Although the Psychometric Hepatic Encephalopathy Score (PHES) remains the gold standard in diagnosing minimal hepatic encephalopathy (MHE), its complexity limits its application in clinical practice. While more convenient tests, such as the Stroop test, Quickstroop, and the 1-min animal naming test (ANT-1), have emerged, they haven't been validated in our setting. Our objective was to validate these tests in our population.
Patients and MethodsThis multicenter, observational, descriptive, and cross-sectional study was conducted in three hospitals in northeastern Mexico. MHE was defined as a PHES <-4. We included patients with cirrhosis aged >15 years without a history of overt hepatic encephalopathy. Data regarding sex, age, education, Child-Pugh/MELD-Na scores, etiology of cirrhosis, diabetes, hypertension, obesity, ascites, and clinically significant portal hypertension was collected. Fisher's exact test, Mann-Whitney U test, and receiver operating characteristic (ROC) curves were used for statistical analysis.
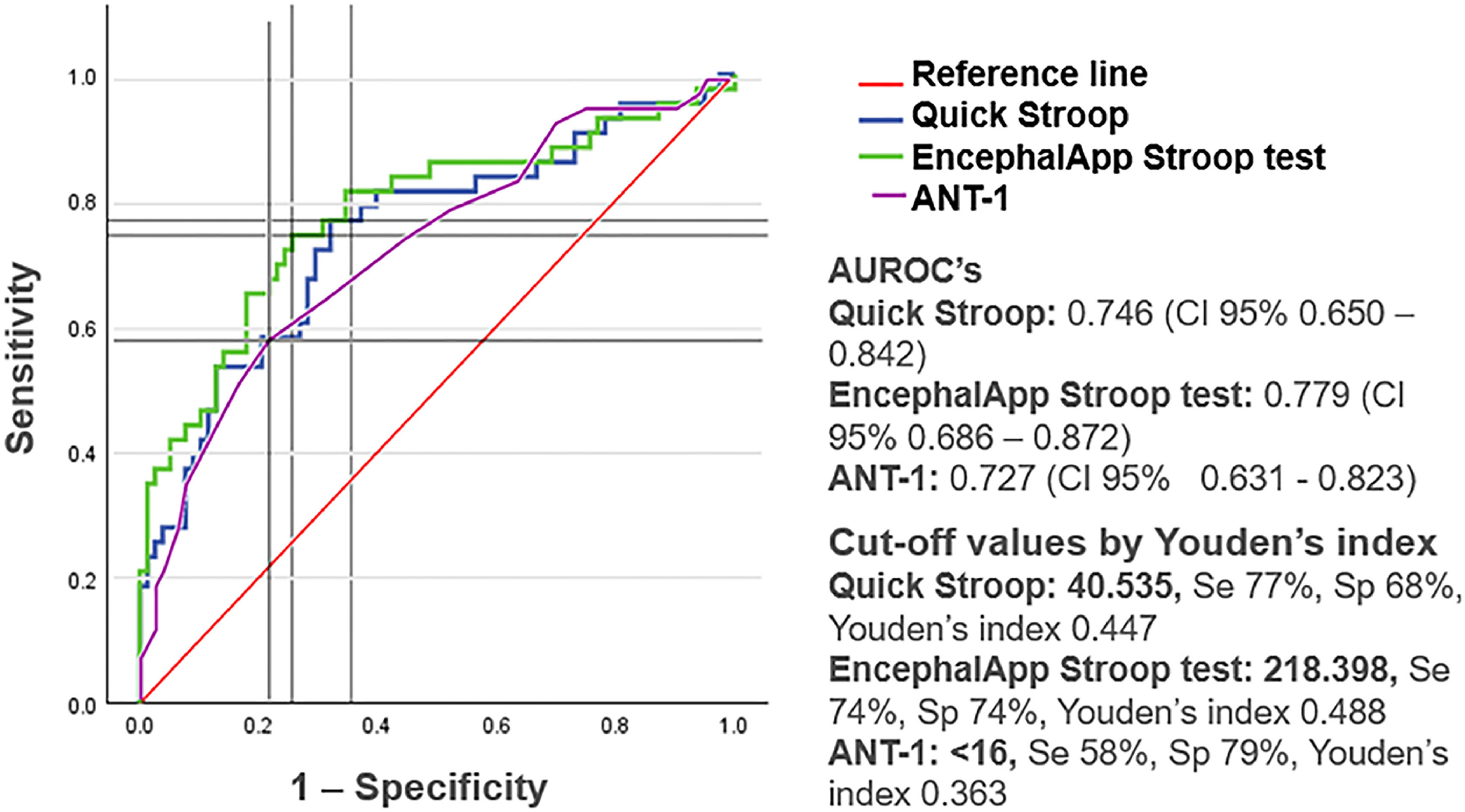
ResultsOf the 121 patients included, 35.5 % were diagnosed with MHE. The presence of MHE was significantly associated with education level, years of study, and scores in the Stroop test, Quickstroop, and ANT-1. The AUROC curves were 77.9 %, 74.6 %, and 72.7 % for the Stroop test, Quickstroop, and ANT-1, respectively. The resulting cut-off points were 218.398 (sensitivity: 74 %; specificity: 74 %), 40.535 (sensitivity: 77 %; specificity: 68 %), and <16 animals (sensitivity: 58 %; specificity: 79 %), respectively.
ConclusionsThese tests are valid diagnostic tools for detecting MHE in our population. Their simpler use and applicability could increase the early diagnosis of MHE and prompt primary prophylaxis initiation for overt hepatic encephalopathy.
Hepatic encephalopathy is a neuropsychiatric syndrome that occurs in patients with acute or chronic liver disease and is classified based on the severity of neuropsychiatric alterations into covert or overt forms. Overt hepatic encephalopathy is easily recognized on physical examination and corresponds to grades II–IV of the West Haven (WH) criteria. Conversely, covert hepatic encephalopathy encompasses minimal hepatic encephalopathy (MHE) and corresponds to WH grade I [1].
