The role of N-acetylcysteine (NAC) in the treatment of acetaminophen induced acute liver injury (ALI) is well established but its role in non-acetaminophen induced ALI is still elusive. We conducted this meta-analysis to evaluate the role of NAC in non-acetaminophen induced ALI. We searched electronic databases for studies published till Oct 25, 2020. We used RevMan v5.4 software to analyze the data extracted from selected studies by using Covidence systematic review software. Outcome estimation was done using Odds Ratio (OR) with 95% confidence interval (CI). The heterogeneity in various studies was determined using the I2 test. A total of 11 studies were included in quantitative analysis. Use of NAC in non-acetaminophen induced ALI showed 53% reduction in mortality compared to standard of care (OR, 0.47; CI, 0.29−0.75) and reduced mean duration of hospital stay by 6.52 days (95% CI, −12.91 to −0.13). Similarly, the rate of encephalopathy was 59% lower in the treatment group (OR, 0.41; CI, 0.20−0.83). However, the risk of developing nausea and vomiting (OR, 3.99; CI, 1.42–11.19), and the need for mechanical ventilation (OR 3.88; CI, 1.14–13.29) were significantly higher in the treatment group. These findings conclude use of NAC decreases mortality and hepatic encephalopathy compared to standard of care in patients with non-acetaminophen induced ALI. Although there is an increased risk of nausea and vomiting with the use of NAC, the majority of adverse events are transient and minor.
Acute liver injury (ALI) is a life-threatening medical emergency which involves multiple organ systems. The synthetic, metabolic and excretory functions of the liver are severely impaired in ALI. ALI is defined as a rapid development of ALI with encephalopathy in patients who previously had normal liver functions [1]. ALI is classified in two types- fulminant or hyperacute ALI and classical ALI. The diagnostic criteria for fulminant liver failure is development of jaundice and encephalopathy within 8 weeks of ALI with an International Normalized Rate > 1.5 [2]. The etiologies of ALI are diverse and include causes such as viral, autoimmune, drugs, toxins among others. Most common among these is drug-induced in USA and Europe, and viral hepatitis in Asia [3,4].
N-acetylcysteine (NAC) is a free radical scavenger which prevents the accumulation of N-acetyl-p-benzoquinone imine (NAPQI) and replenishes mitochondrial and cytosolic glutathione stores. NAC also has anti-inflammatory effects and prevents activation of inflammatory mediators such as cytokines, macrophages and neutrophils. It has inotropic and vasodilatory effect as well, and therefore, improves microcirculation to various organs including the cerebral circulation [5]. The role of NAC in the treatment of acetaminophen induced liver injury is well established [6,7]. Recent studies have shifted their focus in demonstrating the use of NAC for ALI for causes other than acetaminophen. Given its multiple mechanisms of action, small clinical trials have been done to determine its utility in non-acetaminophen induced liver damage. A systematic review done in 2016 concluded that the role of NAC could not be established based on the body of evidence present at that time, and further research in this area was recommended [8].
2ObjectiveThe aim of this study is to determine the utility of NAC in non-acetaminophen induced ALI and gauge its effectiveness in terms of mortality and survival, duration of hospitalization, safety profile and need for other interventions. We intend to objectively analyze studies on use of NAC, considering the most relevant studies published till October 2020 to provide a definitive answer on the role of NAC in treatment of non-acetaminophen induced liver failure.
3MethodsPreferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in the study [9]. The study protocol is registered in International prospective register of systematic reviews (PROSPERO ID: CRD42020222982).
3.1Criteria for considering studies for this review3.1.1Types of studiesWe systematically examined the studies reporting use of NAC in patients with ALI due to causes other than acetaminophen toxicity. Cases series with more than 5 cases, cross-sectional studies, cohort studies and clinical trials, both randomized and non-randomized, that reflected the use of NAC in non-acetaminophen induced ALI were included.
3.1.2Types of participantsPatients diagnosed with ALI due to non-acetaminophen related causes who were treated with NAC, standard of care (SOC) or both were included in our review. Patients receiving NAC were included in the treatment group and those not receiving NAC were included in the control group.
3.1.3Types of interventionsWe included patients receiving NAC alone or NAC in combination with standard of care in the treatment group whereas all patients receiving supportive care (fluids, steroid, antibiotics, vasopressors or fresh frozen plasma) were included in the control group.
3.1.4Types of outcome measuresWe analyzed the patient characteristics at admission including their clinical status, laboratory parameters-liver function tests, renal function tests and electrolytes, in order to look for improvement in such parameters in patients receiving N-acetyl cysteine. Our main aim was to determine clinical improvement/deterioration, length of hospital stay and prognosis during the end of hospital stay in patients receiving NAC as compared to those receiving standard care only.
