Immunotherapy represents an effective and promising option in various cancers, including in hepatocellular carcinoma (HCC). The immune checkpoint inhibitors (ICIs) have shown a remarkable breakthrough in the last decade, in addition to molecular targeted therapy of angiogenesis such as tyrosine kinases inhibitors. ICIs provide new regimen that can be applied in different stages of the disease. In parallel, HCC progression is related to the tumor microenvironment (TME), involving the cross-talk between various cellular and non-cellular components within the TME niche. It appears logical to synergistically target several HCC components to increase the efficacy of the treatment. In this paper, we summarize evidence that the combination therapy of ICIs and angiogenesis inhibitors would be a potentially better strategy for HCC treatment.
Hepatocellular carcinoma (HCC) is one of the global health problems worldwide. In 2018, it accounted for an estimate of 841,000 cases and 782,000 deaths (9.3 cases and 8.5 per 100,000 person-years, respectively) [1]. The similar number between cases and deaths implies a high mortality of this disease, regardless of multiple attempts of prevention and surveillance, diagnosis, and therapy. Even though the chronic infections of hepatitis B virus (HBV) and hepatitis C virus (HCV) remain principal factors for HCC development, the prevalence of the metabolic risk factors such as non-alcoholic fatty liver disease (NAFLD) - recently termed the metabolic-associated fatty liver disease (MAFLD) [2] – is increasing, and may become the major cause of HCC globally [3].
The diagnosis of HCC is usually based on non-invasive criteria and the success of HCC treatment is notably associated with the severity of the disease [4]. When the HCC nodule is small and in early stage, surgery (partial liver resection) gives a good prognosis for the patients. Radiofrequency ablation is a potential option when surgeries are precluded. When the HCC is already in intermediate and advanced stages, chemotherapy and/or molecular therapy become applicable. The use of conventional chemotherapy unfortunately has several issues as it is non-selective and can affect both cancerous and non-cancerous cells and can generate important side effects [5]. Hence, the use of combination therapy to specifically target cancer-promoting cells and reduce toxicity has become a cornerstone for cancer treatment [6]. Also, in cases where monotherapy is ineffective, combination therapy can be applied as an additive and synergistic approach to treatment [7].
In 2007, sorafenib, an oral-systemic tyrosine kinase inhibitor (TKI), was approved for the treatment of HCC patients in advanced stages. Sorafenib has the dual-target function to inhibit the tyrosine kinases in angiogenesis and block the serine-threonine kinase Raf, which is part of the Ras/MEK/ERK signaling pathway in the cancer cell [8]. Sorafenib was reported to extend the overall survival (OS) of the patients for about 3 months [9]. Starting from 2017, other TKIs such as lenvatinib, a multikinases inhibitor targeting VEGFR1-3, FGFR1-4, PDGFRα, RET, and KIT that showed clinical activity and acceptable toxicity profiles in patients with advanced HCC [10], were approved as a first-line therapy [11], while regorafenib [12], cabozantinib [13], ramucirumab [14], as second-line treatment after sorafenib. However, patients who rely on the rather moderate outcome of systemic therapies, often display various side effects, chemoresistance, and dismal prognosis [15]. In an attempt to further improve treatment options, immunotherapy has been added for advanced stages tumors for potentially better results [16].
2Tumor microenvironment and angiogenesisHCC is a complex disease. Due to its various etiologies and long-term disease progression, there is a vast tumoral heterogeneity occuring between patients and in the same tumor. HCC heterogeneity is mirrored by genetic mutations and aberrations, and the various HCC classifications are based on histology, transcriptomic subtypes, and immunologic characteristics [17,18]. However, among the diverse HCC profiles, hypervascularity and vascular abnormalities are common findings in this type of malignancy, since the tumor would rely on the formation of new blood vessels to grow [19].
