We initiated this study to explore the efficacy of camrelizumab combined with transcatheter arterial chemoembolization (TACE) plus sorafenib or lenvatinib versus TACE plus sorafenib or Lenvatinib for unresectable hepatocellular carcinoma (HCC).
Materials and methodsFrom June 2019 to November 2022, 127 advanced HCC patients were retrospectively analyzed in this study. This consisted of 44 patients that received camrelizumab plus TACE plus sorafenib or lenvatinib (triple therapy group) and 83 patients that received TACE plus sorafenib or lenvatinib (double treatment group). The overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) were compared between the two patient groups.
ResultsOur findings demonstrated that patients received the triple therapy exhibited superior median OS (15.8 vs. 10.3 months, P=0.0011) and median PFS (7.2 vs. 5.2 months, P=0.019) compared to the double treatment group. In addition, the triple therapy group exhibited better 6-month (93.5% vs. 66.3%), 12-month (67.2% vs. 36.3%), and 24-month (17.2% vs. 7.6%) survival rates than the double treatment group. However, the ORR (43.2% vs. 28.9%, P = 0.106) and DCR (93.2% vs. 81.9%, P = 0.084) of the two groups were similar. Subgroup analysis showed that compared with the double treatment group, the triple therapy group had a better mOS for HCC with HBV (15.8 vs. 9.6 months, P = 0.0015) and tumor diameter ≥ 5cm (15.3 vs. 9.6 months, P = 0.00055).
ConclusionsCamrelizumab plus TACE and sorafenib or lenvatinib may be a promising treatment approach for the clinical management of unresectable HCC patients.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and ranks as the fourth leading cause of cancer-related deaths worldwide [1,2]. It poses a major health challenge, particularly in regions with a high prevalence of chronic liver diseases such as viral hepatitis and alcoholic liver disease [3-5]. HCC is often diagnosed at advanced stages when curative treatment options, such as surgical resection or liver transplantation, are no longer feasible, leading to a poor prognosis for the affected patients [6-9].
In recent years, targeted therapies have emerged as promising avenues for the treatment of advanced HCC. Sorafenib, an oral multi-kinase inhibitor, is the first systemic therapy that has demonstrated a survival benefit in patients with unresectable HCC, leading to its approval as the standard first-line treatment [10]. Sorafenib blocks the RAF/MEK/extracellular signal-regulated kinase (ERK) pathway on tumor cells, and reduces angiogenesis by inhibiting VEGFR and PDGFR signaling [11,12]. Another notable targeted therapy in the management of advanced HCC is lenvatinib, an oral inhibitor of multiple receptor tyrosine kinases. Lenvatinib's mechanisms of action include the blockage of vascular endothelial growth factor (VEGF) receptors, fibroblast growth factor receptors (FGFR), and other pathways involved in tumor progression and angiogenesis [13,14]. In a phase III study, lenvatinib exhibited a similar overall survival (OS) compared to sorafenib for the treatment of inoperable HCC patients (13.6 vs. 12.3 months) [15]. In a meta-analysis conducted by Jaiswal et al. [16], lenvatinib demonstrated superior progression-free survival (PFS, HR 0.67, P < 0.00001), OS (HR 0.82, P = 0.02), objective response rate (ORR, OR 5.43, P < 0.00001), and disease control rate (DCR, OR 2.35, P < 0.00001) compared to sorafenib in the treatment of advanced HCC.
Despite the significant clinical advancements with sorafenib and lenvatinib, several challenges remain, including the emergence of therapy resistance and disease progression [17,18]. Transcatheter arterial chemoembolization (TACE) has emerged as a widely accepted and established therapeutic strategy for treating unresectable or advanced HCC. A meta-analysis of advanced HCC patients showed that the combination of lenvatinib and TACE further improved the OS (HR=0.48), PFS (HR=0.47), ORR (OR=2.54), and DCR, (OR=2.68) compared to lenvatinib alone [19]. In addition, TACE + sorafenib improved the 5-year disease-free survival (DFS, 100%) and 5-year OS (77.8%) for HCC patients [20].
In recent years, immunotherapy had revolutionized HCC treatment [21]. Camrelizumab is a humanized monoclonal antibody that belongs to the class of immune checkpoint inhibitors, specifically targeting programmed cell death protein 1 (PD-1) [22,23]. Liu et al. [24] conducted a retrospective study to explore the efficacy of camrelizumab combined with sorafenib for the treatment of advanced HCC patients. This study reported an OS of 14.1 months, PFS of 10.2 months, ORR of 17.1%, and DCR of 68.6% for the combination therapy. Xu et al. [25] conducted a phase 2 study and found that the combination of camrelizumab and apatinib could improve the ORR to 34.2%, mPFS to 5.7 months, and OS rate to 74.7% at 12 months in advanced HCC patients.