3.2OutcomesThe primary outcome of interest in our review was the hospital mortality. Other outcomes of interest were transplant-free survival, overall survival, and duration of hospital stay in surviving patients, requirement of ICU admission and mechanical ventilation, and overall rates of adverse events including serious adverse events between the two groups.
3.3Search methods for identification of studiesTwo reviewers performed independent web-based search of electronic databases including Pubmed, PubMed Central, Embase, Scopus and Clinicaltrials.gov for studies published till Oct 25, 2020 using relevant search terms. Search terms included MeSH headings “acetylcysteine”, “liver failure”, “acute”, and “non-acetaminophen”. Title and abstract review of the studies identified by the search was done by two independent reviewers using Covidence systematic review software. Any conflicts arising during selection of studies was resolved by a third reviewer. Full text review was done similarly. Following full-text review, data was extracted for quantitative and qualitative analysis. The assessment of risk of bias and cross-checking of all the selected studies were done by another reviewer.
3.3.1Electronic searchesThe detailed search strategy has been attached in the Supplementary material 1.
3.4Data collection and analysisWe used Review Manager (RevMan) Version 5.4 to analyze the data extracted from selected studies. The heterogeneity in the included studies was determined using the I2 test. Outcomes were measured using a fixed or random effect model for dichotomous variables, or mean difference for continuous variables.
3.5Selection of studiesQualitative analysis was done for all studies in which patients received NAC for treatment of ALI even for studies without a control group. For studies with both treatment and control groups-retrospective cohort or randomized control trials, quantitative analysis was done. We excluded studies with less than 5 cases, editorials, opinions, animal studies, studies in other languages without English translation and letters to editors.
3.6Data extraction and managementWe thoroughly evaluated the quality of the studies and selected studies with data of our interest.
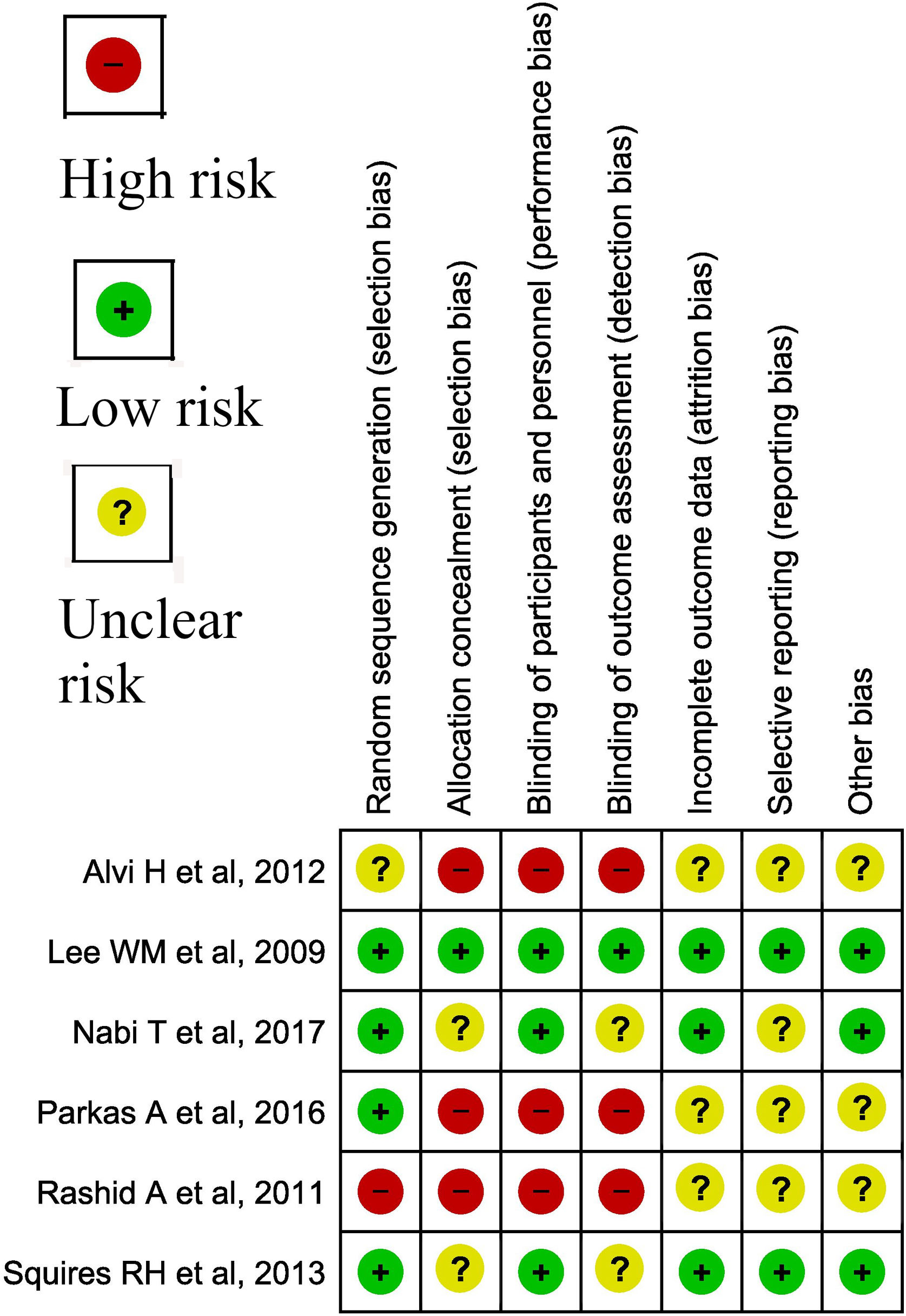
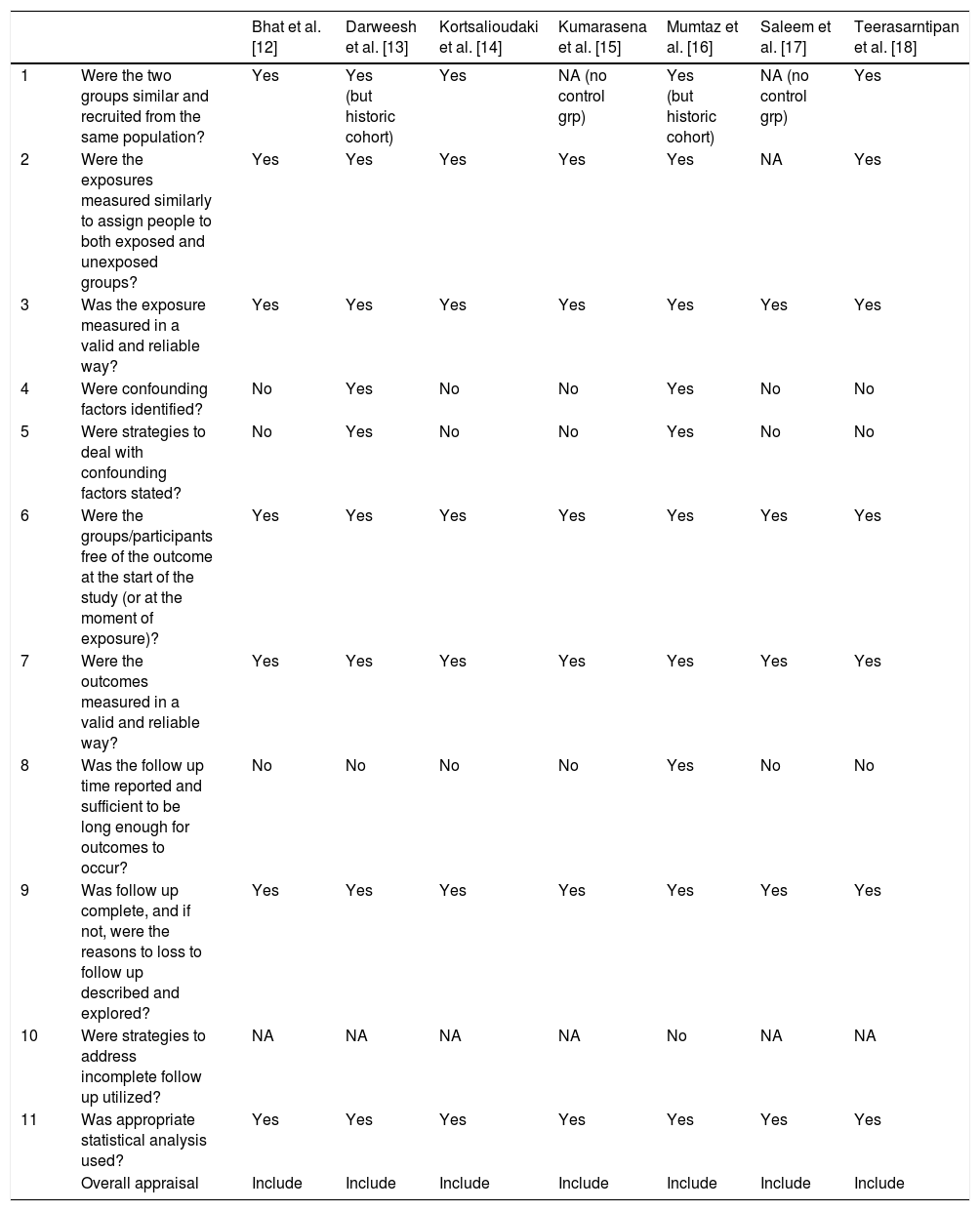
3.6.1Assessment of risk of bias in included studiesCochrane risk of bias (RoB) was used for assessment of bias in trials (Fig. 1), while Joanna Briggs Institute critical appraisal tool was used for cross-sectional and cohort studies (Table 1) [10,11].
JBI Critical Appraisal tool for bias assessment.
| Bhat et al. [12] | Darweesh et al. [13] | Kortsalioudaki et al. [14] | Kumarasena et al. [15] | Mumtaz et al. [16] | Saleem et al. [17] | Teerasarntipan et al. [18] | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Were the two groups similar and recruited from the same population? | Yes | Yes (but historic cohort) | Yes | NA (no control grp) | Yes (but historic cohort) | NA (no control grp) | Yes |
| 2 | Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Yes | Yes | Yes | Yes | Yes | NA | Yes |
| 3 | Was the exposure measured in a valid and reliable way? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4 | Were confounding factors identified? | No | Yes | No | No | Yes | No | No |
| 5 | Were strategies to deal with confounding factors stated? | No | Yes | No | No | Yes | No | No |
| 6 | Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7 | Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8 | Was the follow up time reported and sufficient to be long enough for outcomes to occur? | No | No | No | No | Yes | No | No |
| 9 | Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 | Were strategies to address incomplete follow up utilized? | NA | NA | NA | NA | No | NA | NA |
| 11 | Was appropriate statistical analysis used? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall appraisal | Include | Include | Include | Include | Include | Include | Include |
The I2 test was used for the assessment of heterogeneity using the Cochrane Handbook for Systematic Review of Interventions [19].
3.6.3Assessment of reporting biasesReporting bias was checked by prefixed reporting of the outcome.
3.6.4Data synthesisStatistical analysis was performed using RevMan v5.4. Outcome estimation was done using Odds Ratio (OR) with 95% confidence interval. Based on heterogeneity fixed or random effects models were used. Duration of hospital stay was analyzed using mean and standard deviation, or median and interquartile range, whichever was reported in the studies.
3.6.5Subgroup analysis and investigation of heterogeneityHeterogeneity was assessed using the fixed/random effects model.
3.6.6Sensitivity analysisWe performed sensitivity analysis by analyzing the results of randomized control trials (RCTs) alone excluding the retrospective studies in order to see the effect on desired outcome.
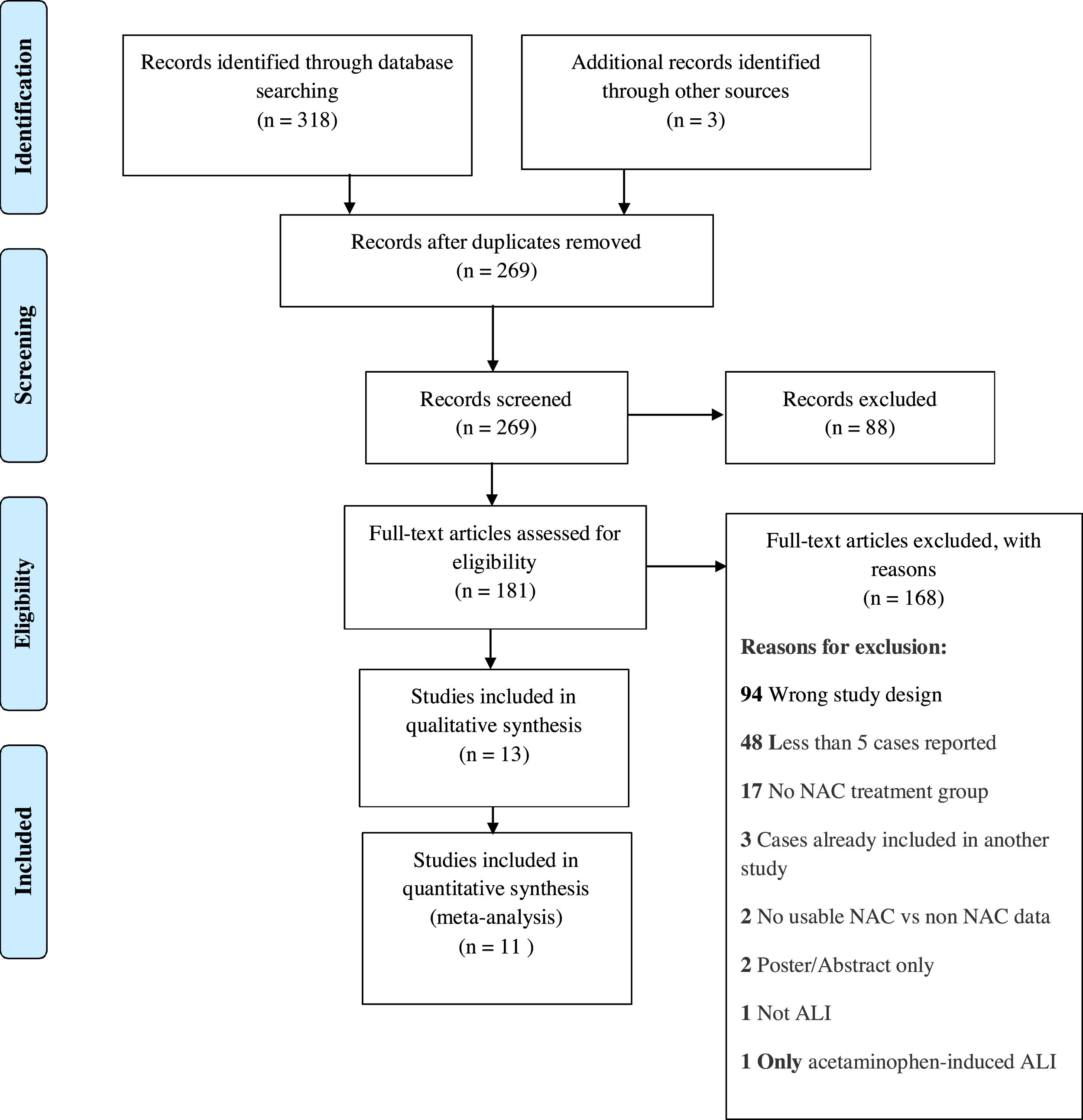
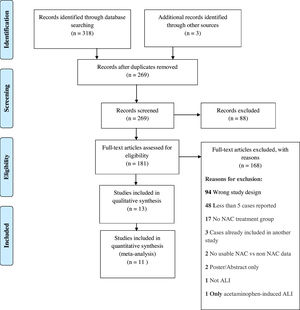
4ResultsWe identified a total of 321 studies after thorough database searching. After removing 52 duplicates, we screened the title and abstract of 269 records. We assessed the full text of 181 studies and excluded 168 studies with definite reasons (Fig. 2). A total of 13 studies were included in qualitative analysis in Table 2. Study baseline characteristics and their criteria of patient enrollment is provided in Supplementary material 2. We included 11 studies in quantitative analysis.
