' definition [8,9]. With the distinct presence of insulin resistance as a major pathway in the pathogenesis of NAFLD, T2DM has been closely linked to NAFLD. A recently updated meta-analysis shows that T2DM is significantly prevalent amongst NAFLD [10] and up to half of T2DM patients have NAFLD [11]. T2DM has additionally been associated with an increased risk of end-organ damage relative to NAFLD patients without T2DM. Yet, due to differing definitions between MAFLD and NAFLD, current estimates have shown a higher prevalence of T2DM in MAFLD [3,12]. The current definition of MAFLD allows any patient with hepatic steatosis and T2DM to be included regardless of alcohol use and despite the significantly high prevalence and the association between the T2DM with fatty liver, the impact of this change remains elusive. Thus, we sought to examine the differences in clinical characteristics and longitudinal outcomes of patients with T2DM and MAFLD relative to patients with T2DM that are excluded from NAFLD within the United States National Health and Nutrition Examination Survey (NHANES) 1999 to 2018.
2Methods2.1Study PopulationThe NHANES study [13] examines aggregated health-related data from a clustered sampled national survey involving general and noninstitutionalized individuals in the United States between 1999-2018. The study involved participants undergoing a comprehensive interview, medical examinations, and laboratory assessments. Data on mortality were obtained by linking the NHANES data to death certificates from the National Death Index. Baseline characteristics such as but are not limited to age, gender, ethnicity, body mass index (BMI), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, total cholesterol, triglyceride, fasting blood glucose, glycohemoglobin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and past medical history (diabetes, hypertension) were collected. [14,15] Information on the outcomes of MAFLD and NAFLD, including Major Adverse Cardiovascular Events (MACE), stroke, chronic kidney disease (CKD), advanced fibrosis by Fibrosis-4 Index (FIB-4), all-cause mortality and cardiovascular disease (CVD) related mortality were also collected.
2.2DefinitionsThe definition of NAFLD was adapted based on the American Association for the Study of Liver Disease (AASLD) guidelines for NAFLD. We defined NAFLD as the presence of steatosis in the absence of substantial alcohol use (≥2 drinks a day in men, ≥3 drinks a day in women). MAFLD was defined as the presence of hepatic steatosis with metabolic dysregulation as proposed by experts from the international expert panel [16,17]. The proposed criteria defined MAFLD as the presence of fatty liver with concomitant type 2 diabetes, overweight or obesity (i.e., BMI 23 kg/m2), or two or more of the following metabolic conditions: (1) waist circumference ≥ 90 cm in men and ≥ 80 cm in women (central obesity), (2) blood pressure ≥ 130/85 mm Hg or receiving antihypertensives, (3) plasma triglycerides ≥ 1.7 mmol/L or receiving specific drug treatment, (4) plasma high-density lipoprotein < 1.03mmol/L in men and < 1.29mmol/L in women. Overweight patients were defined with BMI≥25.0 kg/m2 for Caucasians and BMI≥23.0 kg/m2 for Asians. Obese patients were defined as BMI≥30.0 kg/m2 for Caucasians and BMI≥27.5 kg/m2 for Asians. [18,19] A Fatty Liver Index (FLI) ≥ 6024 or US-FLI ≥ 30 [20] indicates the presence of hepatic steatosis. Diabetes was defined as glycohemoglobin ≥ 6.5%, fasting plasma glucose ≥ 7mmol/l, self-reported diabetes, or the use of anti-diabetic medications. Prediabetes was defined as glycohemoglobin (HbA1c) between 5.7% - 6.5% or fasting plasma glucose between 5.6mmol/l - 7mmol/l [21]. Hypertension was defined as a systolic or diastolic blood pressure ≥140/90 or the use of antihypertensive [22]. Viral hepatitis was defined as the presence of hepatitis B surface antigen and hepatitis C RNA or Anti-HCV. Major Adverse Cardiovascular Events (MACE) include the occurrence of heart failure, stroke, myocardial infarction and mortality. Chronic Kidney Disease (CKD) was defined as the presence of kidney damage or an estimated glomerular filtration rate of ≤60 mL/min/1.73 m2 under the Modification of Diet in Renal Disease (MDRD) equation. [23] Quantification of fibrosis in the liver was examined using the Fibrosis-4 (FIB-4) Index Score, where a score of ≥2.67 was defined as advanced fibrosis. [24]
2.3Statistical analysisThe study population was divided into three groups. Firstly, T2DM patients without fatty liver were classified as MAFLD(-)/NAFLD(-). T2DM patients with fatty liver without substantial alcohol use set forth by the definition of NAFLD were classified as MAFLD(+)/NAFLD(+). Lastly, T2DM patients with a fatty liver that fulfilled the definition of MAFLD but not NAFLD were MAFLD(+)/NAFLD(-). All statistical analysis was performed using STATA (16.1). Continuous variables were examined with Wilcoxon ranked sum test and Kruskal–Wallis analysis of variance, while binary variables were examined with chi-square test and fisher exact where appropriate. Post-hoc analysis was conducted with Dunn's test as an appropriate nonparametric pairwise multiple-comparison procedure in examining the differences in baseline characteristics between MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+). Univariate and multivariate logistic regression analysis was used to obtain odds ratios (OR) for the estimation of comment events and clinical interpretability in the two study groups. Mortality outcomes were examined with a Cox proportional model for hazard ratios (HR) and a separate competing risk analysis was used to examine the risk of CVD-related mortality with the fine grey substruction hazard ratios (SHR). A cluster analysis was also included in OR, HR and SHR analyses based on the year of study to account for heterogeneity introduced by the year of study. The multivariable models in logistic regression and survival analysis were constructed with important traditional confounders that include age, gender, race, smoking and BMI.
2.4Ethical statementsThe study was conducted in accordance with the Declaration of Helsinki. Ethics approval by the Institutional Review Board and written informed consent from patients were exempted due to the anonymous nature of the data made publicly available by the National Centre for Health Statistics (NCHS).