Therefore, camrelizumab has a great therapeutical potential in the treatment of HCC [26,27]. We initiated this retrospective study to explore the efficacy of camrelizumab plus TACE plus sorafenib or lenvatinib versus TACE plus sorafenib or lenvatinib in advanced HCC.
2Materials and methods2.1PatientsFrom June 2019 to November 2022, 127 HCC patients with Barcelona Clinic Liver Cancer (BCLC) stages B/C were retrospectively analyzed from three Chinese tertiary hospitals. This included 44 patients that received camrelizumab plus TACE plus sorafenib or lenvatinib (triple therapy group) and 83 patients that received TACE plus sorafenib or lenvatinib (double treatment group).
The inclusion criteria were: 1) availability of complete clinical data; 2) clinically or pathologically confirmed HCC; 3) Child A/B; 4) BCLC stage B/C; 5) Eastern Cooperative Oncology Group (ECOG) score 0-2.
The exclusion criteria were: 1) diagnosis of hepatic encephalopathy or refractory ascites; 2) simultaneous presence of other malignant tumors; 3) incomplete information; 4) previous history of liver transplant.
2.2Treatment protocol2.2.1TACEIn this study, patients were treated using the c-TACE technique. TACE was recommended for unresectable HCC patients with Child A/B and ECOG 0-2. TACE was performed using a digital subtraction angiography machine. The Seldinger technique facilitated access to the celiac trunk and superior mesenteric artery, enabling catheter angiography for precise assessment of the tumor burden and supplying arteries. Subsequently, a microcatheter was introduced into the tumor-supplying aorta, through which a combination of chemotherapeutic agents (oxaliplatin 100-200 mg + 5-fluorouracil 500 mg + epirubicin 30-50 mg) and embolic agents (iodinated oil emulsion and gelatin sponge) were infused. After confirming the successful embolization via angiography, the catheter and sheath were removed, and pressure bandages were used at the puncture point to control bleeding.
Repeated TACE procedures were performed within 1-2 months in the case of multiple lesions or large lesions. The decision to perform multiple TACE was made by HCC Expert Team.
2.3Sorafenib, lenvatinib, and camrelizumabAll enrolled patients received TACE + sorafenib (400mg, bid) or lenvatinib (<60kg, 8mg/day; ≥60kg, 12mg/day). Clinicians recommended camrelizumab (200mg, intravenous injection once every 3 weeks) to the patients within 1 week after TACE. A final treatment decision was determined by physicians after discussion with the Hospital HCC Expert Team, and taking into account the physician's and patient's preferences, and treatment costs. All patients signed informed consents before they received PD-1 inhibitors. Targeted therapy and immunotherapy were administered to the patients until there was evidence of progressive disease (PD) or the development of intolerable toxic side effects. All the drugs involved in the study (camrelizumab, sorafenib, and lenvatinib) have been approved by the National Authorities in the Research Centers.
2.4Follow-upPatients underwent MRI/CT scans every 2-3 months to assess the efficacy of the treatment. Furthermore, before each cycle of camrelizumab treatment, patients underwent laboratory tests, including the evaluation of alkaline phosphatase (ALP), white blood cells, neutrophils, alpha fetoprotein (AFP), hemoglobin, alanine aminotransferase (ALT), and other necessary assessments. We evaluated patient responses according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [28]. The OS was defined as the time span from the initiation of treatment to either the last follow-up time or the occurrence of patient death due to any cause. On the other hand, PFS was defined as the period between the start of treatment and the confirmation of progressive disease through imaging. The primary endpoint of this study was OS. The secondary endpoints were PFS, ORR, and DCR.
2.5Statistical analysisAll baseline characteristics were presented using categorical variables. Baseline characteristics between the triple therapy group and the control group were compared using the chi-square test. We evaluated the survival of the two groups using Kaplan-Meier analysis and log-rank tests. Subsequently, we introduced variables with P < 0.05 confirmed in univariate analysis into multivariate analysis to identify independent prognostic factors for PFS and OS. All data in this study were processed using SPSS for Windows (version 26.0) and R software (version 3.3.2), where a two-tailed P-value less than 0.05 indicated statistical significance.
2.6Ethical statementThis retrospective study complied with the principles outlined in the Declaration of Helsinki and was approved by the Clinical Trial Ethics Committees, including Luxian People's Hospital, Chongqing General Hospital, and The Affiliated Hospital of Southwest Medical University. Given the retrospective nature of this study, informed consent was waived, and the ethics committees did not require patients to review their medical records. All patient data were kept confidential and anonymous.