Qualitative analysis of Included Studies.
| Study year | Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|
| Alvi et al. [20] | N = 55 (T = 30, C = 25) Gender [M = 35 (T = 19, C = 16); F = 20 (T = 11, C = 9)] Age 32.0 ± 16.8y (range 17−90) C = 31.3 ± 15.0 y (range of 18−85 years)At presentation: Jaundiced T = 29 (96.7%), C = 25 (100%)Etiology HAV (T = 15, C = 14) HBV (T = 3, C = 5) HEV (T = 8, C = 5) Unknown T = 4, C = 1 | Oral NAC at a dose of 140 mg/kg, followed by 70 mg/kg, for a total of 17 doses, 4 h apart within 24 h of admission PLUSContinuous IV dextrose, broad-spectrum prophylactic antimicrobials and PPIs Fresh frozen plasma only in cases which had spontaneous bleed Nasogastric tube for feeding purpose in patients with encephalopathy | Continuous IV dextrose, broad-spectrum prophylactic antimicrobials and PPIs and other supportive care | Patient followed up until hospital discharge or death,Mortality: T = 3/30, C = 4/25 Mean Hospital Stay; T = 8.8 ± 1.9 days C = 10.0 ± 1.9 daysICU stay; T = 2−3 days; C = 4−5 daysAdverse effects of NAC T = 2 (one patient had mild body rashes which was self-limited and 1 had severe vomiting treated symptomatically)Adverse effects in comparison group not mentioned |
| Bhat et al. [12] | N = 100 (T = 26, C = 74)Gender: M = 50 (T = 13, C = 37); F = 50 (T = 13, C = 37)Mean Age; T = 29.88 ± 8.96; C = 27.34 ± 13.41History of alcohol abuse = 29/100 (T = 6/26, C = 23/74)On admission: Mean ALT/AST 306/451 (T = 681/1186, C = 370/468)Discharge ALT/AST was 291/302, and peak ALT/AST was 451/655. | NAC was given at the dose of 150 mg/kg for one hour followed by 50 mg/kg over four hours, followed by 100 mg/kg over 16 h. PLUSSupportive care | Supportive care | Mortality: 18/100 (T = 2/26, C = 16/74) Left against medical advice (LAMA): 7/100 (T = 5/26, C = 2/74)Discharged after recovery: 75/100 (T = 19/26, C = 56/74) |
| Darweesh et al. [13] | N = 155 (T = 85; C = 70)Gender; Male: 93 (T = 51, C = 42)Female: 62 (T = 34, C = 28)Mean age: (T = 33.5 ± 11 y; C = 34.8 ± 8.8 y) Characteristics:Drug abuse T = 5/85 (6.7%) C = 6/70 (10.0%) Encephalopathy at admission T = 24/85 (28.2%) C = 25/70 (35.7%)Etiology of ALI Viral infection T = 41 (46.7%) C = 40 (56.7%)Drugs T = 31 (36.7%) C = 28 (40%) | NAC was given as an infusion of 150 mg/ kg in 100 mL dextrose 5% over 30 min, followed by 70 mg/kg in 500 mL dextrose 5% over 4 h, then 70 mg/kg in 500 mL dextrose 5% over 16 h Subsequently, a continuous infusion of 150 mg/kg in 500 mL dextrose 5% over 24 h was continued until two consecutive INR results of less than 1.3 with improving LFT then the patient was shifted to oral NAC in a dose of 600 mg/day and was discharged 2−3 days after the change to oral NAC | All patients received treatment of symptoms and complications according to AASLD guidelines | Patient followed up until hospital discharge or deathMortality: T = 1/85, C = 16/70 Recovered without transplant: T = 82/85 (96.4%) C = 17 (23.3%) Liver transplantation T = 2 (6.7%) C = 37/70 (53.3%)Hospital stay (days); T = 10.1 ± 3.89; C = 28.0 ± 5.32Encephalopathy during course T = 28/85 (33.3) C = 44/70 (63.3)ICU admission T = 28/85 (33.3) C = 47/70 (66.7)Bleeding T = 20/85 (23.3) C = 47/70 (66.7) RFT during course (normal) T = 56/85 (66.7) C = 16/70 (26.7)Deranged Renal functionT = 29/85, C = 54/70Adverse events Severe allergic reaction to NAC in 2/85 Prolonged cholestasis in T = 82/85 |
| Kortsalioudaki et al. [14] | N = 170 (T = 111; C = 59) C: (1989−1994), (n = 59; 34 [58%] male; median age 2.03 yr, range 0.003−15.8 yr); T: (1995−2004), (n = 111; 57 [51%] male; median age 3.51 yr, range 0.005−17.4 yr).Etiology of ALI Indeterminate: C = 18 (31%), T = 42(38%); Metabolic disorders: C = 11 (19%), T = 16 (14%); Infectious causes: C = 7 (12%), T = 14 (13%); Autoimmune hepatitis: C = 6 (10%), T = 7 (6%); Neonatal hemochromatosis: C = 7 (12%), T = 7 (6%); Drug or toxin: C = 4 (7%), T = 7 (6%); Miscellaneous: C = 6 (10%), T = 18 (16%)Presenting complaints Jaundice C = 55, T = 84; encephalopathy C = 32, T = 60;Lab values on admission: AST (IU/L)C = 560 [36–9706] T = 1446 [37–21,660]; Serum bilirubin ( micromol/L) C = 326 [12–1053] T = 194 [12–1132]; ALP (IU/L) C = 364 [52–2115], T = 287 [18–1917]; Creatinine ( micromol/L) C = 70 [10–357], T = 82 [23–404] | NAC was administered as a continuous intravenous infusion (100 mg/kg/24 h) until normalization of the INR, death, or transplantation. Standard care treatment was similar throughout the study period and was directed at maintaining normal tissue oxygenation and preventing and treating complications of ALI. | Standard care treatment | Patient followed up until hospital discharge or deathMortality: C = 30/59, T = 29/111;Alive with the native liver C = 13/59 (22%) T = 48/111 (43%);ICU admission C = 41/59, T = 85/111; Mechanical ventilation C = 35/59 T = 72/111 Infection C = 10/59 (17%) T = 24/111 (22%); Renal failure C = 17/59 (29%)T = 28/111Death without transplantation (C = 15/59, T = 21/111)Transplant free survival (C = 13/59, T = 48/111) |
| Kumarasena et al. [15] | N = 8(T = 8, C = 0) Gender (M = 5, F = 3) Age 28−64 yearsEtiology: Dengue Hemorrhagic Fever: 2 Dengue Shock Syndrome: 6At presentationSerum bilirubin = 2.7−12.2 mg/dL;ALT = 4070−19,800 IU/L;AST = 4455−26,500 IU/L;Serum creatinine = 0.7−2.5 mg/dL. | NAC 150 mg/kg loading dose by intravenous administration over 15 min followed by 12.5 mg/kg/h for4 h and then 6.25 mg/kg/h for up to 72 h. Other supportive management | None | Patient followed up until hospital discharge or deathMortality: T = 3/8 No patient had adverse effects attributable to NAC. |