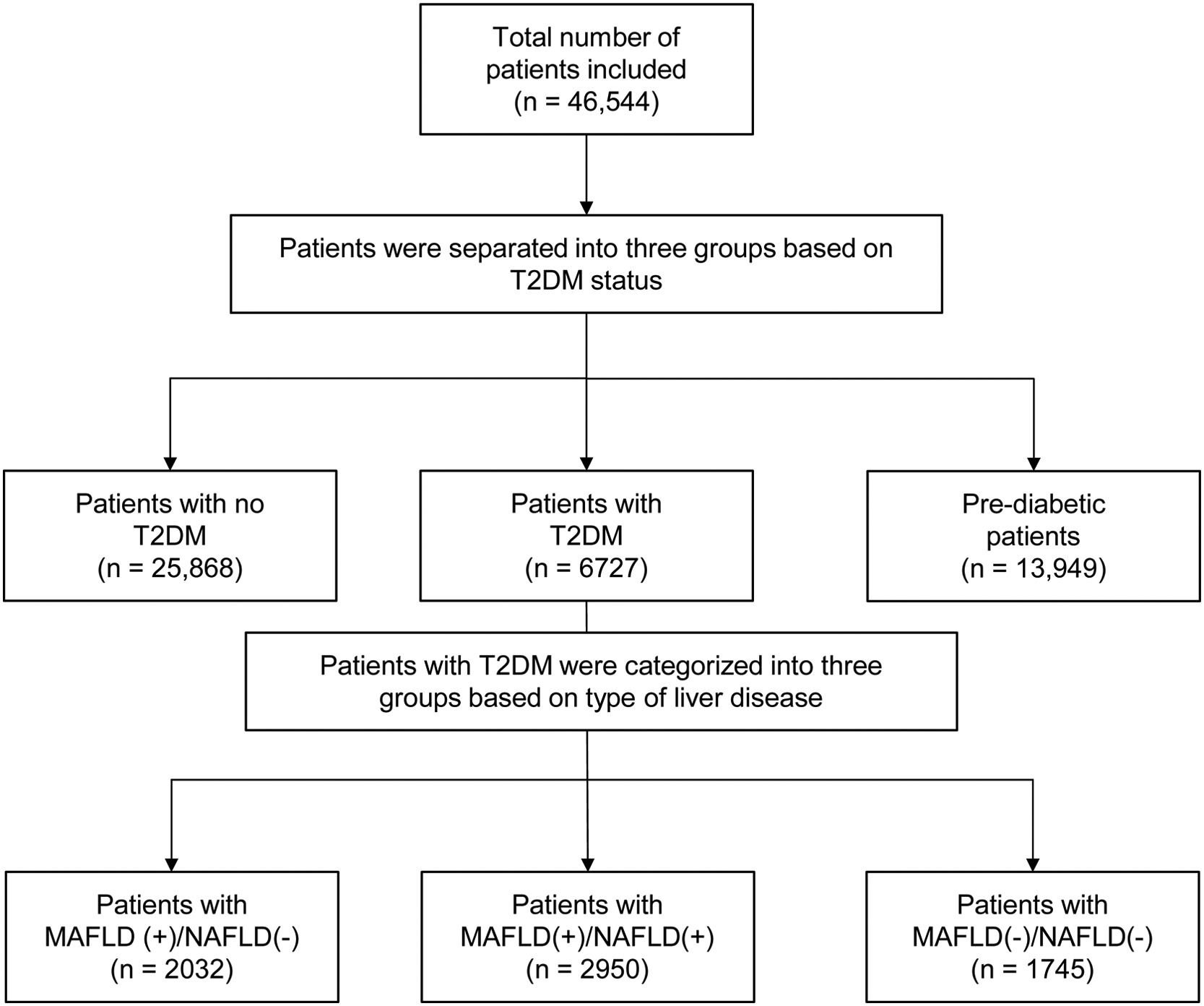
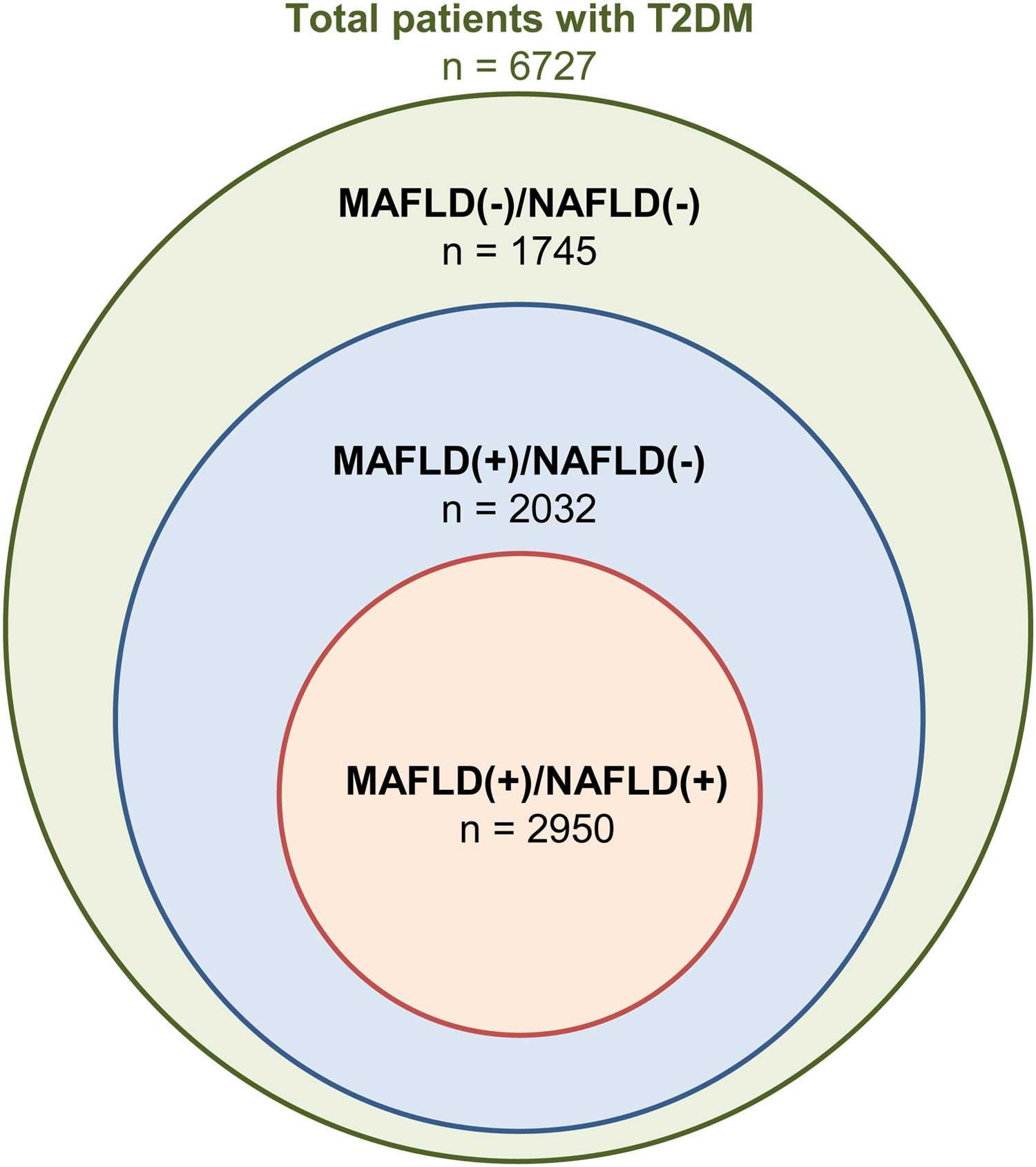
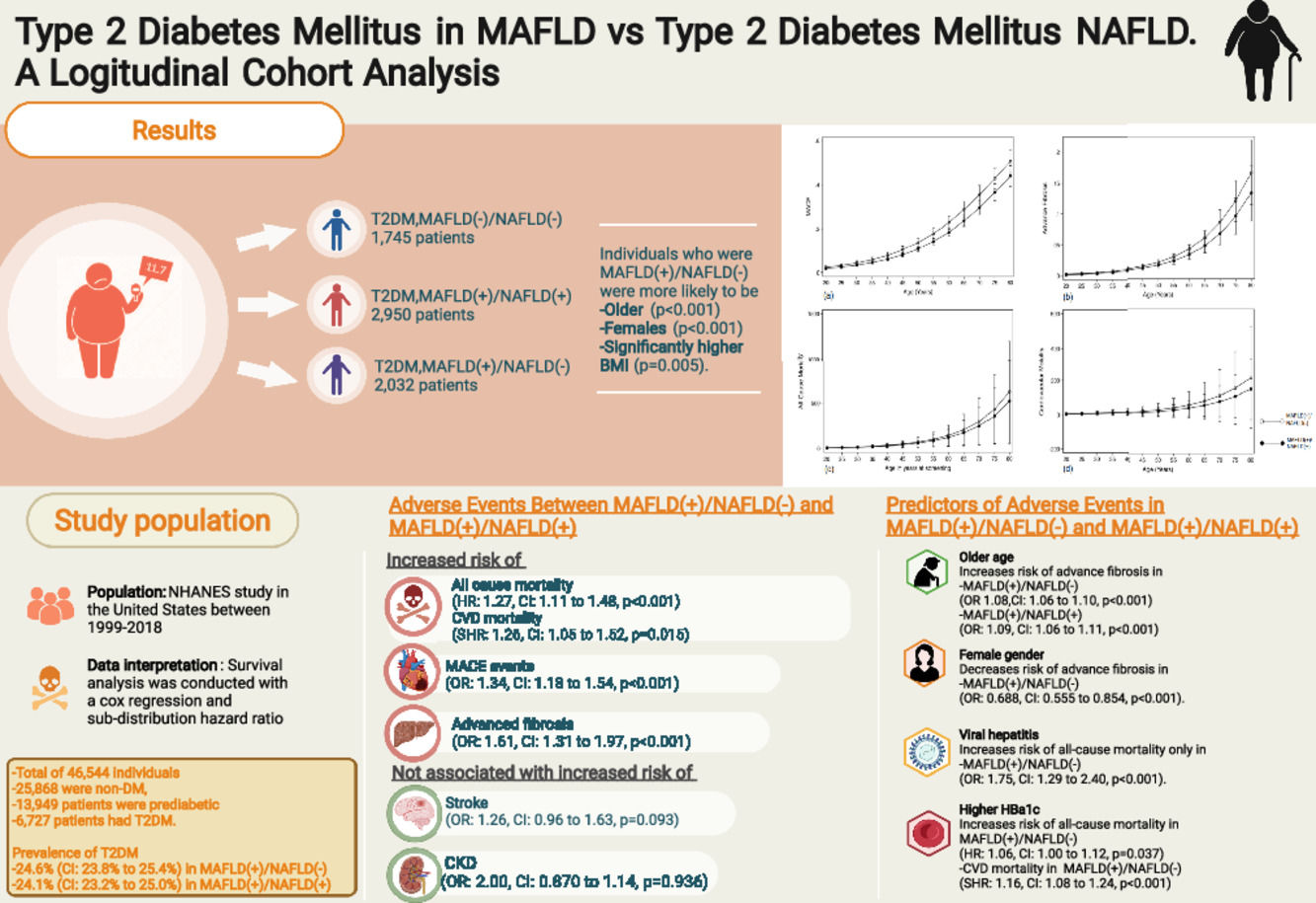
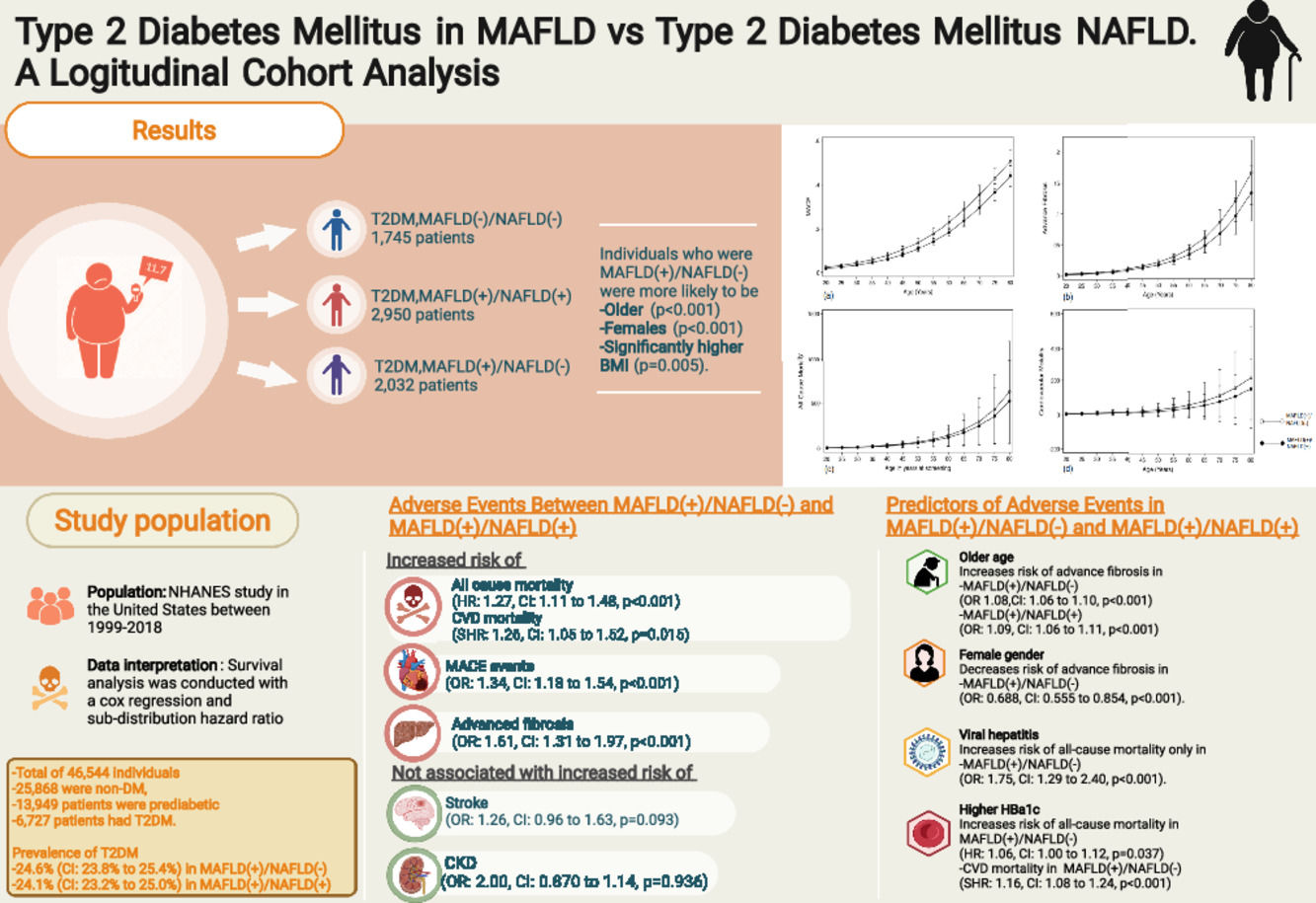
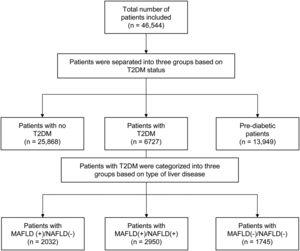
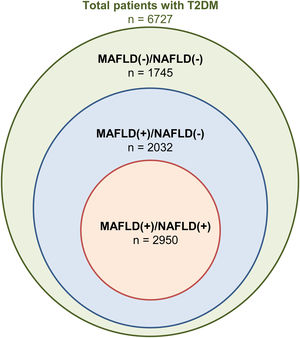
3Results3.1Baseline characteristics of included populationA total of 46,544 individuals were included. 25,868 were non-DM, 13,949 patients were prediabetic and a total of 6727 patients had T2DM. The prevalence of T2DM was 24.6% (95% CI: 23.8% to 25.4%) in MAFLD(+)/NAFLD(-) and 24.1% (95% CI: 23.2% to 25.0%) in MAFLD(+)/NAFLD(+). Amongst patients