With a global prevalence of about 25 %, metabolic dysfunction-associated steatotic liver disease (MASLD) [1], formerly known as non-alcoholic fatty liver disease (NAFLD), has emerged as the most prevalent cause of chronic liver disease globally [2–4]. A large population in Asia, where hepatitis B virus (HBV) infection is common [5–7], may be at increased risk of developing two concomitant liver disorders. The prevalence of biopsy-proven MASLD in chronic hepatitis B (CHB) ranges from 18 to 40 % [8–11].
Regarding the histological distinctions between CHB patients with and without MASLD, there is an ongoing debate on whether two concomitant diseases link to a higher likelihood of hepatocellular carcinoma (HCC), cirrhosis, and death [12]. Whether concurrent MASLD aggravates the disease course in CHB patients remains controversial [13–15]. Even though many studies have looked into how CHB and hepatic steatosis affect liver disease, few have specifically examined biopsy-defined MASLD or the impact of metabolic disorders [16–23].
The effectiveness of treatment for MASLD becomes reduced when the disease reaches its end stage, often necessitating liver transplantation. Thus, intervention and early detection of significant fibrosis are critical. The impact of MASLD and its metabolic criteria on fibrosis progression in CHB has not yet been thoroughly investigated. It is essential to determine whether patients were at higher risk of significant fibrosis when MASLD was considered in patients with CHB.
To this end, the objective of this research was to assess the impact of MASLD on the progress of fibrosis in a large cohort of treatment-naïve Asian CHB patients with histopathological evidence.
2Patients and methods2.1Study designThis was a dual-center, cross-sectional study comprising CHB patients who underwent liver biopsy between January 2009 and December 2021 at XX Hospital, XX University, and XX Hospital, XX University. Liver biopsies due to suspected malignant liver tumors were not within the scope of the study. Hepatitis B surface antigen (HBsAg) persistence for more than six months was considered to be CHB. Individuals with hepatic steatosis with CHB were included based on histological findings. Patients who met the following criteria were excluded: i) antiviral therapy with nucleoside (acid) or interferon; ii) co-infection with other viral hepatitis such as hepatitis C or D; iii) other chronic liver diseases (e.g., autoimmune liver diseases, history of liver transplantation, Wilson's disease, and suspected drug-induced liver disease) or use of steatogenic medications; iv) human immunodeficiency virus co-infection; v) excessive alcohol consumption (men >30 g and women >20 g of alcohol per day, respectively, or other substance abuse); vi) malignant tumor such as hepatocellular carcinoma.
2.2Histological evaluationLiver specimens were collected with percutaneous liver biopsy under transabdominal ultrasound guidance. Hematoxylin-eosin and Masson's trichrome stains were used to stain all slides, and only adequate biopsy samples— those having a minimum of six portal tracts and specimen lengths of ≥10 mm were included. The degree of fibrosis based on METAVIR score [24] were reevaluated by three dedicated and experienced histopathologists who were blinded to clinical, laboratory, and radiographic details. Steatosis grade was recorded as:0, <5 %; 1, 5 %–33 %; 2, 33 %–66 %; 3, >66 %. Batts-Ludwig scores were recorded as minimal 0–1 = 0, mild 2 = 1, moderate 3 = 2, marked 4 = 3. Ballooning was recorded on a 0–2 scale (0, absent; 1 or 2, present). METAVIR fibrosis stages ≥2 and ≥3 were used to categorize significant fibrosis and advanced fibrosis. NAFLD activity score (NAS), which is based on steatosis (on a scale of 1–3), lobular inflammation (on a scale of 0–3), and ballooning (on a scale of 0–2), was utilized in patients with MASLD. MASH was defined as NAS ≥5.
2.3Clinical data and laboratory testAt inclusion, extensive clinical and anthropometric data were gathered. The following information was evaluated for each patient: age, sex, clinical history, drug history, body mass index (BMI), waist circumference (WC), and blood pressure were reviewed. Self-reported data on the frequency and volume of alcohol usage over the past years were collected. At the time of the liver biopsy, information on patients’ clinical characteristics, ultrasonography, and vibration-controlled transient elastography were recorded. Along with platelet count (PLT), liver biochemistry, hemoglobin A1c (HbA1c), total cholesterol (TC), triglycerides (TG), fasting glucose, triglyceride, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), serum creatine, plasma high-sensitivity C-reactive protein level and insulin resistance score were included. All the blood tests were performed within 72h of the liver biopsy. Liver stiffness measurement (LSM) was expressed in kilopascals (kPa). All patients underwent vibration-controlled transient elastography (Fibroscan, Echosens, Paris) examination, which was performed at the time of biopsy by two experienced operators who had performed over 500 TE examinations. XL probe was available for obese patients. Noninvasive tests for liver steatosis and fibrosis, such as the Fatty Liver Index (FLI) [25] and Fibrosis Index-4 (FIB-4) [26], are calculated as follows:
2.4Viral markersQuantitative HBsAg level was measured by Architect (Abbott, Abbott Park, IL) assay (range, 0.05–250 IU/mL), and the samples were diluted and re-tested when the level was higher than 250 IU/mL. Serum HBV DNA levels with a lower quantitative limit of 20 IU/mL and Hepatitis e antigen (HBeAg) were assessed and recorded.
2.5Diagnostic criteria for MASLD and definition of groupsPatients were divided into the CHB + MASLD group and the CHB group according to the MASLD criteria.