| Lee et al. [21] | N = 173 (T = 81; C = 92) C: F = 68%, Median Age = 40.5, Bilirubin level mg/dl = 20.3, Creatinine level, mg/dl = 1, ALT = 756.5, MELD = 33 T: F = 47%, Median Age = 42, Bilirubin level mg/dl = 22.3, Creatinine level, mg/dl = 1.3, ALT = 999, MELD = 32 ;Etiologies: drug-induced liver injury(DILI; n 45), autoimmune hepatitis (n 26), hepatitis B virus (HBV; n 37), and indeterminate cause (n 41) | After randomization, infusion of 5% dextrose with N-acetyl cysteine (Acetadote; Cumberland Pharmaceuticals, Nashville, TN)was begun, with an initial loading dose of 150 mg/kg/h of NAC over 1 h, followed by 12.5 mg/kg/h for 4 h, then continuous infusions of 6.25 mg/kg NAC for the remaining 67 h | Infusion of 5% dextrose (placebo) | Mortality at 3 weeks: T = 24/81, C = 31/92; Overall survival at 3 weeks was 70% (57/81) for NAC and 66% (61/92) for placeboTransplant-free survival T = 40% (32/81) C = 27% (25/92);Hospital stay median T = 9, C = 13 days;ADEs: Overall: C = 41% (38/92). T = 42% (34/81)Infection C = 20% (18/92), T = 20% (16/81);Rash C = 2% (2/92), T = 2% (2/81);Bronchospasm C = 1% (1/92), T = 1% (1/81); Arrhythmia C = 10% (9/92), T = 9% (7/81);Nausea and vomiting C = 4% (4/92), T = 14% (11/81); Other C = 9% (8/92), T = 11% (9/81) Renal Failure: C = 25% (23/92), T = 33% (27/81); |
| Mumtaz et al. [16] | N = 91 (T = 47, C = 44); Male T = 26, C = 24T: Age = 27.7 ± 11.8 years, Bilirubin = 20.63 ± 11.03, PT = 59.55 ± 36.07; ALT = 1926 ± 1374.2; Creatinine = 1.39 ± 0.82; HE I = 6 (12.8%) HEII = 9 (19.1%) HE III = 15 (31.9%) HE IV = 17 (36.2%)C: Age = 37.5 ± 18.8 years, Bilirubin = 14.36 ± 8.90, PT = 53.05 ± 30.57; ALT = 1457.2 ± 1467.8; Creatinine = 1.57 ± 0.90; HE I = 6 (13.6%) HEII = 18 (40.9%) HEIII = 9 (20.5%) HE IV = 11 (25.0%)Etiology of ALI HAV (T = 2, C = 0); HBV (T = 11, C = 14); Drug induced ALI (T = 3, C = 8) | Oral NAC at a dose of 140 mg/kg, followed by 70 mg/kg, for a total of 17 doses, 4 h apart within 6 h of admission. All patients were managed with the standard supportive care treatment, which was similar throughout the study period in both the study groups. | The patients received the standard supportive care treatment. | Patient followed up until hospital discharge or deathMortality T = 25/47, C = 32/44; Renal failure T = 9/47, C = 11/44;Mechanical ventilation T = 36/47, C = 16/44;Infection T = 25/47, C = 22/44 ADEs: T 6/47 (Rash 2; Bronchospasm 1; Vomiting 4)Adverse effects in comparison group not mentioned |
| Nabi et al. [22] | T: M = 17, F = 23, Mean age = 30.60 ± 11.64 years, Bilirubin = 21.12 ± 8.94 AST = 1726 ± 983.4, ALT = 1050.78 ± 717.46; creatinine = 2.83 ± 0.55; HE I = 10 (25%); HEII = 13 (32.5%); HE III = 7 (17.5%); HE IV = 10 (25%)C: M = 24, F = 16; Mean age = 38.48 ± 20.11 years, Bilirubin = 20.67 ± 9.54, ALT = 1055.80 ± 569.04; AST = 1462 ± 678.8; Creatinine = 1.35 ± 0.65 HE I = 21 (52.5%) HE II = 8 (20%) HE III = 7 (17.5%) HE IV = 4 (10%)Etiology: Acute hepatitis E T = 7, C = 7; Acute hepatitis A T = 8, C = 5; Acute hepatitis B T = 8, C = 4; Drug induced ALF T = 15, C = 10, | 40 NAIFHF patients who fulfilled the eligibility criteria were treated with intravenous NAC for duration of 72 h. NAC group were administered intravenous NAC with initial loading dose of 150 mg/kg over 1 h, followed by 12.5 mg/kg/hr for 4 h and then continuous infusion of 6.25 mg/kg/hr for remaining 67 h. | Control group: 40 NAIFHF patients who received 5%dextrose (placebo) infusion for 72 h. | Length of hospital stay in days T = 8.241 ± 2.115, C = 10.737 ± 3.106);Mortality: T = 11, C = 21No adverse effects were noted in patients that could have been attributed to NAC administration.Hospital courseRenal failure T = 6, C = 9; Development of ascites T = 4, C = 7; Infection T = 21, C = 20; Seizure T = 5, C = 10; Mannitol T = 30, C = 37; Hypotension T = 8, C = 10; Mechanical ventilation T = 4, C = 5; UGI bleeding T = 4, C = 6 |
| Parkas et al. [23] | N = 32(M = 22, F = 10) T = 16 (M = 10,F = 6), C = 16(M = 12,F = 4); Mean age T = 7.5 ± 1.36 years; C = 7.6 ± 1.23 yearsEtiologies: T: HAV = 10/16; Non A–E = 3/16; HBV = 2/16; Co-infection with HAV and HEV = 1/16 C: HAV = 13/16 Non A–E = 2/16 HBV = 1/16At presentation:T: Jaundice 13/16; hepatomegaly 11/16; bleeding manifestations 5/16; HE I = 5/16, HE II = 7/16, HE III = 3 /16, HE IV = 1/16Bilirubin = 7.9 ± 5.2 Direct Bilirubin (mg/dl) = 6.1 ± 4.3 AST = 1360.4 ± 540.2 ALT = 1401.9 ± 612.0 Prothrombin Time = 55.00 ± 14.41 Serum Creatinine (mg/dl) = 0.969 ± 0.679 C: Jaundice 16/16; hepatomegaly 12/16; bleeding manifestations 5/16; HE I = 6/16, HE II = 4/16, HE III = 4 /16, HE IV = 2 /16Bilirubin = 7.9 ± 4.5; AST = 1405.6 ± 467.6; ALT = 1446.2 ± 526.8 Prothrombin Time = 61.56 ± 14.21 Serum Creatinine (mg/dl) = 1.2 ± 0.792 | NAC was administered as a continuous intravenous infusion (100 mg/kg/24 h) until normalization of the INR or death. Standard care treatment was similar throughout the study period, including IV dextrose infusion, broad spectrum antimicrobials, antacids, FFP as required | Standard care treatment was similar throughout the study period, including IV dextrose infusion, broad spectrum antimicrobials, antacids, FFP as required | Patient followed up until hospital discharge or deathMortality: T = 5/16, C = 9/16;Survival: T = 11/16, C = 7/16;Length of Hospital Stay T = 14.4 ± 6.7 C = 23.8 ± 4.1 |
| Rashid et al. [24] | N = 60 (T = 30, C = 30) Age T = 8.1 ± 2.2 years, C = 8.3 + 2.2 years,Gender T= (M = 19, F = 11); C= (M = 15, F = 15)Jaundice and encephalopathy: T = 30/30, C = 30/30;Bleeding manifestation T = 7/30; C = 10/30 | N-acetyl cysteine 140 mg/kg loading dose followed by 70 mg/kg four hourly for 17 doses diluted to a 5% solution in sweet fruit juices or carbonated soft drinks. It SAwas given either orally in conscious patients or through nasogastric tube in comatose patients. Appropriate nutrition, IV fluids and drugs like vitamin K, H2 receptor antagonists, antibiotics and lactulose were also given if needed | only IV fluids, appropriate nutrition and drugs if needed | Patient followed up until hospital discharge or deathMortality: T = 6/30, C = 11/30Survival: T = 24/30, C = 19/30Hospital stay T = 7.3 ± 3.4, C = 9.1 ± 3.4,Duration of encephalopathy T = 5.4 ± 2.5, C = 6.4 ± 2.2 |