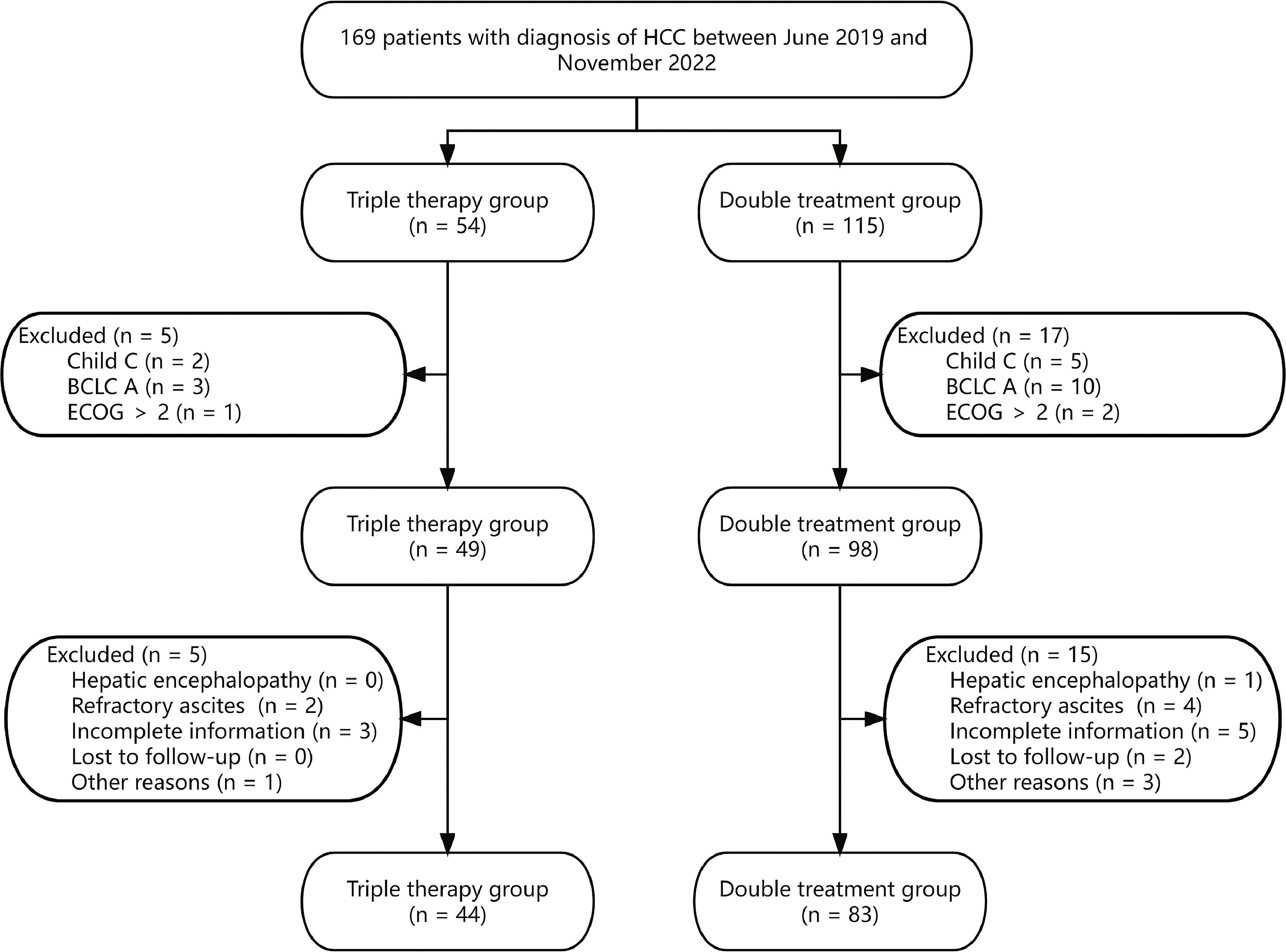
3Results3.1Patient characteristicsThis study included 127 advanced HCC patients (Fig. 1). Among them, 44 patients were treated with camrelizumab plus TACE plus sorafenib or lenvatinib (triple therapy group), while the remaining 83 patients received TACE plus sorafenib or lenvatinib (double treatment group).