Briefly, MHE can be defined as the mildest form in the hepatic encephalopathy spectrum, in which a cognitive and psychomotor deficit occurs in the absence of recognizable clinical symptoms of overt hepatic encephalopathy [2]. The prevalence of MHE varies from 20 to 80 % in patients with cirrhosis, reflecting the variability in its definition and diagnosis. In Mexico, a prevalence of 32.7 % has been reported using the Psychometric Hepatic Encephalopathy Score (PHES) [3].
Once considered harmless, MHE is now known to be associated with a diminished quality of life and an increased risk of experiencing a flare of overt hepatic encephalopathy, with up to 33 % of patients experiencing the latter 1 year after diagnosis [1].
Hepatic encephalopathy affects multiple components of mental function; therefore, the International Society for Hepatic Encephalopathy and Nitrogen Metabolism suggests that the diagnosis of MHE should be based on a combination of >1 test of choice, depending on availability and local experience [4]. However, the concordance between the tests is low since they evaluate different functions [5].
The PHES is currently used as the gold standard for MHE diagnosis, recommended by expert groups and validated in different countries, including Mexico. Nevertheless, it is complex and time-consuming, resulting in low use in clinical practice. Hence, several simpler tests have been developed to optimize screening and improve diagnosis.
The EncefalApp - Stroop test assesses psychomotor speed and cognitive flexibility by interference between the recognition reaction to a color field and a written color name, measuring the inhibitory response. It is available in several languages as an application for smartphones or tablets called EncephalApp Stroop and has been validated in different parts of the world as a diagnostic test for MHE [6-11]. The Quickstroop test, a shorter version of the EncephalApp, detects covert hepatic encephalopathy with similar accuracy in <1 min, using only the first two turns of the Off Time [12]. Finally, the 1-min animal naming test (ANT-1) evaluates the number of animals a patient can name in 1 min and has been previously used for diagnosing MHE [13].