Most HCCs are highly vascularized, emphasizing the significance of angiogenesis in the understanding of the pathogenesis of the disease and the development of therapy. Angiogenesis is acknowledged as being stimulated by hypoxia. However, at least in human HCC, a direct and accurate measurement of oxygen concentration, oxygen pressures, and evaluation of the effect of hypoxia is difficult, particularly at the cellular level and therefore its mechanism is still unclear [20,21].
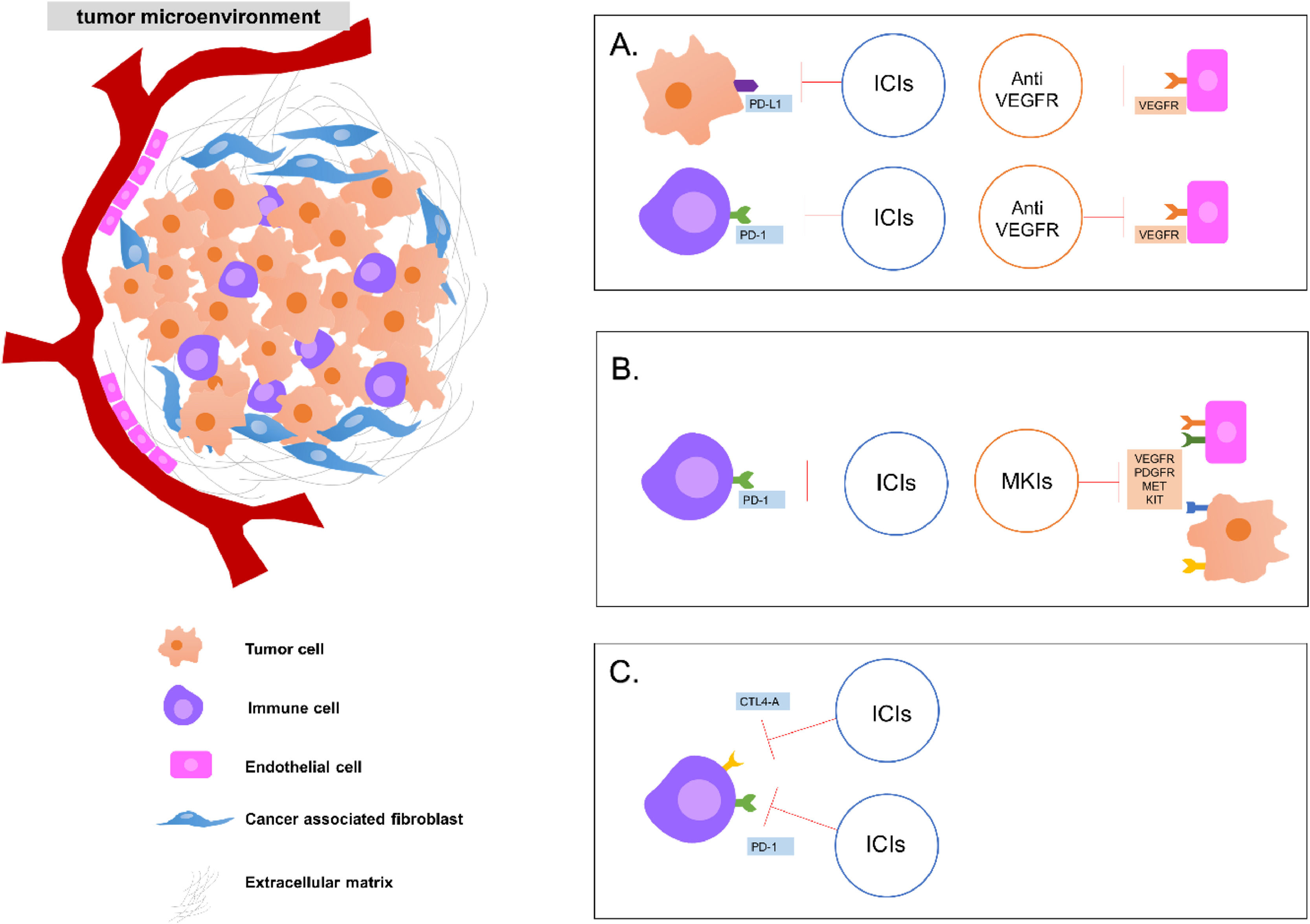
Current combined therapy approaches for the treatment of advanced hepatocellular carcinoma targeting players of the tumor microenvironment. Some strategies of combined therapies: A. Combination of ICIs (either in the tumor cell or in the immune cell) and anti-angiogenesis drugs targeting VEGFR in the vascular endothelial cell. B. Combination of ICIs and MKIs found in endothelial cells and tumor cells and C. Combination of two ICIs targeting immune cells. ICI: immune checkpoint inhibitors; VEGFR: vascular endothelial growth factor receptor; MKI: multikinases inhibitors.