with T2DM, a total of 2032 patients had a diagnosis of MAFLD(+)/NAFLD(-), 2950 patients were MAFLD(+)/NAFLD(+) and 1745 patients with T2DM were MAFLD(-)/NAFLD(-). In turn, there was a 68.89% (n = 2032) increase in the diagnosis of fatty liver amongst individuals with T2DM in the transition to MAFLD (Fig. 1). A total of 117 individuals had a diagnosis of viral hepatitis in MAFLD(+)/NAFLD(-). The baseline characteristics of the population with T2DM are summarized in Table 1 and there were significant differences between MAFLD(+)/NAFLD(-), MAFLD(+)/NAFLD(+) and MAFLD(-)/NAFLD(-). A post-hoc analysis was conducted to examine the differences between MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+) using the Dunn test. Individuals who were MAFLD(+)/NAFLD(-) were more likely to be older (p < 0.001) and females (p < 0.001) gender. By and large, there was no difference in the lipids measures aside from HDL, which was significantly higher in MAFLD(+)/NAFLD(-). Liver enzymes (AST and ALT) were also higher in MAFLD(+)/NAFLD(+) relative to MAFLD(+)/NAFLD(-).
Baseline demographics of patients with T2DM and MAFLD(+)/NAFLD(-), MAFLD(+)/NAFLD(+) or MAFLD(-)/NAFLD(-).