| Saleem et al. [17] | N = 40 (T = 40, C = 0) (Male = 25; Female = 15) Mean age (T = 80 ± 40 months)Characteristics at Presentation: Jaundice 26/40 SGPT (ALT) (n = 40) 2102 ± 1509 SGOT (AST) (n = 35) 2495 ± 2786 PT (n = 40) 45 ± 35 SBR (serum bilirubin, total) (n = 40) 17 ± 14ICU therapy Mechanical ventilation 24 (60%) Renal replacement therapy 5 (13%) Length of ICU stay (days) 3.4 ± 2Causes: HAV = 22/40; HEV = 3/40; Hep A & E = 13/40 Hep B & D = 2/40 | NAC was given intravenously as 21 -h regimen in 3 doses with cumulative dose of 300 mg/kg over 21 h (initial loading dose of 150 mg/kg over one hour, followed by 50 mg/kg for 4 h, then continuous infusion of 100 mg/kg over the next 16 h) | Not applicable | Mortality 15/40; AKI 12/40 Grade-IV coma 10/40 Bleeding 3/40LFT changes after N-acetyl cysteineALT; 24 h post-NAC 1019 ± 816 Before discharge/death 466 ± 495 AST; 24 h post-NAC 528 ± 451 Before discharge/death 435 ± 431 PT; 24 h post-NAC 31.2 ± 30 Before discharge/death 28.3 ± 33 SBR; 24 h post-NAC 17 ± 12 Before discharge/death 14.6 ± 11 |
| Squires et al. [25] | N = 184 (T = 92; C = 92) Gender; Male: 101 (T = 47, C = 54) Age (median, IQR) [T = 3.7 (0.8, 10.5); C = 4.5 (1.0, 9.5)]Diagnosis at hospital discharge: Indeterminate T = 55/92, C = 54/92 Autoimmune T = 8/92, C = 11/92 Infection T = 9/92, C = 6/92 Metabolic T = 13/92, C = 5/92 Other T = 7/92, C = 16/92 | Eligible children were adaptively allocated within strata defined by age (less than 2 years of age or at least 2 years old) and HE (grade 0−1 or 2−4) to receive NAC (150 mg/kg/d) in 5% dextrose (D5W) and water. Volumes were adjusted for small children. Study medications were infused over 24 h for up to 7 consecutive days in a dedicated line without other medications. Treatment was stopped earlier than 7 days in the case of hospital discharge, LTx, or death within 7 days of randomization. Supportive measures, coma, and adverse events were recorded. | Placebo consisting of an equal volume of D5W alone. Volumes were adjusted for small children. Study medications were infused over 24 h for up to 7 consecutive days in a dedicated line without other medications. | 1 year overall Survival T = 73% (67/92), C = 82% (75/92);Mortality: T = 25/92; C = 17/92Encephalopathy 0−1 T = 78% (51/65), C = 85% (58/68); 2−4 T = 63% (17/27), C = 75% (18/24) 1 year spontaneous survival (transplant-free) T = 35% (32/92), C = 53% (49/92) ADEs:At least one AET = 19/92 (21 events); C = 16/92 (17 events). Infection (T = 11/92, C = 8/92) Rash (T = 4, C = 2) Bronchospasm (T = 1, C = 0) Arrhythmia (T = 1, C = 4) Serious adverse events: T = 5/92; C = 4/92 |
| Teerasarntipan et al. [18] | N = 17 (T = 7; C = 10) | 7 patients, 4 with NAC alone, 2 with NAC + single-pass albumin dialysis (SPAD), 1 with NAC + Molecular adsorbent recirculating system (MARS)) | 10 patients, 7 with no liver-specific treatment, 1 with mannitol and steroids, 1 with 3% NaCl, 1 with SPAD | Patient followed up until hospital discharge or deathMortality T = 3/7 C = 7/10 |
Abbreviations: AASLD: American Association for the Study of Liver Diseases, ADE: Adverse Drug Event, AE: Adverse event, ALI: Acute Liver Injury, ALP: Alkaline phosphatase, ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, ATTT: Anti-tubercular therapy toxicity, C = Number of participants in comparison group, D5W: 5% dextrose and water, DILI: Drug-induced liver injury, F: Female, HAV: Hepatitis A virus, HBV: Hepatitis B virus, HDV: Hepatitis D virus, HE: Hepatic Encephalopathy, HEV: Hepatitis E virus, ICU: Intensive Care Unit, INR: International Normalized Ratio, IQR: Interquartile range, IU: International Units, IV: Intravenous, LAMA: Left against medical advice, LFT: Liver Function Test, Ltx: Liver transplant, M: Male, MARS: Molecular adsorbent recirculating system, MELD: Model For End-Stage Liver Disease, N: Total number of participants, NAC: N-acetyl cysteine, NaCl: Sodium Chloride, PPI: Proton Pump Inhibitor, PT: Prothrombin time, SBR: Serum bilirubin, SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase, SPAD: Single-pass albumin dialysis, T: Number of participants in Intervention group, TN: Tennesse.
We included 11 studies in our quantitative synthesis.
- A
Hospital mortality
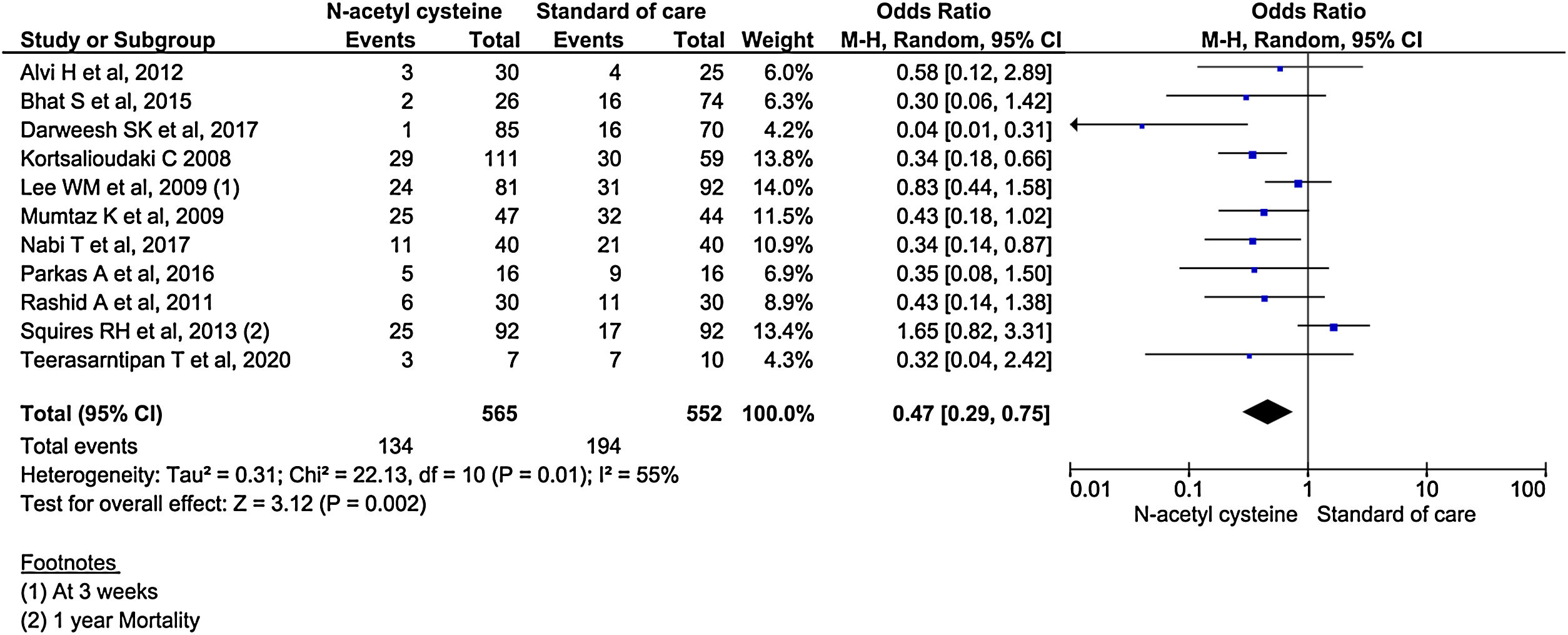
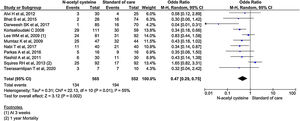
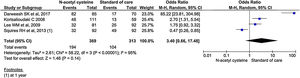
All the 11 studies reported mortality outcomes. The use of NAC in non-acetaminophen induced ALI showed a 53% reduction in mortality compared to standard of care treatment (OR, 0.47; 95% CI, 0.29 to 0.75; n = 1117; P = 0.002; I2 = 55%) (Fig. 3). However, analysis using randomized studies only did not reach a statistically significant level (OR, 0.71; 95% CI, 0.38–1.33; P = 0.29) (Supplementary material 3, Fig. 1).
- B
Length of hospital stay
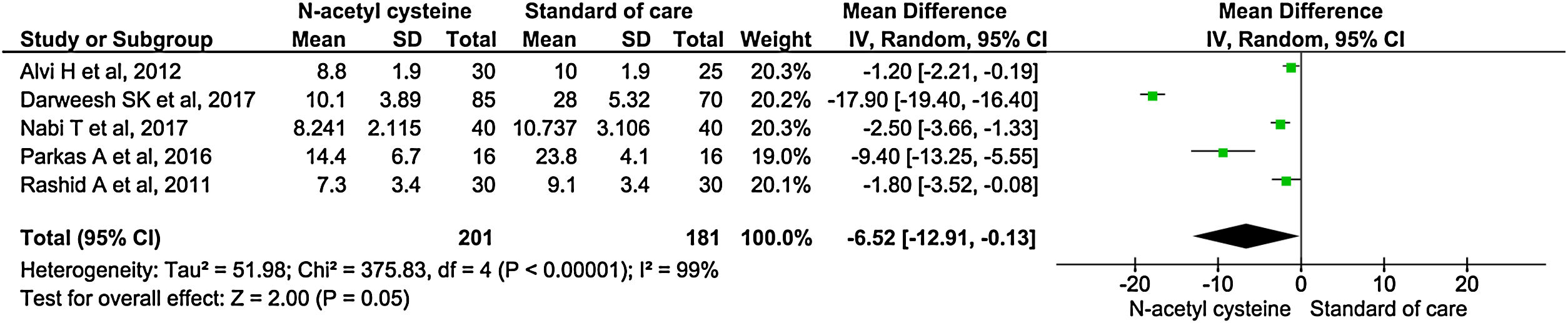
Five studies reported length of hospital stay. There was significantly shortened in mean duration of hospital stay among the treatment group than control group (MD, −6.52; 95% CI, −12.91 to −0.13; P = 0.05) (Fig. 4).
Sensitivity analysis by excluding non-randomized studies also showed some significant shortening in length of hospital stay in the treatment group (MD, −3.50; 95% CI, −6.11 to −0.89; P = 0.009) (Supplementary material 3,Fig. 2).