Patients in CHB + MASLD group was diagnosed by the presence of hepatic steatosis ≥5 % with at least one of the following five cardiometabolic risk factors (CMRF): (1) namely overweight/obesity (BMI ≥ 23 kg/m2 in Asians) or waist circumference ≥90/80 cm in Asian men and women; type 2 diabetes mellitus (DM) or treatment for DM, or prediabetes, or HbA1C>5.7 %; (3) Blood pressure ≥130/85 mmHg or specific antihypertensive drug treatment;(4) TG ≥150 mg/dL or specific drug treatment;(5) plasma high-density lipoprotein-cholesterol <40 mg/dL for men and <50 mg/dL for women or specific drug treatment.
Patients in the CHB group were diagnosed with the presence of steatosis less than 5 % or more than 5 % while without CMRF above.
2.6Statistical analysisStatistical analyses were performed using SPSS 25.0(IBM Corp., Armonk, NY, USA) and R (4.1.2 for Windows). The mean ± standard deviation or median with interquartile range (IQR) for continuous variables was displayed. Depending on whether the variable follows a normal distribution, they were analyzed using either Student's t-test or the nonparametric Mann-Whitney U test. The Chi-square test was used to compare categorical variables, which are given as numbers (percentages). A 2-tailed p < 0.05 was considered statistically significant. The Wilcoxon sign rank test or the paired t-test was used to compare repeated measurement data. To control confounding factors, participants’ characteristics according to the presence or absence of MASLD were compared after pairing. To ascertain if a component was independently associated with significant fibrosis, factors from univariate analyses that with a p < 0.1 were included in a multivariate binary logistic regression. Forward stepwise Logistic regression was performed for factor selection. The results were reported together with the adjusted odds ratio (aOR), 95 % confidence interval (CI), and p values. The Receiver Operating Characteristic curve (ROC) was used to verify the effectiveness of FLI as a predictive index for MASLD. Diagnostic value of FIB-4 for significant fibrosis was calculated, using liver biopsy as the gold standard, and ROC were plotted. The area under the ROC curve (AUC) was computed. Figures and locally estimated scatterplot smoothing (LOESS) were drawn by the ggplot2 package in R.
2.7Ethical statementsThe study was in accordance with the Helsinki Declaration of 1975, as revised in 2000. The study protocol was approved by the Ethics Committee of XX Hospital, XX University (No. DTEC-KT2023-006-01). Written informed consent was obtained from each individual before the liver biopsy.
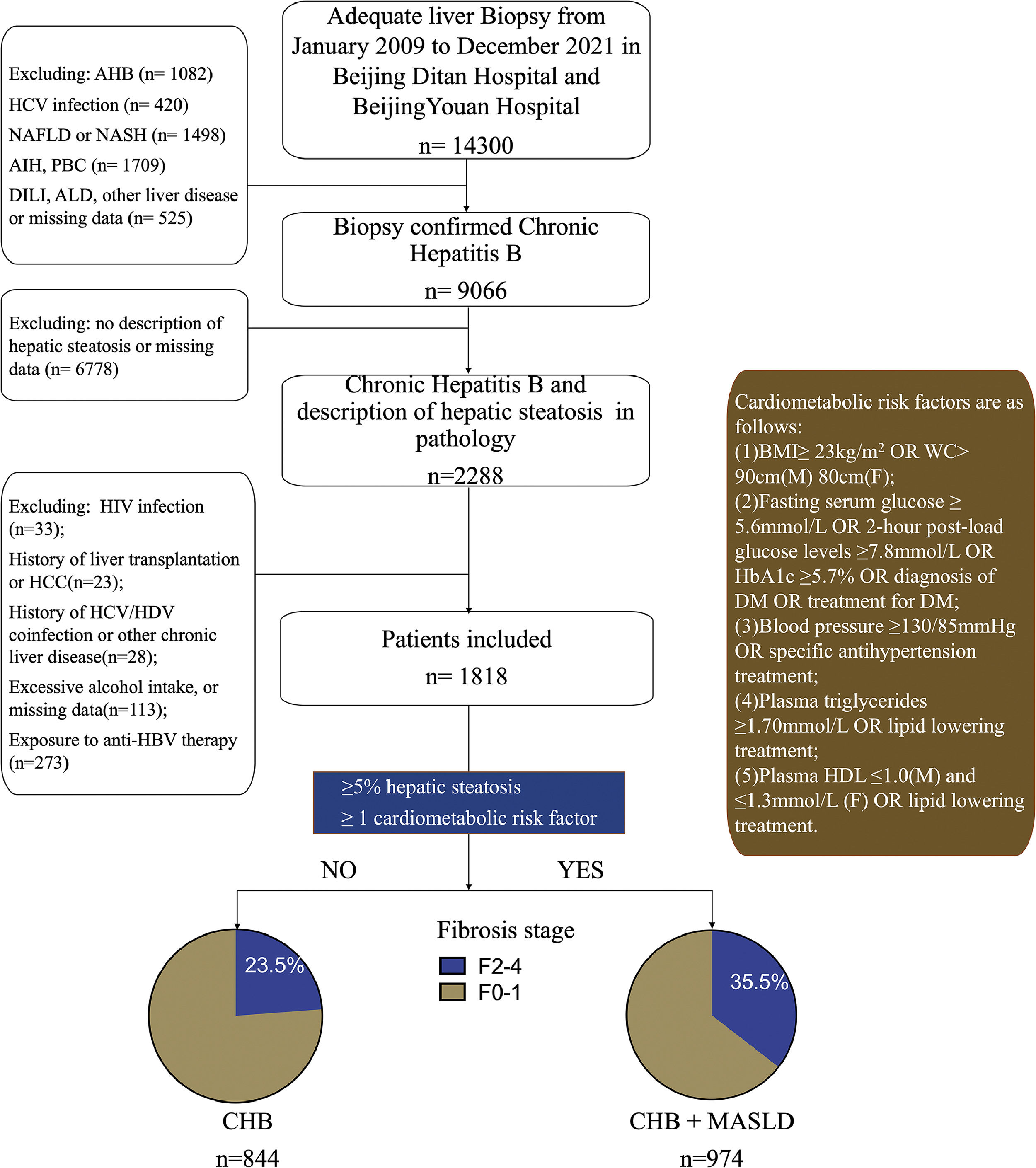
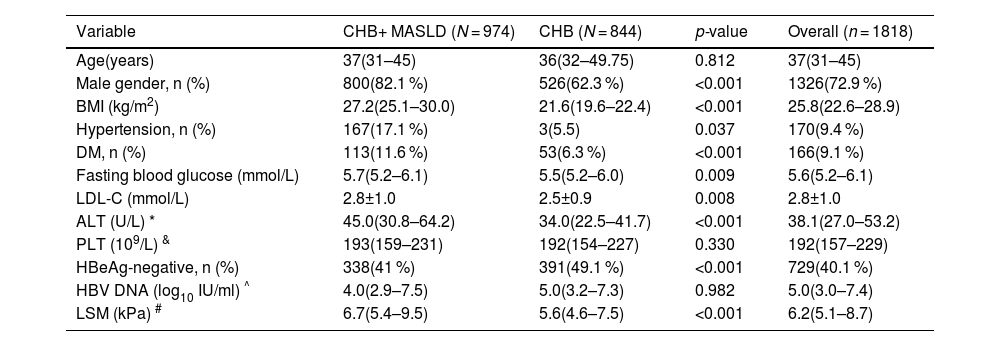