From the molecular level, angiogenesis is prominently regulated by vascular endothelial growth factor (VEGF) produced by cancer cells and VEGF receptor (VEGFR) by vascular endothelial cells. VEGFR is overexpressed in HCC and it leads to an abnormal formation of blood vessels that cause abnormal blood flow resulting in lack of oxygen [22]. High expression and distinctive membrane localization of VEGFR in HCC cells had been associated with HCC progression and worse outcomes [23].
Several other pro-angiogenic factors such as fibroblast growth factors (FGF), platelet-derived growth factors (PDGF), angiopoietins, hepatocyte growth factor (HGF), endoglin (CD105), and others have also been significantly involved (reviewed in [24]). The binding between the pro-angiogenic factors (e.g. VEGF) and its receptor (e.g. VEGFR) leads to phosphorylation of tyrosine kinases stimulating a cascade of intracellular pathways. This induces the proliferation and migration of endothelial cells that subsequently results in the formation of new blood vessels [24,25].
Together with hypoxia, persistent inflammation is also a solid stimulus for pathological angiogenesis and vascular remodeling [26]. Hypoxia also affects epithelial-mesenchymal transition and mainly changes the expression of immune checkpoint molecules as PD-L1, CD47, PD-1, and HLA-G [27]. It is known that HCC is mostly an immunologic cancer where the development of malignancy is closely associated with its tumor microenvironment (TME), accompanied by chronic liver inflammation and cirrhosis, shown by the high infiltration of immune cells in the TME.
TME is composed of cellular (e.g. immune cells, fibroblast, and endothelial cells) and non-cellular components (e.g. extracellular matrix (ECM), cytokines, and growth factors) that together establish a bidirectional communication to support the growth of the tumor and metastasis [28]. During its progression, HCC cells interact closely with cellular and non-cellular components in the TME. Besides supporting the growth of HCC tumor cells, TME has significant immunosuppressive elements that may affect response to immunotherapy [29].
The infiltration of specific immune cells in the TME, such as CD163+ macrophages and CD8+ T cells is also associated with HCC prognosis [30]. For instance, tumor-infiltrating CD8+ T cells and the expression of programmed cell death protein 1 (PD-1), whereas HCCs with PD-1-high cells population were characterized to be aggressive with higher levels of predictive biomarkers of response to anti-PD-1 therapy [31,32].
As reviewed previously [33], the immune factors in the TME plays a significant function in HCC growth and response to therapy. The immune tolerance of TME is due to the presence of myeloid-derived suppressor cells (MDSCs), T regulatory (Treg) cells, and tumor-associated macrophages (TAMs). To add, the interactive mechanism between alterations of immune checkpoint molecules, reduction of anti-tumor effector cells (natural killer cells and dendritic cells), as well as changes in levels of cytokines contribute to the TME's ability to promote tumor immune escape [33,34]. The presence of VEGF in TME also has an immunosuppressive effect. The release of VEGF can increase the number and function of immunosuppressive cells, including Tregs, MDSCs and TAMs [35].