- C
ICU admission
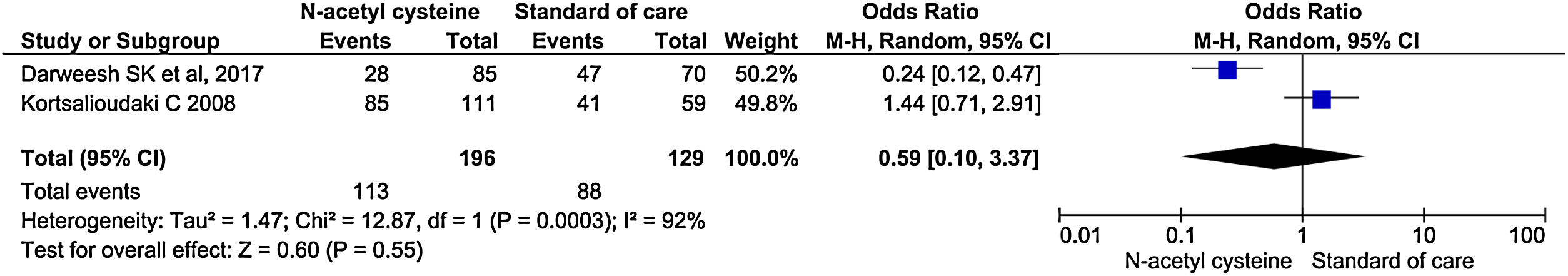
Two studies reported the rate of ICU admission. There was no significant difference in the rate of ICU admission between the groups (OR, 0.59; 95% CI, 0.10–3.37; P = 0.55) (Fig. 5).
- D
Infection
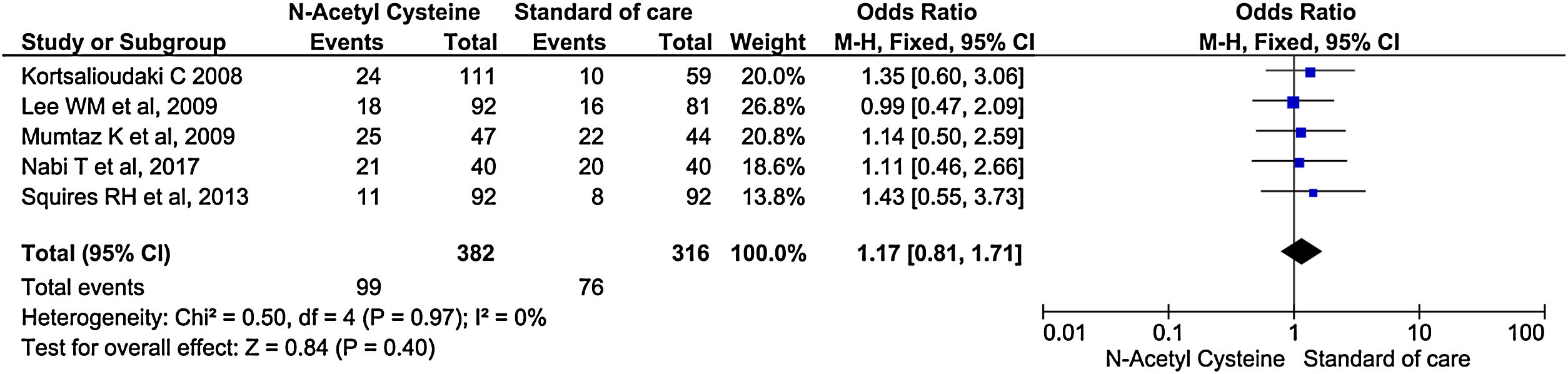
Five studies reported the rate of new infection during the treatment and follow up periods. It did not show significant difference in the rate of infection between treatment and control arm (OR, 1.17; 95% CI, 0.81–1.71; P = 0.40) (Fig. 6).
Sensitivity analysis by excluding non-randomized studies showed no significant differences in rate of infection between two groups (OR, 1.13; 95% CI, 0.69–1.84; P = 0.63) (Supplementary material 3,Fig. 3).
- E
Encephalopathy
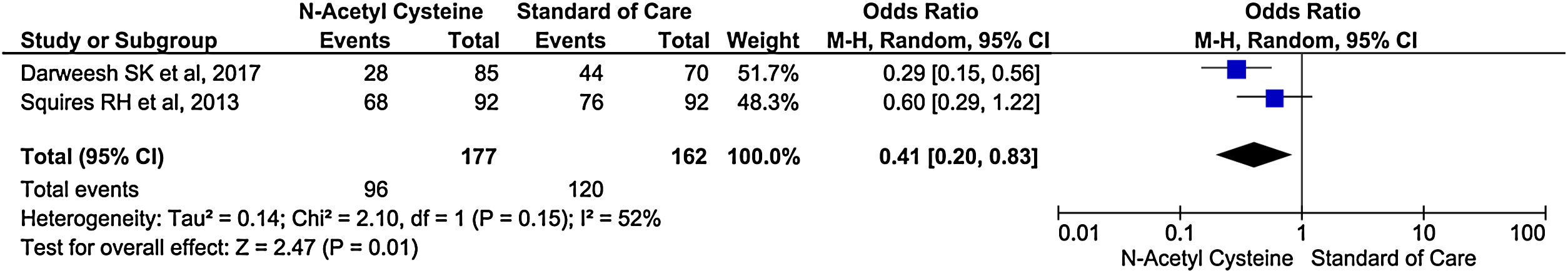
Only two studies reported encephalopathy during treatment. There was 59% reduction in rate of encephalopathy in treatment group than control group (OR, 0.41; 95% CI, 0.20–0.83; P = 0.01) (Fig. 7).
- F
Overall Adverse events
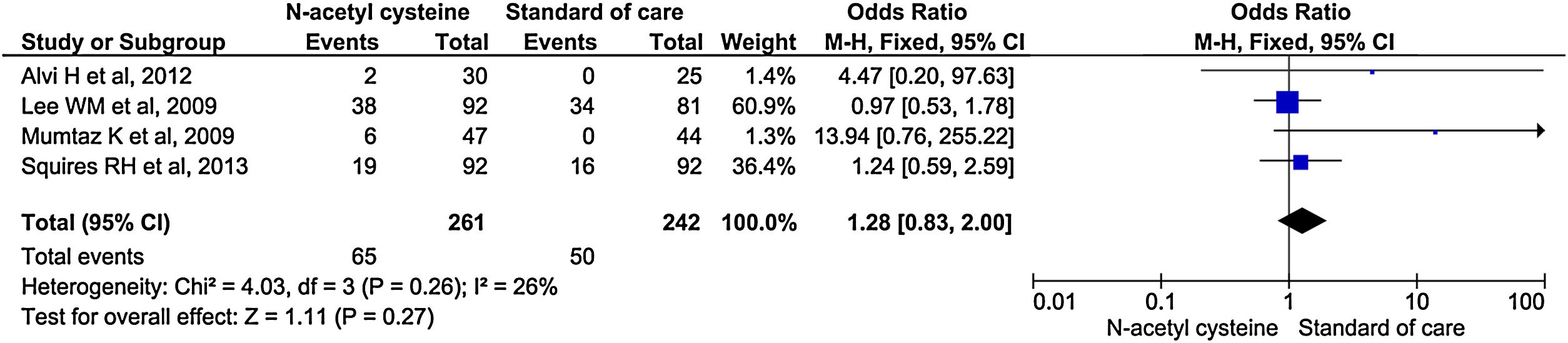
Four studies reported overall adverse effects during treatment. There were no significant differences in the rate of adverse events between the two groups (OR, 1.28; 95% CI, 0.83–2.00; P = 0.27) (Fig. 8). Analysis with random effect model due to considerable heterogeneity also did not show any significant differences between the two groups (OR, 1.28; 95% CI, 0.69–2.40; P = 0.44) (Supplementary material 3,Fig. 4). Similarly, studies including RCTs only did not reach statistical significance (OR, 1.12; 95% CI, 0.71–1.77; P = 0.63) (Supplementary material 3,Fig. 5).