3Results3.1Patient characteristicsA total of 1818 patients with CHB were included in the study (Fig. 1). The participant characteristics are summarized in Table 1. Men made up 72.9 % of the entire cohort, with a median age of 37.
Study design, * MASLD criteria was based on Rinella ME, et al. J Hepatol. 2023 Jun 20:S0168-8278(23)00418-X., AHB, acute hepatitis B; ALD, alcoholic liver disease; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; AIH, autoimmune hepatitis; PBC, Primary Biliary Cholangitis; DILI, drug induced liver injury; BMI, body mass index; WC, waist circumference; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; HbA1C, Glycosylated hemoglobin type A1C; CHB, chronic hepatitis B; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; MASLD, metabolic dysfunction-associated steatotic liver disease.
Demographic Data of CHB patients with Versus without MASLD
| Variable | CHB+ MASLD (N = 974) | CHB (N = 844) | p-value | Overall (n = 1818) |
|---|---|---|---|---|
| Age(years) | 37(31–45) | 36(32–49.75) | 0.812 | 37(31–45) |
| Male gender, n (%) | 800(82.1 %) | 526(62.3 %) | <0.001 | 1326(72.9 %) |
| BMI (kg/m2) | 27.2(25.1–30.0) | 21.6(19.6–22.4) | <0.001 | 25.8(22.6–28.9) |
| Hypertension, n (%) | 167(17.1 %) | 3(5.5) | 0.037 | 170(9.4 %) |
| DM, n (%) | 113(11.6 %) | 53(6.3 %) | <0.001 | 166(9.1 %) |
| Fasting blood glucose (mmol/L) | 5.7(5.2–6.1) | 5.5(5.2–6.0) | 0.009 | 5.6(5.2–6.1) |
| LDL-C (mmol/L) | 2.8±1.0 | 2.5±0.9 | 0.008 | 2.8±1.0 |
| ALT (U/L) * | 45.0(30.8–64.2) | 34.0(22.5–41.7) | <0.001 | 38.1(27.0–53.2) |
| PLT (109/L) & | 193(159–231) | 192(154–227) | 0.330 | 192(157–229) |
| HBeAg-negative, n (%) | 338(41 %) | 391(49.1 %) | <0.001 | 729(40.1 %) |
| HBV DNA (log10 IU/ml) ^ | 4.0(2.9–7.5) | 5.0(3.2–7.3) | 0.982 | 5.0(3.0–7.4) |
| LSM (kPa) # | 6.7(5.4–9.5) | 5.6(4.6–7.5) | <0.001 | 6.2(5.1–8.7) |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CHB, chronic hepatitis B; HBeAg, hepatitis B e-antigen; HBV, hepatitis B virus; LDL-C, low-density lipoprotein cholesterol; LSM, Liver stiffness measurement; PLT, platelet; MASLD, metabolic dysfunction-associated steatotic liver disease; DM, type 2 diabetes mellitus.
While comparing the characteristics of patients with MASLD to those without MASLD, it was shown that patients with CHB + MASLD were more likely to be men, obese, and had a higher percentage of hypertension, diabetes, and HBeAg positivity. In comparison to the CHB group, those patients also tended to have higher LSM, alanine aminotransferase (ALT), aspartate aminotransferases (AST), gamma-glutamyl transferase (GGT). Age and HBV DNA levels, however, did not significantly differ between groups. The FLI and FIB-4 were significantly higher in the CHB+ MASLD group (Table 1).