The inhibition of angiogenesis also exerts a profound effect on the TME [36]. It is reported that TME factors, including hypoxia, angiogenesis, and various immune responses are key contributors to tumor progression after locoregional treatment [37]. In cancer cells, NF-κβ (nuclear factor kappa β) and HIF-1α (hypoxia-inducible factor 1α) were shown to upregulate programmed cell death ligand 1 (PD-L1) expression [38]. Increased HIF-1α level is also associated with high PD-L1 expression and increased risk of cancer recurrence and metastasis of HCC [39].
3Immune checkpoint inhibitions: monotherapy vs. combined therapyAs briefly mentioned above, various TKIs as molecular targeted agents have been under clinical trials phase III for the treatment of HCC, including sorafenib and lenvatinib as first line treatment [24]. In the last decade, however, immunotherapy provides another attractive option for cancer therapy.
Among various approaches of immunotherapies, including adoptive cell transfer, cancer vaccine, and others, the therapy with immune checkpoint inhibitors (ICIs) has shown a remarkable breakthrough in various types of cancers. Targeting mutual interaction (the checkpoint) between immune and tumoral cells is beneficial since it can be applied in various stages of cancer. Several selected studies of ICIs therapies on advanced HCC, either as monotherapy or as combined therapy with anti-angiogenesis drugs are listed in Table 1.
Selected studies of combined therapies using immune checkpoint and angiogenesis inhibitors in patients with advanced HCC.
| Trial | Agents | Target | N | Result | Refs. |
|---|---|---|---|---|---|
| Monotherapy | |||||
| CheckMate 040 (NCT01658878), phase 1/2 | nivolumab | PD-1 | 262 | OS: 15.0 monthsORR: 15-20% | [40] |
| Keynote 224 (NCT02702414), phase 2 | pembrolizumab | PD-1 | 104 | OS: 12.9 monthsORR: 17%PFS: 4.9 months | [41] |
| CheckMate 459 (NCT02576509), phase 3 | Nivolumab vs sorafenib | PD-1 vs VEGFR, PDGFR, Ras/Raf/Mek/Erk | 743 | OS: 16.4 vs 14.7 monthsORR: 15% vs 7% | [45] |
| Combined therapy | |||||
| VEGF Liver 100 (NCT03289533), phase 1b | avelumab + axitinib | PD-1, VEGFR | 22 | ORR: 13.6% PFS: 5.5 months | [48] |
| RESCUE (NCT03463876), phase 2 | camrelizumab + apatinib | PD-1, VEGFR | 190 | First line: ORR 34.3%, mPFS 5.7 months; Second line: ORR 34.3% PFS 5.5 months | [53] |
| KEYNOTE524 (NCT03006926), phase 1b | pembrolizumab + lenvatinib | PD-1, VEGFR, FGFR, PDGFR, RET, KIT | 104 | ORR 46%, PFS 9.3 months, OS 22 months | [57] |
| CaboNivo (NCT03299946), phase 1b | nivolumab + cabozantinib | PD-1, VEGFR, AXL, MET | 15 | Enhanced resectability | [56] |
| CheckMate 040 (NCT01658878), phase 1/2 | nivolumab + ipilimumab | PD-1, CTLA-4 | 148 | ORR: between 27% and 32%OS: between 12.5 and 22.8 month | [59] |
| Combined therapy vs monotherapy | |||||
| GO301240 (NCT02715531), phase 1b | atezolizumab + bevacizumab vs atezolizumab | PD-L1, VEGFR vs PD-L1 | 223 | PFS: 5.6 vs 3.4 months | [42] |
| IMbrave150 (NCT03434379), phase 3 | atezolizumab + bevacizumab vs sorafenib | PD-L1, VEGFR vs VEGFR, PDGFR, Ras/Raf/Mek/Erk | 501 | OS: 19.2 vs 13.2 months PFS: 6.8 vs 4.3 months | [50] |
| COSMIC-312 (NCT03755791), phase 3 | atezolizumab + cabozantinib vs sorafenib | PD-L1, VEGFR, AXL, MET vs VEGFR, PDGFR, Ras/Raf/Mek/Erk | 837 | PFS: 6.8 vs 4.2 months OS: 15.4 vs 15.5 months | [55] |
| HIMALAYA (NCT0329845), phase 3 | tremelimumab + durvalamab vs durvalumab vs sorafenib | CTLA-4, PD-L1 vs PD-L1 vs VEGFR, PDGFR, Ras/Raf/Mek/Erk | 1171 | ORR: 20.1 vs 17.0 vs 5.1%, 24-month OS: 40% vs 39.6 vs 32.6 months | [63] |