- G
Specific adverse events
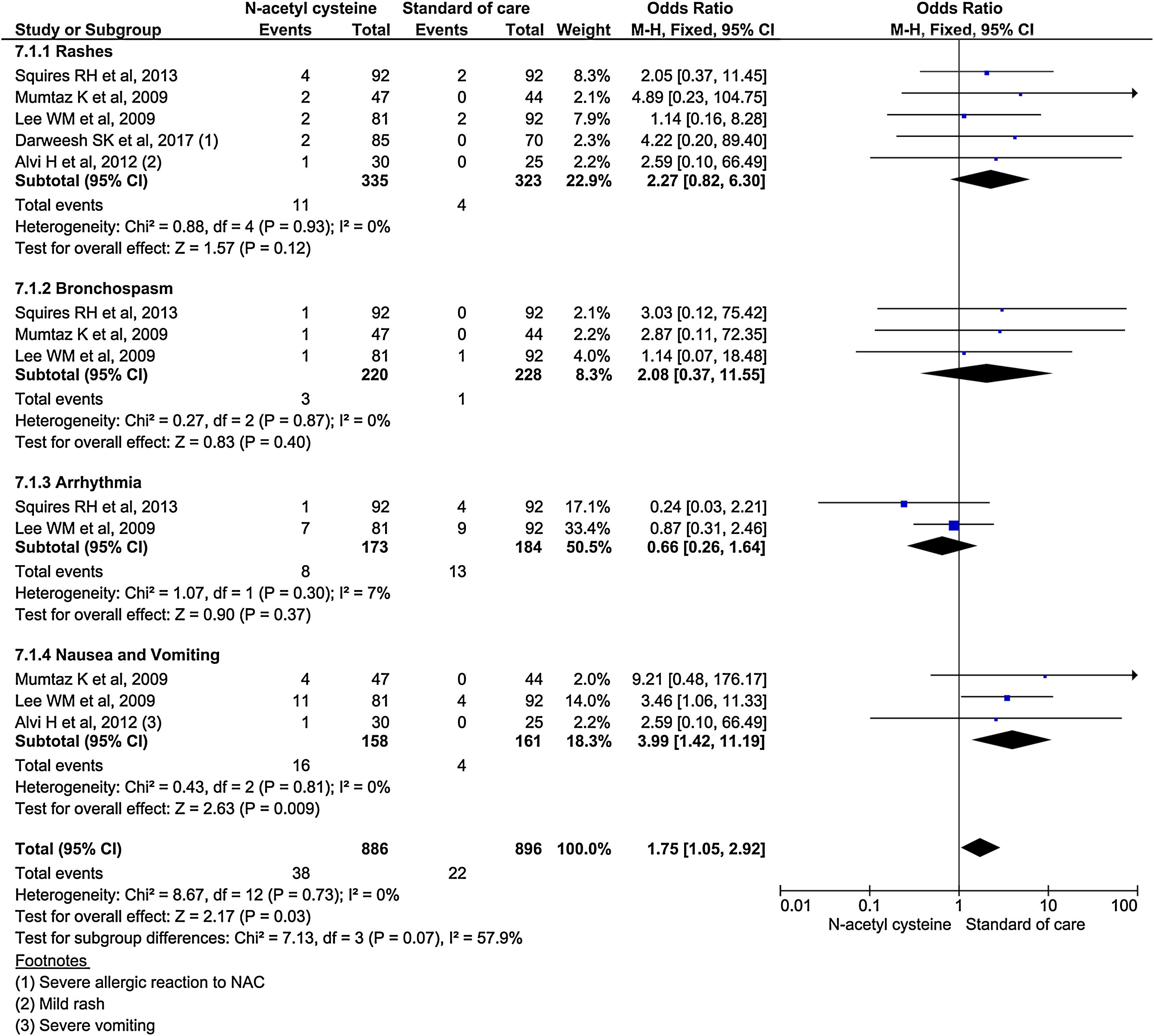
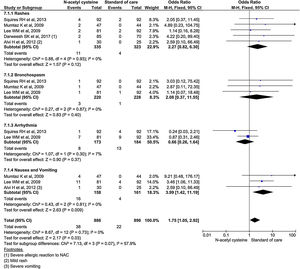
Subgroup analysis using specific adverse events showed nausea and vomiting was almost 4 times higher in NAC (OR, 3.99; 95% CI, 1.42–11.19; P = 0.009). Although, other adverse events (rash, bronchospasm, and arrhythmia) occurred higher in NAC group, it was no statistically significant. Analysis showed 2.27 odds for rash (95% CI, 0.82–6.30; P = 0.2); 2.08 odds for bronchospasm (95% CI, 0.37–11.55; P = 0.4); and 0.66 odds for arrhythmias (95% CI 0.26–1.64; P = 0.37) (Fig. 9).
- H
Mechanical ventilation
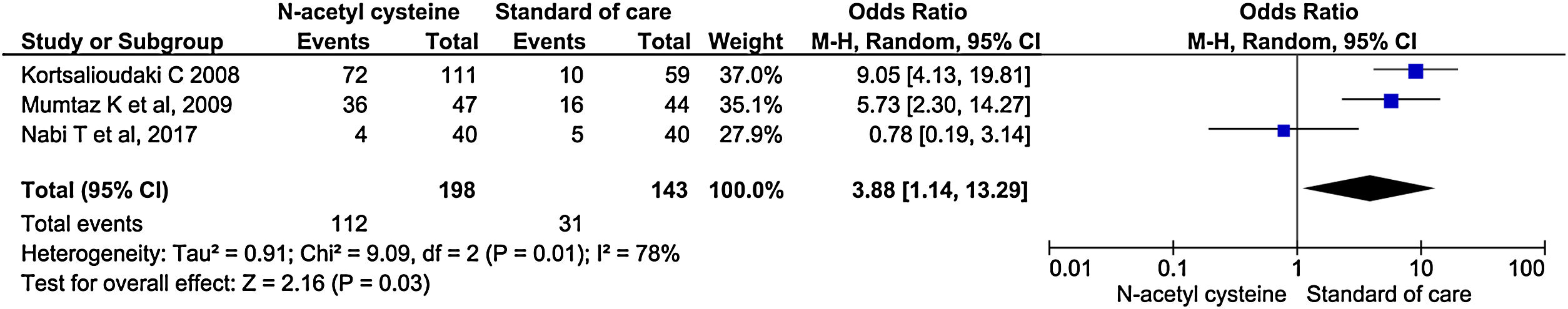
Three studies reported mechanical ventilation during treatment. NAC group had statistically significant higher need for mechanical ventilation than the control group (OR, 3.88; 95% CI, 1.14–13.29; P = 0.03) (Fig. 10).
- I
Transplant-free survival
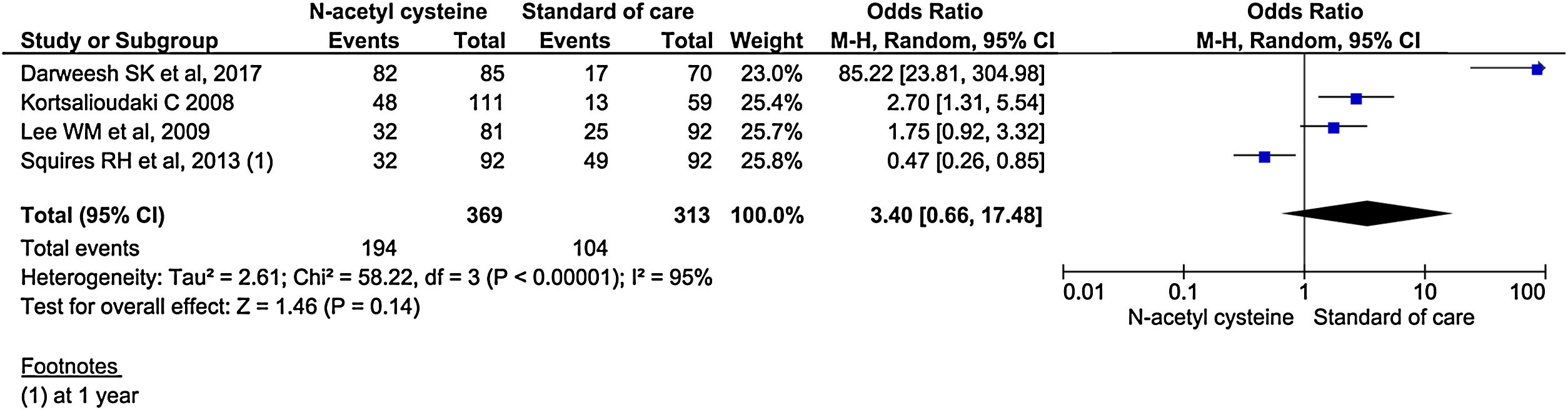
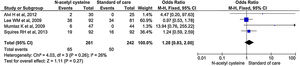
Four studies reported transplant-free survival. Among reported studies, the transplant-free survival was 3.4 times in the treatment group compared to control group, but it did not reach statistical significance (95% CI, 0.66–17.48; P = 0.14) (Fig. 11). Analysis of transplant-free survival using RCTs only did not show statistical significance between the two groups. (OR, 0.90; 95% CI, 0.25–3.28; P = 0.14) (Supplementary material 3,Fig. 6).
- J
Renal failure
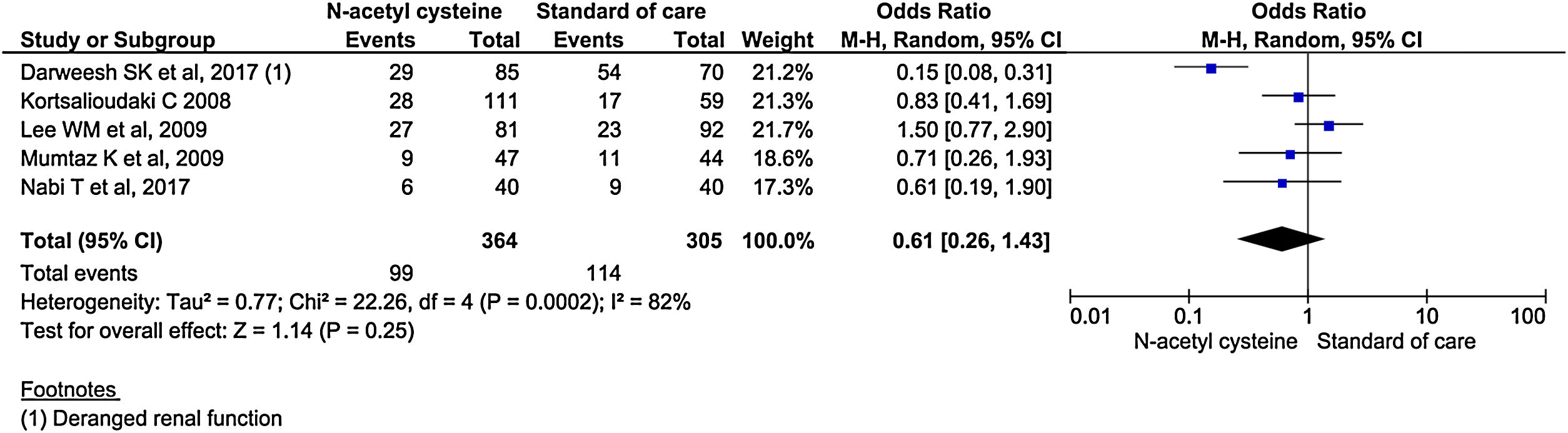
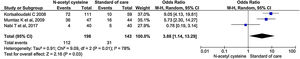
Five studies reported renal failure/impairment during the study period. There was no statistical difference in the rate of development of renal failure/impairment between the two groups. (OR, 0.61; 95% CI, 0.26–1.43; P = 0.25) (Fig. 12).