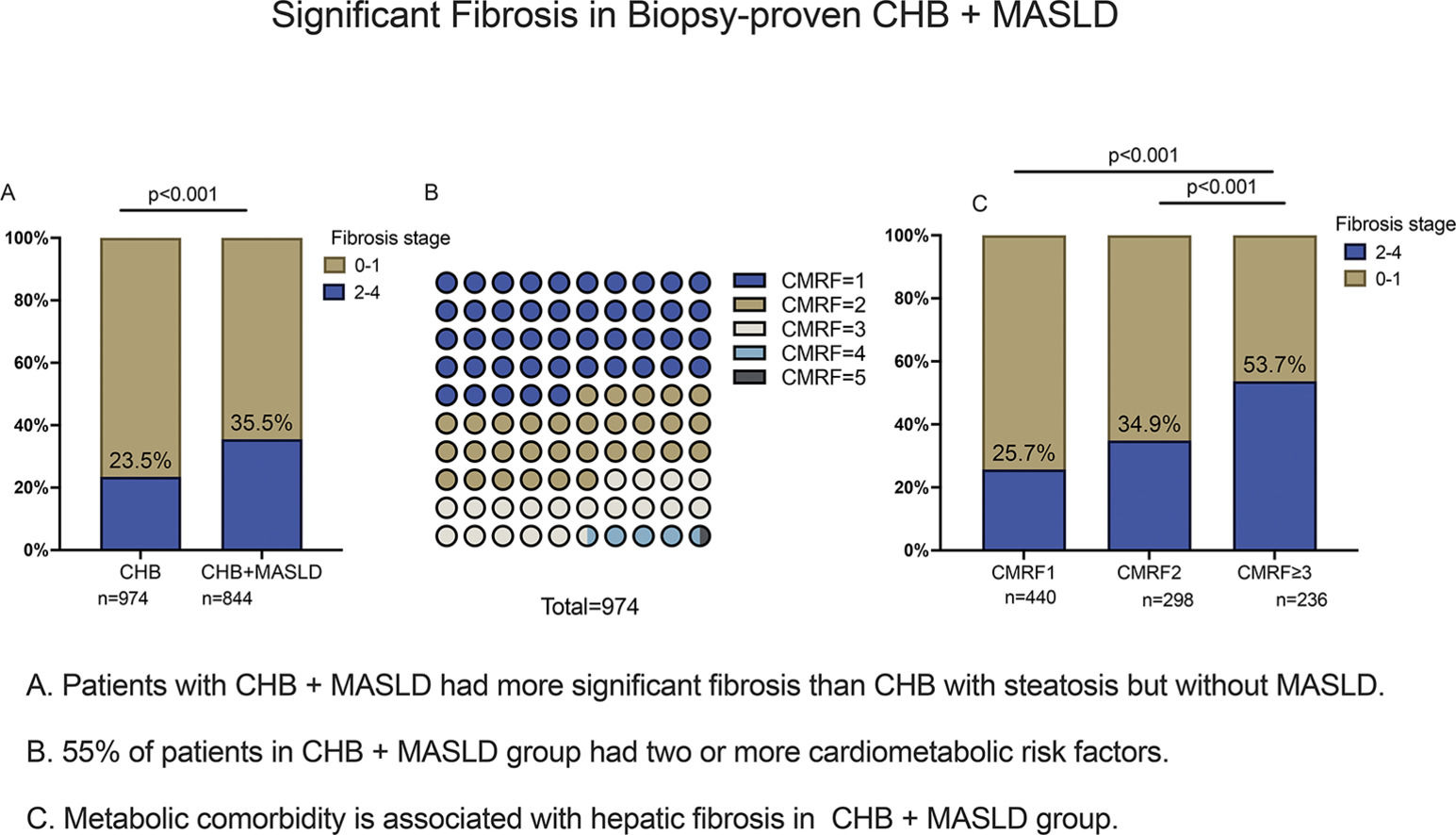
3.3Comparison of liver pathology between patients with and without MASLDThere is a list of pathological scores in Supplementary Table S1. Significant liver fibrosis was more likely to occur in CHB + MASLD patients. In the CHB + MASLD group, 35.5 % of patients had definite fibrosis stage F2-4, which was significantly higher than the CHB group's 23.5 % (p < 0.001). Besides fibrosis stages, there were higher scores of lobular inflammations and ballooning changes in the CHB + MASLD group (p < 0.001). Scores of ballooning change and lobular inflammation were mainly grade 1 in both groups. MASH (NAS≥5) compromise 26.4 % of patients in CHB + MASLD group. After age, sex, and HBeAg were matched, ALT levels (p = 0.003) and the proportion of significant fibrosis were significantly higher in the CHB + MASLD group than those of the CHB group (36.5 % vs. 19.0 %, p = 0.002), which was in line with the findings before matching (Supplementary Table S2).