| MAFLD(+)/NAFLD(-) | MAFLD(+)/NAFLD(+) | MAFLD(-)/NAFLD(-) | P-Value^ | P-Value$ | |
|---|---|---|---|---|---|
| Sample Size | n = 2032 | n = 2950 | n = 1745 | ||
| Age (years) | 62 (IQR: 52 to 70) | 61 (IQR: 50 to 69) | 65 (IQR: 55 to 75) | <0.001* | <0.001* |
| Gender (male) | 46.5 (95%CI: 44.7 to 48.3) | 59.9 (95%CI: 57.8 to 62.1) | 52.7 (95%CI: 50.3 to 55.0) | <0.001* | <0.001* |
| BMI (kg/m2) | 33.1 (IQR: 29.9 to 37.7) | 32.7 (IQR: 29.2 to 37.7) | 25.2 (IQR: 23.2 to 27.3) | <0.001* | 0.005 |
| Hypertension (%) | 83.0 (95%CI: 81.6 to 84.4) | 82.4 (95%CI: 80.7 to 84.1) | 76.6 (95%CI: 74.5 to 78.6) | <0.001* | 0.607 |
| Waist Circumference (cm) | 112 (IQR: 105 to 122) | 112 (IQR: 104 to 122) | 93.1 (IQR: 87.2 to 98.2) | <0.001* | 0.195 |
| Past Smoker (%) | 31.5 (95%CI: 29.8 to 33.2) | 41.7 (95%CI: 39.6 to 43.9) | 29.0 (95%CI: 26.9 to 31.2) | <0.001* | <0.001* |
| Current Smoker (%) | 12.2 (95%CI: 11.0 to 13.4) | 21.8 (95%CI: 20.0 to 23.6) | 16.4 (95%CI: 14.8 to 18.3) | <0.001* | <0.001* |
| Viral hepatitis (%) | 5.75 (95%CI: 4.82 to 6.86) | NA | 2.17 (95%CI: 15.8 to 2.98) | <0.001* | NA |
| Platelet count (1000 cells/uL) | 243 (IQR: 202 to 293) | 236 (IQR: 197 to 288) | 233 (IQR: 194 to 278) | <0.001* | <0.001* |
| Glycohemoglobin (%) | 6.9 (IQR: 6.2 to 8.0) | 7.0 (IQR: 6.3 to 8.3) | 6.7 (IQR: 6.0 to 7.7) | <0.001* | 0.005 |
| FBG (mmol/L) | 7.88 (IQR: 6.83 to 9.94) | 7.94 (IQR: 6.83 to 10.5) | 7.30 (IQR: 6.33 to 8.94) | <0.001* | 0.167 |
| Total bilirubin (umol/L) | 10.3 (IQR: 6.84 to 13.7) | 10.3 (6.84 to 13.7) | 10.3 (IQR: 8.55 to 13.7) | 0.003 | 0.254 |
| Total Cholesterol (mg/dL) | 188 (IQR: 161 to 220) | 187 (IQR: 159 to 219) | 178 (IQR: 151 to 211) | <0.001* | 0.131 |
| LDL-Cholesterol (mg/dL) | 105 (IQR: 82 to 131) | 104 (IQR: 80 to 130) | 99 (IQR: 75 to 127) | 0.005* | 0.279 |
| Direct HDL-Cholesterol (mg/dL) | 45 (IQR: 38 to 54) | 42 (IQR: 37 to 51) | 52 (IQR: 44 to 64) | <0.001* | <0.001* |
| Triglycerides (mg/dL) | 170 (IQR: 119 to 251) | 173 (IQR: 119 to 259) | 108 (IQR: 78 to 152) | <0.001* | 0.181 |
| AST (IU/L) | 23 (IQR: 19 to 28) | 23 (IQR: 19 to 30) | 21 (IQR: 18 to 26) | <0.001* | 0.071 |
| ALT (IU/L) | 23 (IQR: 17 to 31) | 23 (IQR: 18 to 34) | 19 (IQR: 15 to 24) | <0.001* | <0.001* |
| GGT (IU/L) | 27 (IQR: 19 to 41) | 30 (IQR: 21 to 50) | 18 (IQR: 13 to 26) | <0.001* | <0.001* |
| Income (%) | <0.001* | <0.001* | |||
| 0 to 10,000 | 8.28 (95%CI: 7.27 to 9.42) | 10.8 (95%CI: 9.47 to 12.4) | 8.66 (95%CI: 7.34 to 10.2) | ||
| 10,000 to 25,000 | 29.1 (95%CI: 27.3 to 30.9) | 35.4 (95%CI: 33.2 to 37.7) | 32.6 (95%CI: 30.2 to 35.0) | ||
| 25,000 to 45,000 | 26.7 (95%CI: 25.0 to 28.5) | 26.0 (95%CI: 24.0 to 28.1) | 24.6 (95%CI: 22.5 to 26.8) | ||
| 45,000 to 75,000 | 19.5 (95%CI: 18.0 to 21.1) | 18.3 (95%CI: 16.6 to 20.2) | 19.4 (95%CI: 17.5 to 21.5) | ||
| >75,000 | 16.4 (95%CI: 15.1 to 17.9) | 9.42 (95%CI: 8.14 to 10.9) | 14.8 (95%CI: 13.1 to 16.7) | ||
| Ethnicity (%) | <0.001* | <0.001* | |||
| Caucasian | 38.5 (95%CI: 36.7 to 40.2) | 32.1 (95%CI: 30.1 to 34.1) | 33.0 (95%CI: 30.8 to 35.2) | ||
| African American | 24.9 (95%CI: 23.4 to 26.5) | 25.2 (95%CI: 23.4 to 27.2) | 24.6 (95%CI: 22.7 to 26.7) | ||
| Mexican American | 21.1 (95%CI: 19.7 to 22.6) | 26.6 (95%CI: 24.7 to 28.6) | 14.3 (95%CI: 12.7 to 16.0) | ||
| Hispanic | 9.22 to (95%CI: 8.23 to 10.3) | 9.25 (95%CI: 8.07 to 10.6) | 10.1 (95%CI: 8.76 to 11.6) | ||
| Others | 6.31 (95%CI: 5.48 to 7.24) | 6.79 (95%CI: 5.78 to 7.97) | 18.1 (95%CI: 16.3 to 19.9) |
T2DM, Type 2 Diabetes Mellitus; MAFLD, Metabolic Associated Fatty Liver Disease; NAFLD, Non-alcoholic Fatty Liver Disease; BMI, Body Mass Index; FBG, Fasting Blood Glucose; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; GGT, Gamma Glutamyl Transferase; IQR, Interquartile range; 95%CI, 95% Confidence Interval; * bolded p-value ≤0.05 denotes statistical significance.