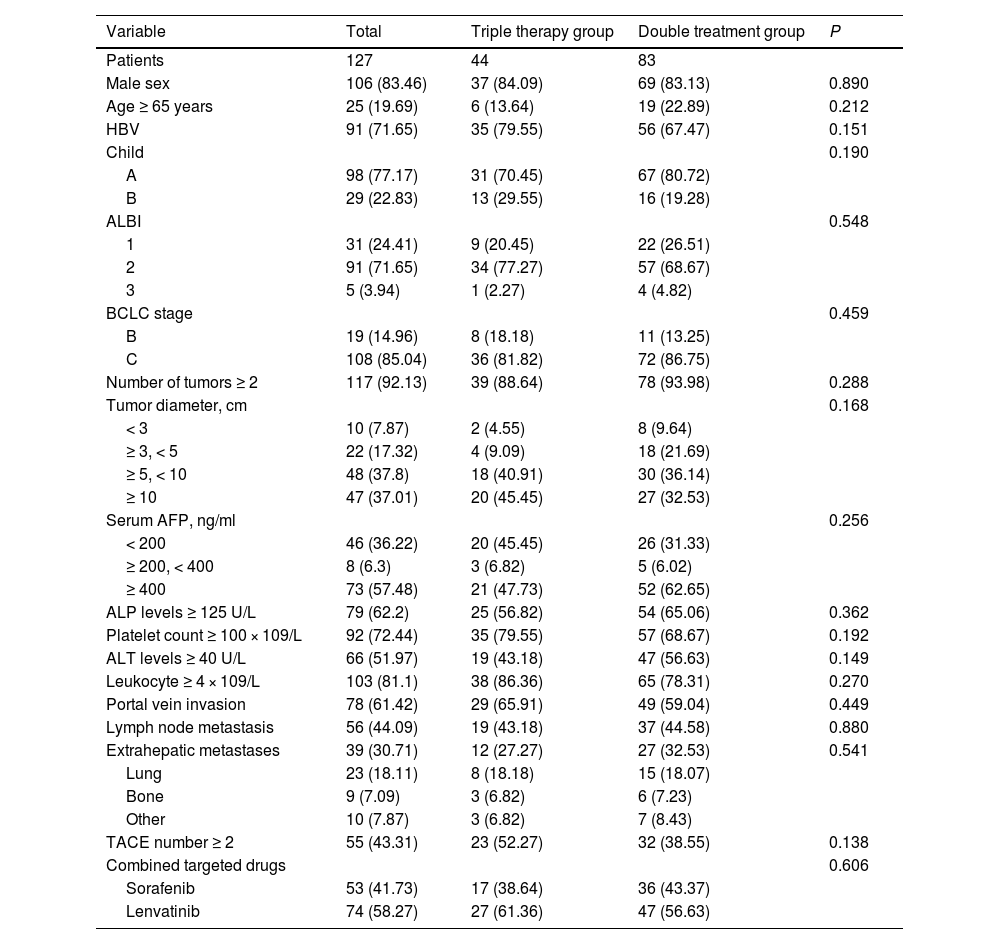
The majority of patients in this study were male (83.5%), with hepatitis B virus (HBV) infection (71.7%), classified as Child A (77.2%), and diagnosed with BCLC C (85.0%). Furthermore, there were 10 (7.9%), 22 (17.3%), 48 (37.8%), and 47 (37.0%) patients with tumor diameters < 3cm, 3-5 cm, 5-10cm, and ≥ 10cm, respectively. Additionally, among the HCC patients included in this study, 78 (61.4%) patients were diagnosed with portal vein tumor thrombosis (PVTT), 56 (44.1%) patients with lymph node metastasis, and 39 (30.7%) patients with extrahepatic metastasis. In addition, in the triple combination therapy group and the double treatment group, the proportions of patients with TACE numbers ≥ 2 were 52.3% and 38.6% respectively. The baseline differences between the triple therapy group and the double treatment group were examined, and it was observed that all covariates were comparable (all P < 0.05, Table 1).
Baseline characteristics of the patients
Abbreviations: HBV, hepatitis B virus; ALBI, albumin–bilirubin; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; TACE, transcatheter arterial chemoembolization.