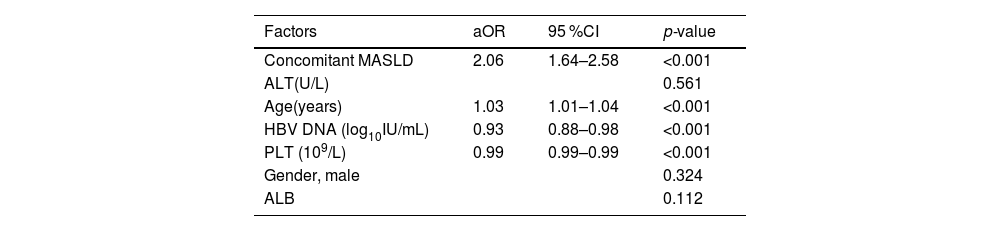
Table 2 displays the relationships between significant fibrosis with MASLD as well as additional patient features. Concurrent MASLD (aOR 2.06,95 %CI 1.64–2.58; p < 0.001) was significantly associated with fibrosis grading, as was age (aOR 1.03,95 %CI 1.01–1.04; p < 0.001), PLT (aOR 0.99,95 %CI 0.99–0.99; p < 0.001), and HBV DNA (aOR 0.93, 95 %CI 0.88–0.98; p < 0.001).
Factors Associated with Significant Fibrosis in All patients: Multivariate Analyses
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; aOR, adjusted odds ratios; CI, confidence interval; HBV, hepatitis B virus; MASLD, metabolic dysfunction-associated steatotic liver disease; PLT, platelet.
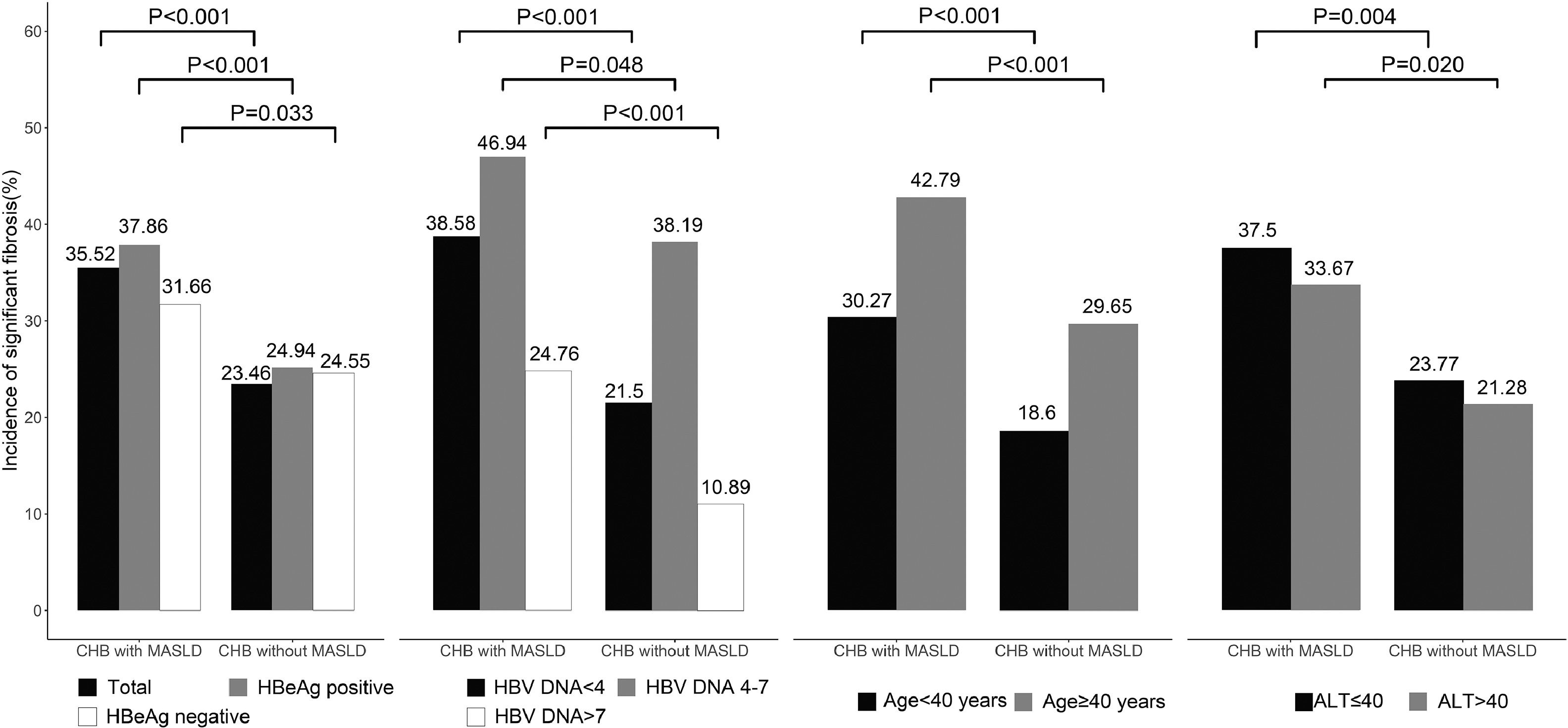
Further analyses were carried out independently in two groups since virological parameters such as HBeAg, HBV DNA, and age are related to the risk of adverse outcomes, as shown in Fig. 2. In patients with MASLD, higher fibrosis stages were more prevalent, regardless of age, HBV DNA, ALT level, and HBeAg.
Comparison of significant fibrosis in subgroups between CHB patients with versus without MASLD, The association between MASLD and significant fibrosis was consistently observed across pre-specified subgroups by HBV DNA level, HBeAg, age, and ALT levels. ALT, alanine aminotransferase; CHB, chronic hepatitis B; HBeAg, hepatitis B e-antigen; HBV, hepatitis B virus; MASLD, metabolic dysfunction-associated steatotic liver disease.