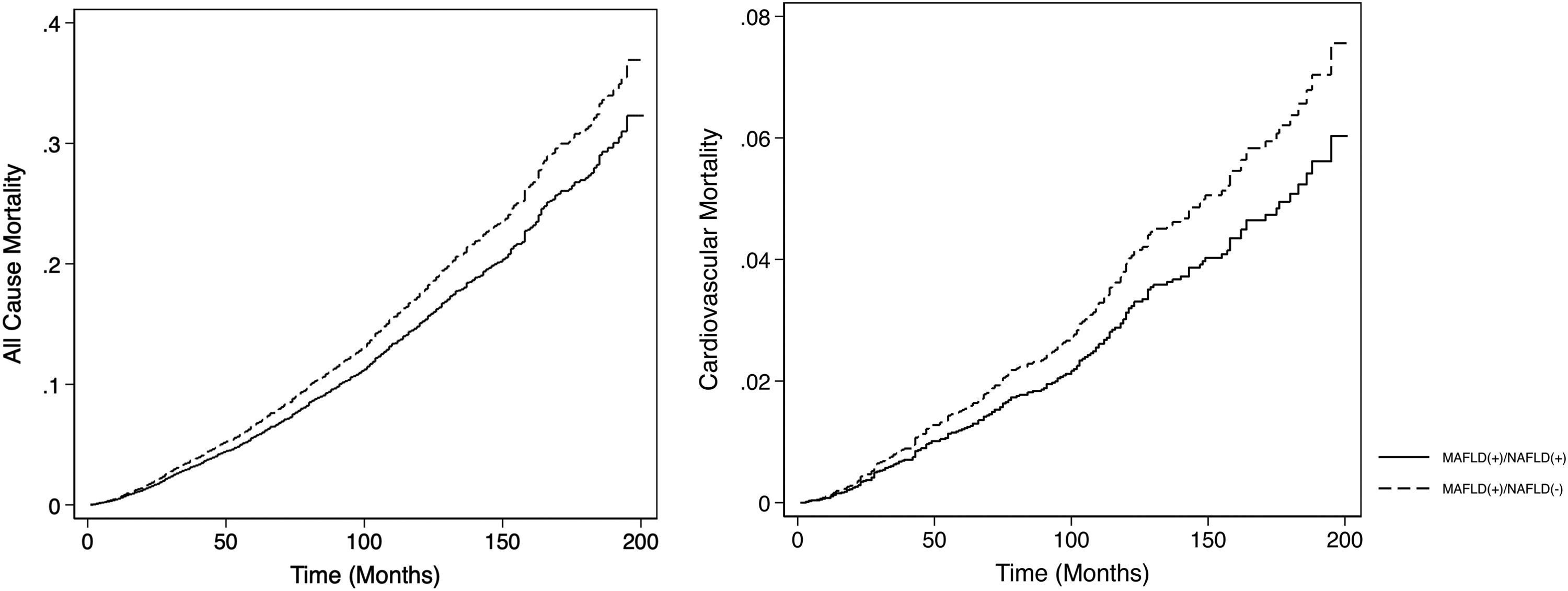
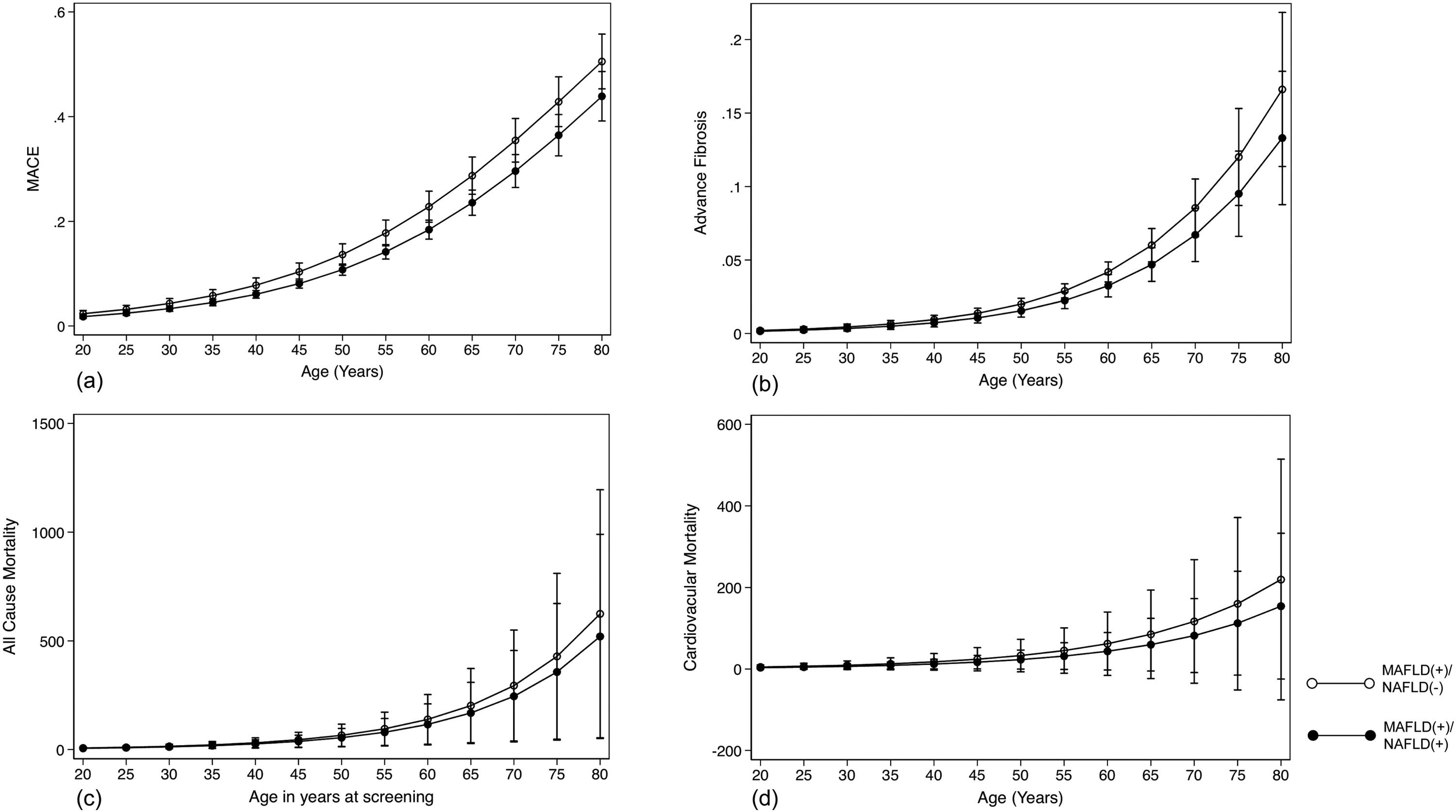
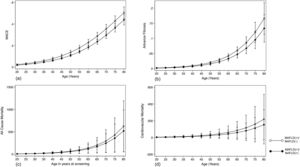
The summary of events is tabulated in Table 2. An unadjusted logistic regression analysis clustered on the study year was conducted to examine the difference between MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+). In a multivariate logistic regression analysis clustered on the year of study, MAFLD(+)/NAFLD(-) was associated with a higher odds of MACE events (OR: 1.34, CI: 1.18 to 1.54, p < 0.001) but not CKD (OR: 2.00, CI: 0.870 to 1.14, p = 0.936) relative to MAFLD(+)/NAFLD(+). There was no associated increased risk of stroke (OR: 1.26, CI: 0.96 to 1.63, p = 0.093) between MAFLD(+)/NAFLD(-) relative to MAFLD(+)/NAFLD(+). There was, however, an associated increase odds of advanced fibrosis by FIB-4 (OR: 1.61, CI: 1.31 to 1.97, p < 0.001) in MAFLD(+)/NAFLD(-) compared to MAFLD(+)/NAFLD(+). In the assessment of mortality, there was an increased risk of all-cause mortality (HR: 1.27, CI: 1.11 to 1.48, p < 0.001, Fig. 3) and CVD mortality (SHR: 1.26, CI: 1.05 to 1.52, p = 0.015, Fig. 3). A marginal model was conducted as a post-hoc analysis to evaluate the impact of age and gender on the predicted probabilities of MACE (Fig. 4a), advanced fibrosis by FIB-4 (Fig. 4b), all-cause mortality (Fig. 4c), and CVD related mortality (Fig. 4d). Significantly, age and gender resulted in a non-linear change in events of MACE, advanced fibrosis by FIB-4, all-cause mortality, and CVD related mortality.
Effect size of adverse events in MAFLD(+)/NAFLD(-) with reference to MAFLD(+)/NAFLD(+).
| Unadjusted Logistic Regression | Multivariate Logistic Regression† | |||
|---|---|---|---|---|
| Effect Size (95%CI) | p value | Effect Size (95%CI) | p value | |
| All-cause Mortality | 1.20 (1.08 to 1.33) | 0.001* | 1.27 (1.11 to 1.48) | <0.001* |
| CVD Mortality | 1.25 (1.00 to 1.56) | 0.023* | 1.26 (1.05 to 1.52) | 0.015* |
| MACE | 1.30 (1.16 to 1.45) | <0.001* | 1.34 (1.18 to 1.54) | <0.001* |
| CKD | 0.890 (0.743 to 1.07) | 0.211 | 2.00 (0.870 to 1.14) | 0.936 |
| Stroke | 1.23 (0.920 to 1.64) | 0.162 | 1.26 (0.96 to 1.63) | 0.093 |
| Advanced Fibrosis | 1.50 (1.24 to 1.81) | <0.001* | 1.61 (1.31 to 1.97) | <0.001* |