In HCC, ICIs targeting the immune cell molecules such as the PD-1 using nivolumab [40] and pembrolizumab [41] and its ligand the PD-L1 using atezolizumab [42] as well as the cytotoxic T lymphocyte antigen 4 (CTLA-4) using tremelimumab [43,44] had given promising results in HCC patients with or without previous sorafenib treatment. Nivolumab showed a manageable safety profile, including acceptable tolerability with the objective response rate (ORR) ranging between 15% and 20% [40].
Currently, two ICIs, nivolumab and pembrolizumab, are already approved as second-line therapy after sorafenib for the treatment of advanced HCC [16]. The first evidence of the safety and efficacy of nivolumab in HCC patients was assessed in a Phase 1/2 randomized trial Checkmate 040 study. Based on these results, the FDA accelerated the approval of nivolumab as a conditional treatment for patients treated with sorafenib. A subsequent randomized, multicenter phase 2 study (CheckMate 459) looked into the efficacy of nivolumab as a first-line treatment vs sorafenib. This study resulted in a not statistically significant result, with the median OS of 16.4 vs 14.7 months for nivolumab and sorafenib, respectively, despite a higher ORR in nivolumab (15%) as compared to sorafenib-treated patients (7.0%) [45].
Another anti-PD-1, pembrolizumab, was also evaluated for its safety and efficacy in advanced HCC. A non-randomized, multicenter phase 2 clinical trial KEYNOTE-224 demonstrated an ORR of 17% with the median of time to progression (TTP) and progression free survival (PFS) of 4.9 months with an OS of 12.9 months [41]. A double-blind, randomized phase 3 KEYNOTE 240 study evaluated the safety and efficacy of pembrolizumab in 413 advanced HCC patients in119 medical centers in 27 countries. The ORR was 18.3% vs 4.4%, and OS of 13.0 vs 10.6 months for pembrolizumab vs placebo [46].
For anti PD-L1, the ICI against the PD-L1 atezolizumab without or in combination with bevacizumab in untreated patients with unresectable HCC showed an acceptable low side-effect profile and promising antitumor activity. However, the atezolizumab monotherapy alone had the PFS of 3.4 months vs 5.6 months in atezolizumab plus bevacizumab [42].
At the moment, numerous clinical trials of phase I/2 are underway to determine the potentiality of other ICIs, such as spartalizumab (PDR001, anti PD-1) [47], avelumab (anti PD-1) [48], camrelizumab (anti-PD-1), durvalumab (anti-PD-L1) [49], and tremelimumab (anti CTLA-4) [43].
3.2Combined therapy: ICIs and specific VEGFR inhibitorsUp to now, one of the most encouraging clinical trial data for HCC is the combined therapy using anti-PD-L1 atezolizumab and anti-VEGFR bevacizumab. The results of the phase 1b trial GO301240 for unresectable HCC resulted in PFS of 5.6 months of combined therapy compared to 3.4 months in atezolizumab monotherapy alone [42]. This study was then followed by the IMbrave150, a global, open-label, phase 3 trial, involving 501 unresectable HCC patients at 111 sites in 17 countries. This study looked into the antitumor effects of this combined therapy compared to sorafenib alone, resulting in a median PFS of 6.8 vs 4.3 months for combination therapy vs sorafenib, as well as a better OS of 19.2 vs 13.2 months. This study concluded that in patients with unresectable HCC, atezolizumab combined with bevacizumab resulted in better overall and progression-free survival outcomes than sorafenib [50,51].