To stratify significant injury in CHB + MASLD, we compared the characteristics of pre-specified subgroups by fibrosis stage F0-1 and F2-4. In comparison to patients without significant fibrosis, patients with significant fibrosis were older, had greater BMI, TC, TG, AST/ALT ratio, GGT, and lower HBV DNA level, albumin (ALB), and platelet levels (Supplementary Table S3). There was no statistically significant difference in sex or the proportion of HBeAg positivity between the two subgroups. There was a trend for significant fibrosis in patients over 40 years compared to patients under 40 (42.8 % vs. 30.3 %; p < 0.001), but not between different sexes (Supplementary Fig. S1).
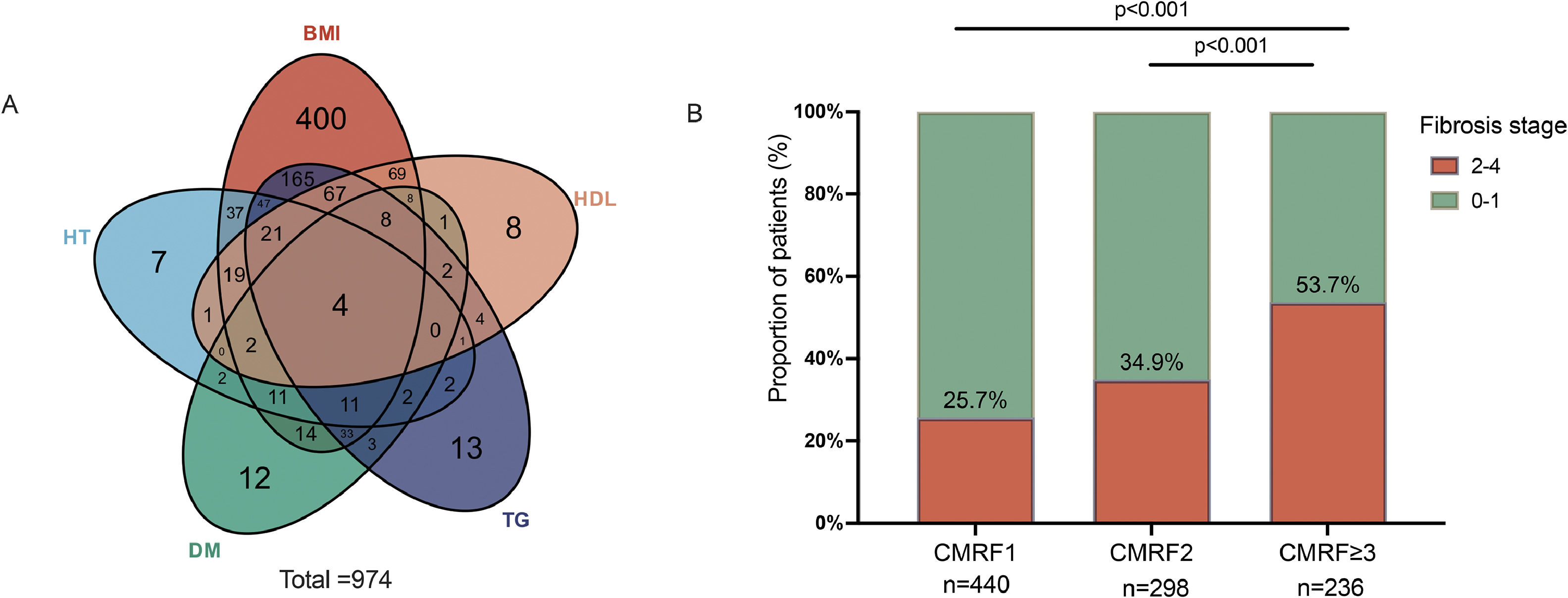
Overweight/ higher waist circumference (BMI group) and higher TG were the most two common CMRFs in patients with CHB + MASLD as shown in Fig. 3. To clarify the impact of CMRFs on significant fibrosis, patients in CHB + MASLD groups were further stratified by the numbers of CMRF. 55 % (n = 534) of patients had more than two CMRFs. There was a trend for patients with more CMRFs to have a higher prevalence of significant fibrosis:(25.7 % in CMRF1 subgroup v.s. 34.9 % in CMRF2 subgroup v.s. 53.7 % in CMRF≥ 3 subgroup, p < 0.001).
3.5Factors associated with significant fibrosis in patients with MASLDHistologically, those with significant fibrosis exhibited a statistically higher grade of inflammatory activity but not steatosis. Using LOESS and bivariate correlation analysis, no clear linear correlation between the score of steatosis and fibrosis stage was found (Pearson correlation coefficient=−0.077, p = 0.016).