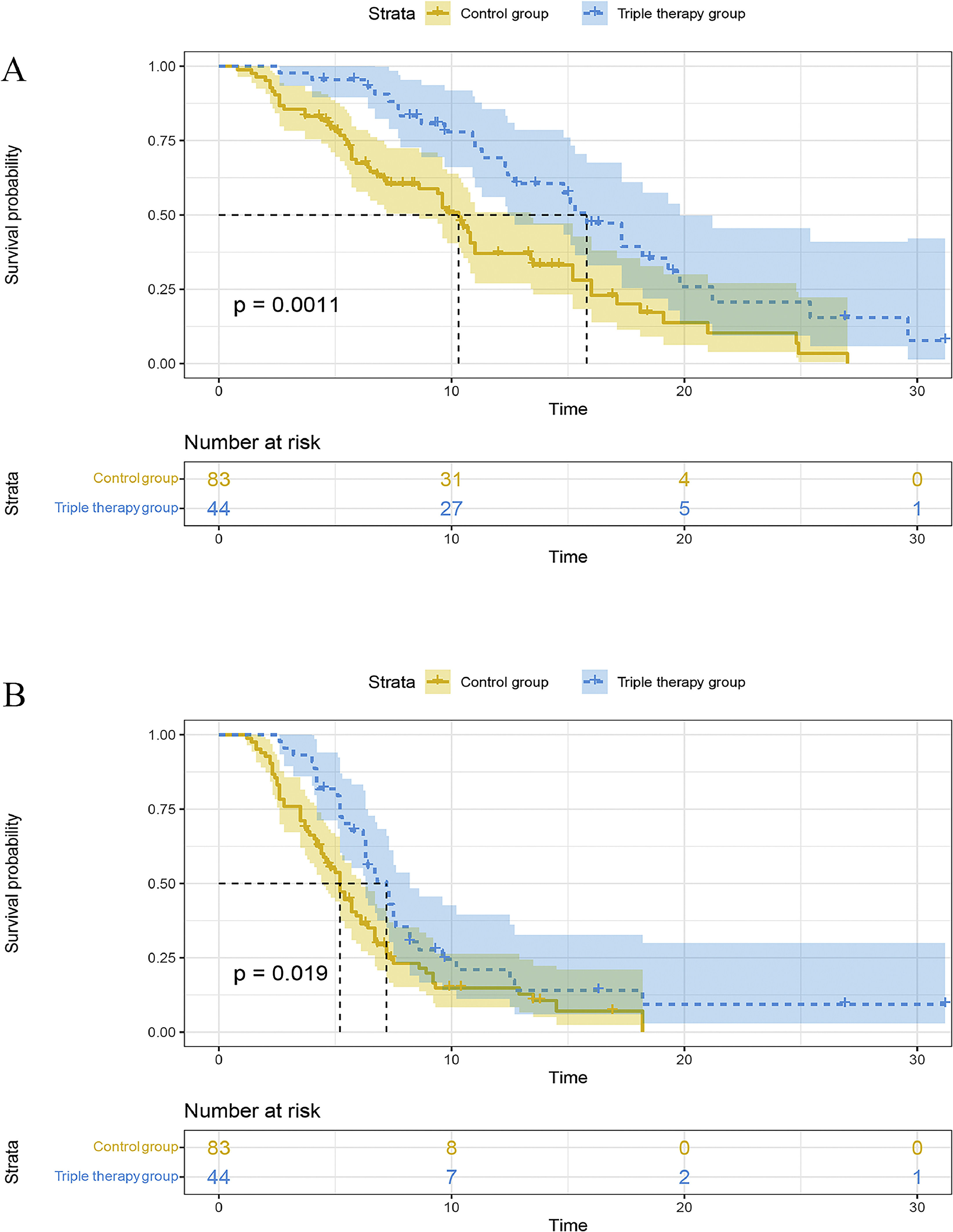
The TACE plus sorafenib or lenvatinib group had a median OS (mOS) of 10.3 (95CI% 8.6-13.4) months, whereas the camrelizumab plus TACE plus sorafenib or lenvatinib group showed a mOS of 15.8 (95CI% 12.4-19.8) months, with statistically significant differences between the two groups (P=0.0011). In addition, the triple therapy group showed better 6-month (93.5% vs. 66.3%), 12-month (67.2% vs. 36.3%), and 24-month (17.2% vs. 7.6%) survival rates than the double treatment group (Fig. 2A).
Moreover, the triple therapy group exhibited a superior median PFS (mPFS) compared to the double treatment group [7.2 (95CI% 6.3-8.2) vs. 5.2 (95CI% 4.5-6.1) months, P=0.019, Fig. 2B].
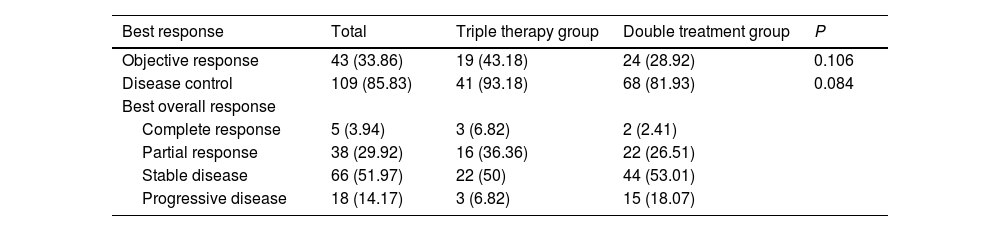
3.3Tumor responseWithin the triple therapy group, the distribution of patients was as follows: 3 (6.8) patients achieved complete response (CR), 16 (36.4) patients achieved partial response (PR), 22 (50.0) patients had stable disease (SD), and 3 (6.8) patients experienced PD. In contrast, the double treatment group had 2 (2.4) patients with CR, 22 (26.5) patients with PR, 44 (53.0) patients with SD, and 15 (18.1) patients with PD. The ORR (P=0.106) and DCR (P=0.084) observed in both groups of patients were similar (Table 2).