Several other clinical studies also used the combination of anti PD-1 and VEGFR inhibitors. The phase 1b clinical trial VEGF Liver 100, explored the combinatory effects of avelumab and axitinib (VEGFR1-3 inhibitor) [52] in naïve, advanced HCC patients. Results showed an ORR of 13.6% while and a median PFS of 5.5 months. The safety profile of the combined drugs is comparable to the safety profiles of the drugs when administered as monotherapies for HCC management. The follow-up study of this clinical trial is currently ongoing [48].
Another study is the phase 2 clinical trial for the combination of camrelizumab and apatinib (VEGFR-2 inhibitor) in the first (treatment naïve) and second-line setting (intolerant/refractory to targeted therapy) for advanced HCC. The study resulted in an ORR of 34.3% and 22.5% and a median PFS of 5.7 and 5.5 months for the first-line and second-line, respectively, [53].
3.3Combined therapy: ICIs and multikinases inhibitorsThe combination between ICIs and multikinases inhibitors (MKIs) targeting several molecules in angiogenesis are also underway. One of the most encouraging studies is the combination between atezolizumab and cabozantinib. Cabozantinib is a multikinases inhibitor against VEGFR2, AXL, and c-MET [13]. Like VEGF, the receptor tyrosine kinases MET and AXL is induced by tumor hypoxia [54].
A phase 3 study COSMIC-312 was conducted in 837 treatment-naïve patients, and compared three arms: the combination therapy of cabozantinib and sorafenib alone. Results showed a median PFS of 6.8 months vs 4.2 months and the median OS of 15.4 months vs 15.5 months for cabozantinib + atezolizumab vs sorafenib, respectively. Combination therapy significantly improved PFS vs OS [35,55].
Another combination using cabozantinib with anti PD-1 was shown in another clinical trial. The phase 1b clinical trial CaboNivo used a combination of nivolumab and neoadjuvant cabozantinib in HCC patients including ones outside of traditional resection criteria. Following treatment, 12 patients out of 15 (80%) had radiographic tumor changes after the neoadjuvant treatment which allowed enhanced resectability and underwent successful margin-negative resection [56].
Another first line treatment, lenvatinib is also used in combination with pembrolizumab. In advanced HCC, together with sorafenib, lenvatinib was also evaluated in the first-line setting for the treatment [10]. Result of the phase 1b clinical trial KEYNOTE524 study following the dose-limiting toxicity revealed an ORR of 46%, median PFS of 9.3 months and OS of 22 months by the mRECIST criteria. The combination showed a promising antitumor effects where toxicity was manageable and no unexpected safety signals were observed [57]. Across various tumor types, the LEAP clinical trial program is evaluating the safety and efficacy of lenvatinib and pembrolizumab [58].
3.4Combined therapy: combination of two ICIsSeveral trials also combined two ICIs to simultaneously target the immune cells. The CheckMate 040 cohort 4 trial is a randomized clinical which looked into the anti-tumor combined effects of two ICIs, nivolumab and ipilimumab in advanced HCC patients that were previously treated with sorafenib. Both the inhibitors act in a complementary mechanism as PD-1 and CTLA-4 inhibitors, respectively, [59]. In this study, patients were grouped into three experimental arms with different concentrations of nivolumab and ipilimumab. The results showed, an ORR between 27%and 32% and a median OS between 12.5 and 22.8 months [59]. Based on the results, the accelerated approval from FDA was granted to nivolumab and ipilimumab with the dosage recommendation for advanced HCC patients progressed after sorafenib treatment [60].