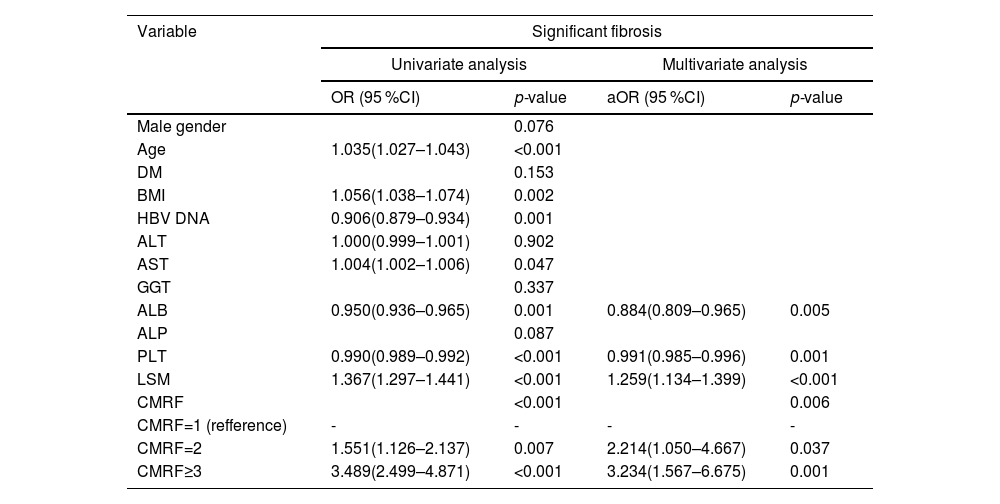
To further stratify the fibrosis stage in patients with MASLD, the multivariable analysis revealed that the coexistence of CMRF≥ 3 was associated with a 3-fold increase in significant fibrosis than patients with one CMRF (Table 3). ALB (aOR=0.88,95 %CI, 0.81–0.97; p = 0.005) and PLT (aOR=0.99,95 %CI, 0.99–1.00; p < 0.001) was negatively associated with significant fibrosis. LSM was positively correlated with both significant fibrosis (aOR=1.39, 95 %CI, 1.28–1.51; p < 0.001) and advanced fibrosis (aOR = 1.28, 95 %CI, 1.21–1.34; p < 0.001).
Univariate and multivariate analyses of factors associated with significant fibrosis in CHB + MASLD
Abbreviations: aOR, adjusted odds ratios; ALB, albumin; ALT, alanine aminotransferase; ALP alkaline phosphatase; AST aspartate aminotransferase; BMI, body mass index; CHB, chronic hepatitis B; CI, confidence interval; GGT gamma glutamyl transferase; HBV, hepatitis B virus; LSM, Liver stiffness measurement; MASLD, metabolic dysfunction-associated steatotic liver disease; PLT, platelet; DM, type 2 diabetes mellitus.
Considering the clinical scenario where liver biopsy is unavailable, we attempted to use the non-invasive diagnostic model FLI to stratify the risk in CHB patients and evaluate its accuracy in diagnosing MASLD among the CHB population. FLI was calculated in all 1818 patients (Table 1). The AUC of the FLI for predicting MASLD was 0.911 (95 %CI 0.893–0.928), with the sensitivity of 79.9 % and specificity of 89.6 % (Supplementary Fig. S2).
To evaluate the noninvasive test for accurate staging and risk stratification in patients with MASLD, FIB-4 was calculated to predict liver fibrosis 974 CHB +MASLD patients. Supplementary Table S3 showed that FIB-4 was significantly higher in patients with fibrosis (1.09 v.s 0.78 kpa, p < 0.001). The AUC of the FIB-4 (Supplementary Fig. S3)for predicting significant fibrosis was 0.679 (95 %CI 0.647–0.709) with a sensitivity of 60.38 % and specificity of 64.78 %. However, the AUC of the FLI predicting significant fibrosis in CHB +MASLD patients was 0.523 (95 %CI 0.476 - 0.569).
4DiscussionThe primary finding of this large cross-sectional cohort study of CHB patients, based on liver biopsy data, is that those with MASLD exhibited higher hepatic fibrosis compared to those without MASLD. From a holistic healthcare perspective, MASLD is a significant concern in CHB patients, particularly given the concurrent presence of multiple metabolic risk factors.
Our study possesses several strengths. Notably, we found that CHB patients with MASLD have a twofold increase in the prevalence of significant fibrosis compared to those with liver steatosis but without MASLD. Previous research suggests that hepatic fibrosis is associated with higher BMIs and waist circumferences, and that steatohepatitis is linked to a 1.33-fold higher Ishak fibrosis score and a 1.68-fold higher risk of advanced fibrosis, which aligns with our findings [27]. However, there is limited evidence to support the notion that treating MASLD improves the likelihood of a functional cure in the CHB population [28–30]. Additionally, previous studies indicated no correlation between steatosis and fibrosis in two meta-analyses of CHB patients with available histology or LSM data [22,31–34]. It's important to note, however, that some of these studies did not distinguish between individuals with and without metabolic dysregulation. On the other hand, metabolic syndrome (OR 4.379) has been associated with fibrosis progression after ten years of follow-up [24,35]. In contrast to CHB patients with steatosis who do not meet the criteria of metabolic syndrome, a greater proportion of CHB patients with MASLD have advanced fibrosis or cirrhosis [13], which corroborates our findings and suggests that the diagnosis of MASLD may have significant implications for the clinical outcomes of CHB patients.
Additionally, we focused on comparing CHB patients with hepatic steatosis who did or did meet the criteria for metabolic dysfunction. No associations were found between steatosis and fibrosis. However, higher fibrosis stages were observed in patients with MASLD who had more CMRFs. In patients with a higher stage of fibrosis, hepatic fat tends to decrease as part of the natural course of MASLD [36]. Those with “burnt-out” steatosis at enrollment would not be overlooked when CMRFs are considered within MASLD criteria, provided patients were regularly monitored for the development of metabolic risk factors. There is still uncertainty regarding the effect of MASLD on liver fibrosis in CHB patients. This may be partly due to the varying severity of steatotic liver disease across research groups, which leads to different degrees of liver injury and subsequent fibrosis. Prospective studies are needed to confirm the role of early screening and follow-up strategies in monitoring for liver-related complications.