Tumor response assessed by mRECIST
Abbreviation: mRECIST, modified Response Evaluation Criteria in Solid Tumors.
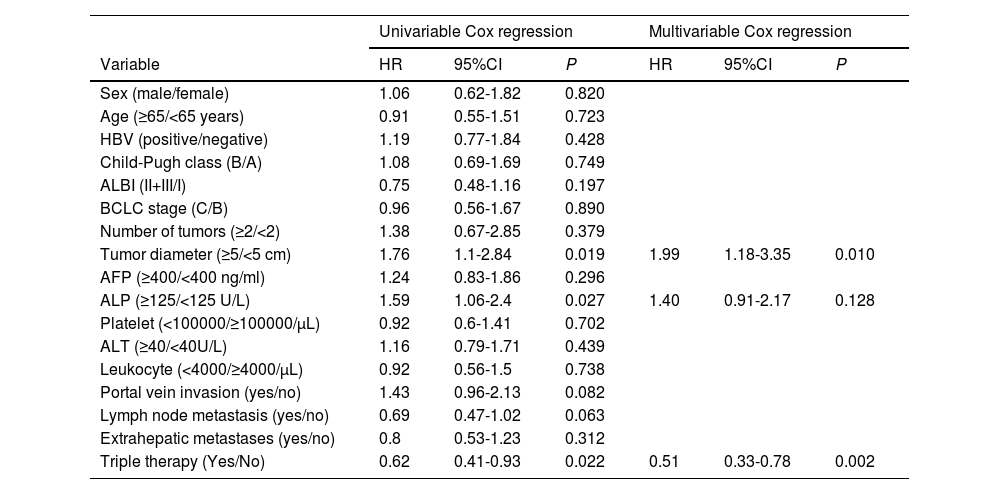
Cox analysis was performed to investigate the risk factors influencing PFS and OS. Univariate Cox analysis revealed that tumor diameter (HR 1.764, 95CI% 1.096-2.838, P=0.019), ALP levels (HR 1.592, 95CI% 1.055-2.403, P=0.027), and receiving triple therapy (HR 0.620, 95CI% 0.411-0.934, P=0.022) were risk factors that affected PFS. Subsequently, multivariate Cox analysis confirmed that tumor diameter and triple therapy were independent predictors of PFS (Table 3).
Univariate and multivariate Cox regression analysis of progression-free survival
Abbreviations: HBV, hepatitis B virus; ALBI, albumin–bilirubin; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferas.
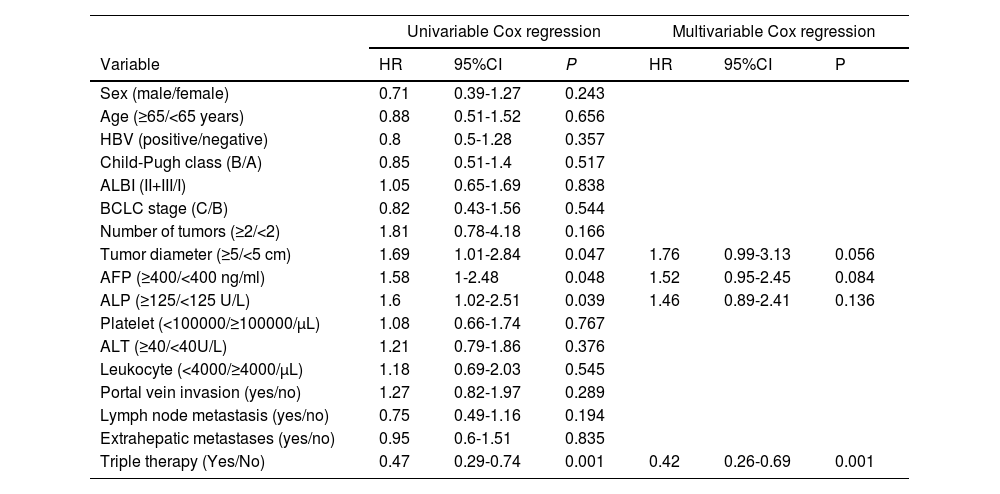
Univariate Cox analysis revealed that tumor diameter (HR 1.692, 95CI% 1.007-2.843, P=0.047), AFP (HR 1.579, 95CI% 1.004-2.483, P=0.048), ALP levels (HR 1.603, 95CI% 1.023-2.512, P=0.039), and receiving triple therapy (HR 0.465, 95CI% 0.290-0.744, P=0.001) were risk factors that affected OS. In multivariate analysis, only triple therapy emerged as an independent predictor of OS (Table 4).