Recently, nivolumab and ipilimumab were reported to achieve durable antitumor activity and to encourage survival outcomes with acceptable toxicity in patients with advanced HCC who had prior treatment with ICIs [61]. Another study, the PRIME-HCC a two-part, multi-center, phase 1b study is currently undergoing to assess the safety and bioactivity of the nivolumab/ipilimumab combination before liver resection in early-stage HCC [62].
The efficacy of the combination between tremelimumab (anti-CTLA-4) and durvalamab (anti-PD-L1) is shown in the phase 3 HIMALAYA (NCT0329845) clinical study. To date, HIMALAYA is one of the largest phase III clinical trials with long-term follow-up and diverse representative of unresected HCC, looked into 1171 naïve patients [63]. This study compared the STRIDE regimen (tremelimumab + durvalamab) vs durvalamab or sorafenib. The resulting ORR was highest in the STRIDE group followed by Durvalamab, and least was in the Sorafenib group (20.1 vs 17.0 vs 5.1%, respectively). The 24-month OS showed 40.5% with the STRIDE group, 39.6% with durvalumab, and 32.6% with sorafenib while the 36-month OS rates were 30.7%, 24.7%, and 20.2%, respectively. The result of this study demonstrated superior efficacy of STRIDE vs sorafenib and can be considered as first-line therapy for unresectable HCC [63].
4Remarks, conclusion and perspectivesAssessing available information on HCC molecular variations and clinical data, it is rather clear that the combination therapies can be the most potent approach for advanced HCC treatment, at least at this moment. In particular, a collaborative approach to targeting the immune checkpoint and angiogenesis in HCC can be a powerful strategy. HCC treatment should not only focus on the tumor cells, and considering a combined approach on the role of TME in the development of future treatments is therefore essential (Figure 1).
One of the interesting data was observed from the work of Ho et.al. using the combination of nivolumab and cabozantinib. The study showed that cabozantinib improved the TME by enhancing systemic and local antitumor T cell responses. Analysis of peripheral blood mononuclear cells from paired pre- and post-cabozantinib samples showed an increase in the number of most effector and memory T cell subtypes. This included cell subtypes which are important signatures in antitumor immunity [56]. It shows one of the underlying rationales to target both immune and angiogenesis factors in HCC.
However, we also need to address that HCC is vastly heterogeneous. HCC heterogeneity, also in term of genomics, plays a crucial role in resistance to therapy and limits the success of available treatments. For instance, the sensitivity of ICIs against PD-1 and PD-L1 is also associated with genetic polymorphisms and molecular aberrations as we reviewed previously [64]. PD-L1 can also be activated by aberrant oncogenic signaling pathways which may take part in angiogenesis including Ras/Raf/MEK/ERK, which are targets of sorafenib [65]. Thus, to overcome the complexity of genomic aberrations in HCC, combination therapy to target multiple molecules in one hit, as our title “it takes two to tangle” would be valuable.
The fast development of immunotherapy for cancers, including for HCC, is evident. However, at least until now, there is still limited solid data on the benefits of this option for the prognosis of the patient. We need to keep ahead with different combinations of treatment regimens to define the most potent strategy for therapy. It would be beneficial if a wider cohort of studies will follow, including clinical trials for treatment naïve HCC as well as HCC in early stages to see the significance of this choice of treatment to improve the prognosis and the life quality of the patients.
Availability of data and materialsNot applicable
FundingCHCS is funded by 2020/2022 grant of the Fondazione Umberto Veronesi, Milan, Italy and LKDC by a fellowship of the Department of Science and Technology and the Philippine Council for Health Research and Development (DoST - PCHRD). The publication of this article was funded by Fundación Clínica Médica Sur.
Author's contributionsCaecilia Sukowati, Loraine Kay Cabral and Claudio Tiribelli contributed to the conceptualization and writing - review and editing. All authors read and approved the final manuscript.