Various imaging modalities are increasingly used to evaluate MASLD, with their diagnostic efficacy largely based on the detection of lipid accumulation. In our study, FLI showed good consistency with MASLD confirmed by liver histopathology. We found that patients with MASLD predominantly exhibited abnormalities in BMI and WC, with less than 10 % presenting with other CMRFs without being overweight or having increased WC. Asians are prone to visceral fat accumulation despite having a lower BMI [37]. The new diagnostic criteria for MASLD-BMI group, which include both BMI and WC, reflect the accumulation of visceral fat and are less likely to overlook patients than NAFLD definition [38]. The calculation formula of FLI also includes WC, contributing to its high diagnostic accuracy [39]. FLI has been externally validated and is associated with cardiovascular, liver, and cancer-related mortality. It also correlates with decreased insulin sensitivity, an increased risk of DM, accelerated atherosclerosis, and heightened cardiovascular risk [40]. In large epidemiological studies where imaging modalities might not be available, FLI can be an alternative method for diagnosing steatosis [41]. However, the FLI can only assess whether CHB patients have concurrent MASLD and does not reliably indicate the degree of fibrosis. The assessment of hepatic fibrosis still primarily relies on liver biopsy. Serum-based scores are relevant, depending on the availability of transient elastography. FIB-4 was initially considered the optimal non-invasive diagnostic algorithm in early studies [42]. Our data align with recent studies showing the similar performance of FIB-4 in diagnosing liver complications and advanced fibrosis [43,44]. Elevated levels of ALT and AST, along with reduced platelet counts in CHB + MASLD, may contribute to higher scores on non-invasive models, thus improving fibrosis detection. However, the AUC, sensitivity, and specificity of FIB-4 remain unremarkable. Considering that MASLD is becoming more prevalent among patients with CHB, these findings underscore the urgent need to develop and validate novel scoring systems tailored to assess fibrosis in the MASLD population.
We also acknowledge the following limitations. First, as a cross-sectional study, patients who had histologically confirmed MASLD as an entry criterion implied that clinically significant hepatic injury was suspected by physicians at the time of liver biopsy, thus initiating a bias of selection. However, we are confident that this bias does not impact the validity of our histopathological findings. Additionally, the results may not be generalizable to other racial groups, as our study cohort consisted solely of Asian individuals. Asians are known to have a higher propensity for central fat deposition and metabolic disorder despite a lower BMI [45]. In this study, overweight was defined as 23 to <25 kg/m2, and obesity as ≥25 kg/m2, according to International Diabetes Federation [46]. The use of stricter BMI and WC criteria reduces the risk of underdiagnosing metabolic disorders. Nonetheless, the generalizability of our conclusions requires further validation through studies involving more diverse populations. Furthermore, the utility of LSM was limited to a small subset of patients in both groups because it was promoted and included in medical insurance in both two centers for less than a decade. The Chinese Society of Hepatology developed the first edition of guidelines for the treatment of CHB in 2005 and updated them in 2010, 2015, and 2019 [47–50]. Significant advances have been made in both basic and clinical research over the past decades. According to the guidelines before, antiviral treatment should be based on HBeAg status, HBV DNA levels, or age, with liver biopsy being the gold standard for assessing the severity of hepatic fibrosis in CHB [51]. For patients in the “grey zone” of typical CHB clinical phases or those requiring differential diagnosis for elevation of ALT, it is necessary to perform liver biopsy to accurately identify patients who require close monitoring or antiviral therapy, resulting in a large sample of patients with evidence of hepatic pathology before antiviral treatment during the past years. With the gradual understanding of the natural history of CHB, indications for antiviral treatment have also shifted over time [52]. However, this is a common limitation in retrospective studies. Further confirmation of this association using a prospective study design is warranted.
5ConclusionsIn conclusion, MASLD was an independent risk factor for significant fibrosis and could speed up the progression of fibrosis. Clinicians should intensify monitoring for liver fibrosis and implement early interventions for fibrosis in patients with CHB who have concurrent MASLD, especially those with three or more CMRFs. Monitoring metabolic disorders in CHB patients helps to stratify the risk for CHB patients better.
Author contributionsAll authors contributed to the study's conception and design. Shan Hong: acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis; Yiwei Hao: statistical analysis, critical revision of the manuscript, obtained funding; Lei Sun: interpretation of data, critical revision of the manuscript for important intellectual content; Ping Li, Junru Yang, Fuyang Zhang and Lingling He: acquisition of data; Jing Zhang and Hongshan Wei: study concept and design, obtained funding, material support and study supervision.
The manuscript has been read and approved by all the authors, and the requirements for authorship as stated earlier in this document have been met. Each author believes that the manuscript represents honest work.
FundingThis paper was supporteded by grants from the National Natural Science Foundation of China [grant no. 82170541), the Capital Foundation for Clinical Characteristic Applied Research Projects [grant no. Z181100001718084], and Beijing Hospitals Authority Youth Programme [QML20231806].
We are grateful to Zifan Hong from Tomsk State University, Russia, who helped with the implementation of the computer code used in this study.