MAFLD, Metabolic Associated Fatty Liver Disease; NAFLD, Non-Alcoholic Fatty Liver Disease; 95%CI, 95% Confidence Interval; CVD, Cardiovascular Disease; MACE, Major Adverse Cardiac Events; CKD, Chronic Kidney Disease; VCTE, Vibration-Controlled Transient Elastography; BMI, Body Mass Index.
A multivariate analysis adjusted for age, gender, race, smoking, BMI clustered on the year of study was conducted to examine for associated factors of advanced fibrosis, all-cause mortality and CVD related mortality in MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+). Supplementary table 1 summarizes the findings of MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+). An older age was a significant factor for advanced fibrosis in both MAFLD(+)/NAFLD(-) (OR 1.08, CI: 1.06 to 1.10, p < 0.001) and MAFLD(+)/NAFLD(+) (OR: 1.09, CI: 1.06 to 1.11, p < 0.001). However, the female gender was associated with a decrease odd of advanced fibrosis only in MAFLD(+)/NAFLD(-) but not observed in MAFLD(+)/NAFLD(+) with T2DM (Supplementary table 1). Patients with viral hepatitis were associated with a 6.77 (CI: 3.92 to 11.7, p < 0.001) times increase odds of advanced fibrosis.
Adjusted effect size of risk factors associated with adverse events in patients with MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+)
MAFLD, Metabolic Associated Fatty Liver Disease; NAFLD, Non-alcoholic Fatty Liver Disease; Hba1c, Hemoglobin A1C; BMI, Body Mass Index; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; FIB-4, Fibrosis-4 score; IQR, Interquartile range; 95%CI, 95% Confidence Interval; * bolded p-value ≤0.05 denotes statistical significance.
In all-cause mortality, there were shared risk factors between the two diseases, including age, gender, and smoking history (supplementary table 1) between MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+). However, ethnicity influence MAFLD(+)/NAFLD(-) differently from MAFLD(+)/NAFLD(+). Additionally, a higher Hba1c level was associated with a higher risk of all-cause mortality (HR: 1.06, CI: 1.00 to 1.12, p = 0.037) and CVD mortality (SHR: 1.16, CI: 1.08 to 1.24, p < 0.001) in MAFLD(+)/NAFLD(-) but not in MAFLD(+)/NAFLD(+). While alcohol consumption did not increase the risk of all-cause mortality and cardiovascular mortality, a diagnosis of viral hepatitis significantly increases the risk of all-cause mortality in MAFLD(+)/NAFLD(-) (HR: 1.75, CI: 1.29 to 2.40, p < 0.001).