Univariate and multivariate Cox regression analysis of overall survival
Abbreviations: HBV, hepatitis B virus; ALBI, albumin–bilirubin; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferas.
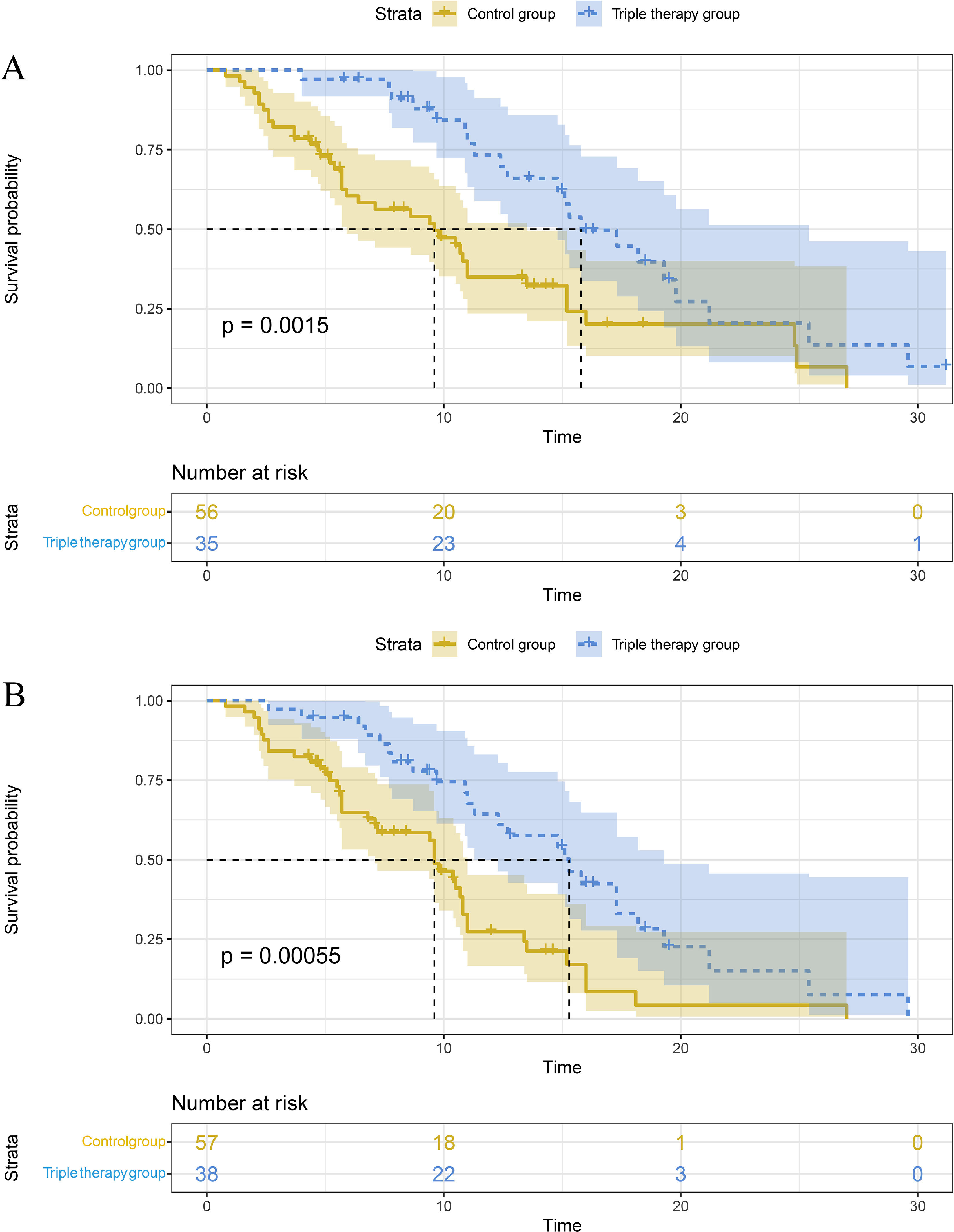
We further investigated the baseline heterogeneity between the HBV group and the tumor diameter ≥ 5 cm group. It was confirmed that there were no significant differences in all baseline characteristics between the triple treatment group and the double treatment group. The results showed that compared with the double treatment group, the triple therapy group had a better mOS for HCC with HBV [15.8 (12.6-19.0) vs. 9.6 (5.4-13.8) months, P = 0.0015; Fig. 3A] and tumor diameter ≥ 5cm [15.3 (11.6-19.0) vs. 9.6 (8.5-10.8) months, P = 0.00055; Fig. 3B].