- K
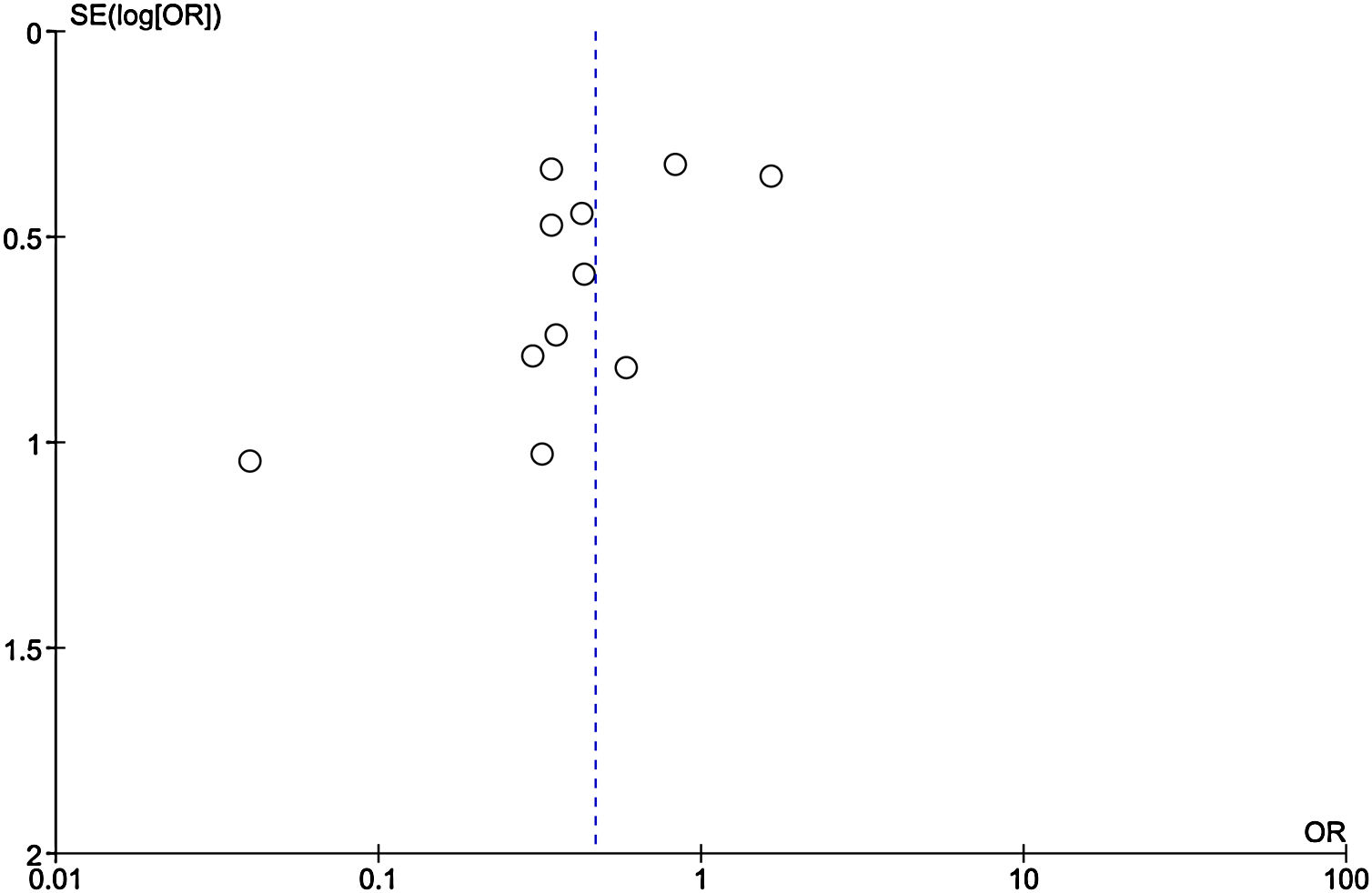
Publication bias
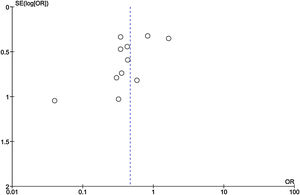
Among the included studies, analysis for publication bias using Funnel plot showed some publication bias among the included studies reflected by asymmetry of study distribution (Fig. 13).
Our meta-analysis is the most comprehensive analysis published to date regarding the use of NAC in non-acetaminophen induced ALI. Prior analyses were small scale studies which were underpowered and relied more on non-randomized studies [26,27]. The major findings of our study were 53% reduction in mortality, 59% reduction in hepatic encephalopathy and 4 times increased risks of nausea and vomiting in patients receiving N-acetyl cysteine compared to standard of care. We did not find any difference in length of hospital stay, ICU admission rate and infections in patients receiving N-acetyl cysteine.
Our finding of statistically significant decreased mortality in NAC group is in agreement with two prior meta-analyses done by Khan et al. and Amjad et al. [26,27]. However, no differences in mortality and overall survival in patients receiving NAC were reported by the analysis done by Hu et al. and Obaid et al. [28,29]. All these prior meta-analyses were conference abstracts and were non-peer reviewed studies. The analysis done by Hu et al. was based on four randomized controlled trials and revealed no difference in overall survival between the two groups [28]. We performed a subgroup analysis including six randomized controlled trials and it confirms the finding of Hu et al. The inclusion of retrospective studies to our analysis might have led to decreased mortality in patients receiving N-acetyl cysteine. However, it is hard to reach a definite conclusion in the absence of large scale double blinded randomized controlled trials in this clinical setting.
We found no difference in length of hospital stay in the NAC group compared to control arm receiving standard of care. This is in contrast to other studies which showed decreased length of hospital stay in the N-acetyl cysteine group [27,29]. However, the analysis done by Walayat et al. showed increased length of hospital stay [30]. The length of hospital stay is typically dependent on different factors like comorbidities, cause of ALI, age of patients and immunological status of patients. It is hard to ascertain the benefit on length of hospital stay with NAC alone in patients of ALI given the multitude of other factors that impacts recovery. We found reduction in hepatic encephalopathy in patients receiving NAC, however, the result was based on analysis of only two studies. The odds of adverse events were 1.28 in patients receiving NAC but this finding was not statistically significant. This was similar to the study done by Obaid et al. which showed 1.64 increased odds of adverse events [29]. Our study showed increased risks of nausea, vomiting, and rash in the treatment group and this was similar to the study by Hu et al. [28]. Nonetheless, majority of adverse events were minor and self-limited.
There was 3.4 times increased transplant-free survival in patients receiving NAC although this was not statistically significant. The analysis done by Khan et al. and Obaid et al. supported our finding while the study by Amjad et al. found no improvement in transplant-free survival. On the contrary, study by Walayat et al. showed improvement in transplant-free survival in control group [26,27,29,30]. The role of NAC in non-acetaminophen induced ALI remains one of the most interesting topic currently and our findings were encouraging for more studies.
The clinical benefit of NAC in terms of mortality and incidence of hepatic encephalopathy seen in our study can be attributed to several plausibility. 1) etiology and etiopathogenesis of ALI is diverse, 2) ALI can include a conundrum of organ failure, and only a minority of patients die from insupportable hepatic dysfunction, while others die from other organ failures and their etiology such as sepsis, thus NAC therapy alone may not modify the pathophysiology, 3) co-administration of neuropsychiatric medications and occurrence of delirium in the critically ill patients could have impacted the hepatic encephalopathy incidence, 4) Lastly, the studies included in our study had a heterogeneous patient population with diversity in the cause and severity of ALI, and different age groups [31]. Although the propensity-matched analysis was used in a few studies, it is difficult to overcome this limitation.
Our study had several limitations. There are small number of randomized prospective studies in this topic. The studies included have heterogeneity in the study designs and populations along with their inherent limitations. Detailed baseline characteristics, specifically an evaluation of degree of organ dysfunction were not uniformly reported across studies. Various doses, duration, and route of administration of NAC were included that prevents granularity of the data in assessing their individual influence on outcomes. The course of hospitalization in these patients could have a significant influence on the clinical outcomes, although these data were not uniformly reported in the included studies.
6ConclusionWe found a decreased rate of mortality and hepatic encephalopathy in patients with non-acetaminophen induced ALI receiving NAC compared to standard of care. Although increased risks of nausea and vomiting were seen in the NAC group, the majority of adverse events were transient and minor. Although our analysis results are encouraging, further large scale randomized clinical trials needs to be done to accept or refute these findings.AbbreviationsALI Acute liver injury Alanine Aminotransferase Aspartate Aminotransferase Confidence Interval Hepatitis A virus Hepatitis B virus Hepatic Encephalopathy Intensive Care Unit Mean Difference Model for End-Stage Liver Disease N-acetylcysteine Odds Ratio Standard of Care
Not applicable.
Consent for publicationNot applicable.
Availability of data and materialsThe datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interestsThe authors declare that they have no competing interests.
FundingThis article did not receive any specific grant from funding agencies in the public, commercial, or any other sectors.
Authors' contributionsDBS, PB, and YRS contributed to the concept and design, analysis, and interpretation of data. DBS, PB, AA, AP, and BA contributed to the literature search, data extraction, review and initial manuscript drafting. YRS, and RB interpretation of data, revising the manuscript for important intellectual content and approval of the final manuscript.
All authors were involved in drafting and revising the manuscript and approved the final version.