4DiscussionThe debate on the most appropriate definition of fatty liver has resulted in significant divides in the international community [25,26]. While previous studies have compared outcomes between MAFLD and NAFLD, there has yet to be a specific analysis within the population with T2DM despite the prevalent nature of fatty liver amongst patients with T2DM. T2DM and fatty liver share commonalities in pathogenesis and are predisposed to developing hepatic steatosis. De-novo hepatic de-novo lipogenesis is a major pathway in fatty liver development and hyperglycaemia induces transcription factor carbohydrate response element binding protein while hyperinsulinemia increases transcription factor Sterol regulatory element-binding protein 1 activity, resulting in hepatic lipogenesis from the increased abundance of fatty acyl-CoAs [8,27]. The MAFLD criteria allow for any individual with T2DM and hepatic steatosis to be included in MAFLD, while NAFLD can only be made with the exclusion of other chronic liver diseases and the absence of significant alcohol use. As a result, our study shows that the change in criteria had a 68.89% (n = 2032) increase in fatty liver diagnosis in T2DM.
Previous estimates have suggested a hepatic steatosis prevalence upwards of 75% in the population with T2DM [28,29]. However, a recent study conducted to differentiate subtypes of MAFLD also found significant variance within MAFLD where metabolically healthy individuals made up 68% of MAFLD in the general population [30]. Our study supports the notion and shows that even in individuals with T2DM who are categorized as metabolically unhealthy, there were distinct differences in patients who were MAFLD(+)/NAFLD(-) and were at a higher risk of MACE, advanced fibrosis, all-cause and cardiovascular-related mortality compared to MAFLD(+)/NAFLD(+) [31,32]. While NAFLD focuses on the liver [3], MAFLD, in turn, seeks to capture systemic factors associated with hepatic steatosis, with T2DM forming a major factor for inclusion [25,31,33,34]. In turn, the increased risk of adverse events might not be an accurate representation of a derivative risk from fatty liver but a result of systemic commodities associated with the condition. The two conditions also differ in predictors of adverse events where a higher Hba1c was associated with MAFLD(+)/NAFLD(-) for all-cause and cardiovascular-related mortality, but the same was not observed in MAFLD(+)/NAFLD(+).
The presence of viral hepatitis amongst patients with T2DM was additionally found to significantly increase the risk of advanced fibrosis and all-cause mortality. This is unsurprising given the presence of dual liver aetiology resulting in higher mortality. A major point against the change of definition relates to the inclusion of hepatic steatosis from viral hepatitis, which has been linked to a higher prevalence of fibrosis in liver biopsy [35]. The presence of hepatic steatosis in viral hepatitis is also associated with a higher risk of overall mortality, HCC and decompensation compared to non-MAFLD viral hepatitis [36–38] and the inclusion of viral hepatitis into MAFLD may omit important differentiating factors of chronic liver disease. Additionally, male patients in MAFLD(+)/NAFLD(-) were found to be at an increased odds of advanced fibrosis. This finding is interesting given the notion that females have conventionally been linked to a higher risk of advanced fibrosis in NAFLD. The finding may be explained by the inclusion criteria where MAFLD(+)/NAFLD(-) patients are more likely to be males with increased rates of metabolic dysfunction, which influences the risk of advanced fibrosis.
MAFLD is not without its benefits and allows for the inclusion of all hepatic steatosis in individuals with T2DM without the nuisance of ruling out other chronic liver disease causes, which might be beneficial to non-hepatologist [39]. Nevertheless, classifying all T2DM patients with liver steatosis under an umbrella term may result in significant heterogeneity between different subcategories of patients and increase the hurdle in the ongoing effort for NAFLD drug development. These individuals may respond to treatment differently from what has been proposed in traditional NAFLD [40–42] and may benefit from a focus on the reduction of systemic events rather than liver fibrosis. Additionally, the change should be conducted with caution as it results in a 68.89% increase in fatty liver diagnosis amongst individuals with T2DM and would, in turn, require better risk stratification to prevent an over-inclusion of fatty liver.
4.1LimitationsThis paper explores the differences in clinical characteristics and longitudinal outcomes in T2DM patients who have MAFLD(+)/NAFLD(-) and MAFLD(+)/NAFLD(+). However, there are several limitations to the current study. Even though an imaging-based diagnosis of fatty liver is preferred in the setting of population studies, blood-based non-invasive tests were employed for the diagnosis of hepatic steatosis in this study due to its practicality in data availability. While imperfect, the fatty liver index is commonly used in population-based studies [43–45] for identifying hepatic steatosis and has an AUROC of 0.83 relative to biopsy [46]. We could also not explore the effect of post-menopausal women in the dataset, given the lack of coding in the primary data frame.
Additionally, the low rates of CKD at a population level may limit the power of the study to detect statistical significance. Furthermore, quantifying alcohol intake through self-report questionnaires rather than monitor-based measures invites room for misclassification, recall and social desirability biases. Finally, to improve the rigors of the study, results of cancers were omitted as the relevant data in NHANES is based on an interview-based recollection by patients rather than a formal diagnosis of cancer, hence subjecting the data to potential bias.
5ConclusionsThe change from NAFLD to MAFLD significantly affects the population with T2DM with a 68.89% increase of fatty liver diagnosis amongst patients and results in an over-diagnosis and exaggerated end-organ complications with MAFLD. In turn, the change in definition creates much heterogeneity in fatty liver and may also require a refocus on the prevention of systemic events, given the significant comorbidities amongst patients with T2DM.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data AvailabilityData were retrieved from the National Health and Nutrition Examination Survey Registry.
Author ContributionsConceptualization and Design: Cheng Han Ng, Mark Muthiah; Acquisition of Data: Cheng Han Ng, Wen Hui Lim, Kai En Chan, Clarissa Elysia Fu; Analysis and Interpretation of Data: Cheng Han Ng, Wen Hui Lim, Darren Jun Hao Tan, Kai En Chan, Clarissa Elysia Fu, Bryan Tan; Writing – original draft: Cheng Han Ng, Wen Hui Lim, Kai En Chan, Darren Jun Hao Tan, Clarissa Elysia Fu; Writing – review & editing: Benjamin Nah, Eunice Tan, Yock Young Dan, Nicholas WS Chew, Nicholas Syn, Jiong-Wei Wang, Nilofer Sayed, Mohammad Shadab Siddiqui, Arun J. Sanyal, Mazen Noureddin, Mark Muthiah, Gwyneth Kong, Jieling Xiao, Jie Ning Yong. All authors approve the final version of the manuscript, including the authorship list and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.