4DiscussionHCC continues to be a major global health concern, primarily due to its elevated rates of incidence and mortality [3,29]. The combination of TACE with targeted therapy is currently a common therapeutic approach for treating patients with advanced HCC [30-32]. Therefore, we initiated this retrospective study to investigate the prognosis of camrelizumab plus TACE plus sorafenib or lenvatinib compared to TACE plus sorafenib or lenvatinib in patients with advanced-stage HCC.
Despite efficient local control of TACE, it doesn't guarantee complete eradication of the tumor. Even with the obstruction of hepatic artery post-TACE, the tumor can continue to receive nutrients through the portal vein, which may facilitate tumor cell survival and recurrence [33]. Our findings revealed that patients treated with triple therapy had superior mOS (15.8 vs. 10.3 months, P=0.0011) and mPFS (7.2 vs. 5.2 months, P=0.019) compared to those in the double treatment group. Additionally, the survival rates at 6-, 12-, and 24- months were notably higher in the triple therapy group, suggesting its potential role in improving short-term outcomes.
This might be related to the long-term immune response mediated by camrelizumab. Camrelizumab is known to inhibit the binding of PD-1/PD-L1, activate T cells and enhance their ability to kill tumors [34]. Research indicates that TACE not only reduces the release of regulatory T cells but also induces immunogenic cell death in tumors, releasing tumor antigens and thereby modulating the tumor immune microenvironment [35-37]. In addition, the use of targeted drugs can enhance T-cell infiltration by normalizing tumor blood vessels [38]. Therefore, the combination of these three treatments has a solid theoretical rationale for cancer therapy. Additionally, some recent published papers suggested that different immune subsets, including myeloid cells, tumor associated macrophages or exhausted T cells could affect the efficacy of immunotherapy in HCC [39-41].
Currently, various combination treatment strategies are being explored to improve the therapeutic outcome for HCC patients. Finn et al. [42] conducted a trial and reported that the combination of lenvatinib and pembrolizumab enhanced the mPFS to 9.3 months and mOS to 22 months in inoperable HCC patients. In the clinical study conducted by Yau et al. [43], advanced HCC patients that received nivolumab plus cabozantinib and ipilimumab had a mPFS of 5.1 months, mOS of 22.1 months, and ORR of 29%. In the LAUNCH study [44], it was observed that a combined first-line treatment with TACE and lenvatinib for advanced HCC significantly improved mOS (17.8 vs. 11.5 months, P < 0.001), mPFS (10.6 vs. 6.4 months, P < 0.001), and ORR (54.1% vs. 25.0%, P < .001) in comparison to patients that received lenvatinib as a monotherapy. In addition, Zhu et al. confirmed that TACE combined with immunotherapy and targeted therapy can improve the mPFS to 9.5 months, mOS to 19.2 months, and ORR to 60.1% in HCC patients [45]. TACE plays a crucial role in the comprehensive treatment of HCC [46,47].
Our study identified tumor diameter as a crucial prognostic factor affecting both PFS and OS. Larger tumor sizes were associated with poorer outcomes, highlighting the importance of early detection and intervention in HCC management [48]. Furthermore, our research also confirmed the association between elevated levels of ALP and a worse prognosis. A previously published study has also reported comparable findings [49].
Overall, our findings indicate that camrelizumab combined with TACE and targeted therapy may offer a potential therapeutic strategy for advanced HCC patients. However, these results are preliminary, and further large-scale, randomized controlled trials are necessary to validate our findings and to determine the optimal treatment regimen for improving patient outcomes and quality of life. Continued research into novel treatment modalities and personalized therapeutic approaches is essential to potentially enhance therapeutic outcomes and prognosis for advanced HCC patients in the future.
The limitations of this study are significant and must be considered. Firstly, as a multicenter, retrospective study, potential heterogeneity within the research cannot be fully excluded. Secondly, our study is constrained by a small sample size, which may introduce variability into the results. Finally, treatment decisions were influenced by the physician's and patient's preferences, as well as treatment costs, leading to a high probability of selection bias. Consequently, the results should be interpreted as exploratory and preliminary.
5ConclusionsCamrelizumab plus TACE plus sorafenib or lenvatinib may be a potential treatment for unresectable HCC patients.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author ContributionsXiumei Jiang, Pan Wang, and Ke Su participated in the writing, data curation, supervision and validation. Fei Wang, Hao Chi, and Han Li participated in the data curation and formal analysis. Fei Wang participated in methodology and software. Ke Xu and Yu Liu designed the study. All authors approved the final version of the manuscript.
Data availability statementAll data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author (nsmcxuke@163.com).
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.