Therefore, our objective was to validate the EncephalApp-Stroop test, Quickstroop test, and ANT-1 for diagnosing MHE in our population.
2Patients and methods2.1Study design and patient selectionThis observational, descriptive, cross-sectional study was conducted in three hospitals in Mexico, including “Unidad Medica de Alta Especialidad No. 25", “Hospital General de Zona No. 33", and “Hospital General de Zona No.17.” In the first hospital, both inpatients and outpatients were included. In contrast, in the other centers, only outpatients were evaluated. A finite sample size calculation was computed based on the number of patients with cirrhosis treated in the three hospitals and the reported prevalence of MHE in Mexico. With N = 900, 95 % confidence interval, 5 % alpha error, p of 90 %, and q of 10 %, a sample size of 120 patients was obtained.
We included patients diagnosed with cirrhosis of any etiology, without a previous diagnosis of overt hepatic encephalopathy, and aged >15 years. Patients under treatment with lactulose, l-ornithine-l-aspartate, rifaximin, antibiotics, and psychoactive agents, those with active infections, a history of transjugular intrahepatic portosystemic shunt placement, a visual, physical, or neuropsychiatric disability that does not allow the correct performance of the tests, color blindness to red or green colors, illiterate, and pregnant women were excluded.
From December 2022 to July 2023, outpatients who met the inclusion criteria were invited via telephone to participate in the study, and an appointment was arranged for the application of the tests. Patients who met the inclusion criteria and had no exclusion criteria performed the following tests: PHES, ANT-1, and EncephalApp-Stroop test. The PHES test, composed of five subtests, was used as the gold standard with a cut-off point of <−4 for diagnosing MHE [14].
The Stroop test includes two modes: Off mode, which consists of matching the color of symbols #### with the colors below, and On mode, which consists of matching the name of the color presented in discordant words with the colors below. Each mode consists of two practice laps and five test laps, which are used for the analysis. The sum of the total time (five Off Time + five On Time) is used for MHE diagnosis. Using the same EncephalApp-Stroop test application, the Quickstroop test consisted of the sum of the times of the first two laps in Off time. Prior to the application of the Quickstroop and Stroop tests, color blindness was ruled out using a smartphone application called Color Test based on Ishihara's charts.
For the ANT-1, an audio recording of the test was made using a smartphone to verify and exclude repeated answers.
2.2Definition of variablesVariables included age, sex, education, Child-Pugh, Model for End-Stage Liver Disease–Sodium (MELD-Na), etiology of cirrhosis, presence of diabetes, systemic arterial hypertension, obesity, clinically significant portal hypertension (defined by transient elastography >25 Kpa, endoscopy with varices or High Productivity Computing Systems prediction models [anticipate/anticipate-NASH/Fib4+]), history of ascites, and scores obtained in the three tests to be validated.
2.3Statistical analysisVersion 29.0.1.0 of the IBM SPSS Statistics software was used for analyses. Results were analyzed using measures of central tendency for descriptive statistics. The presence or absence of MHE was compared using the Mann-Whitney U test for quantitative variables and Fisher's exact test for qualitative variables. Receiver operating characteristic (ROC) curves were created to determine the diagnostic efficacy of the tests applied, and a cut-off point was obtained based on Youden's index.
2.4Ethical considerationsThis study was approved by the local ethics committee and the local health research committee (CLIS) 1901 of the Mexican Institute of Social Security (IMSS). The study was conducted in accordance with the principles of the 1975 Declaration of Helsinki and the IMSS institutional ethical and regulatory standards, adhering to the general health law on research and good clinical research practices. Informed consent was obtained in duplicate from the patients. The authors declare that this article does not contain personal information that would allow identification of the patients.
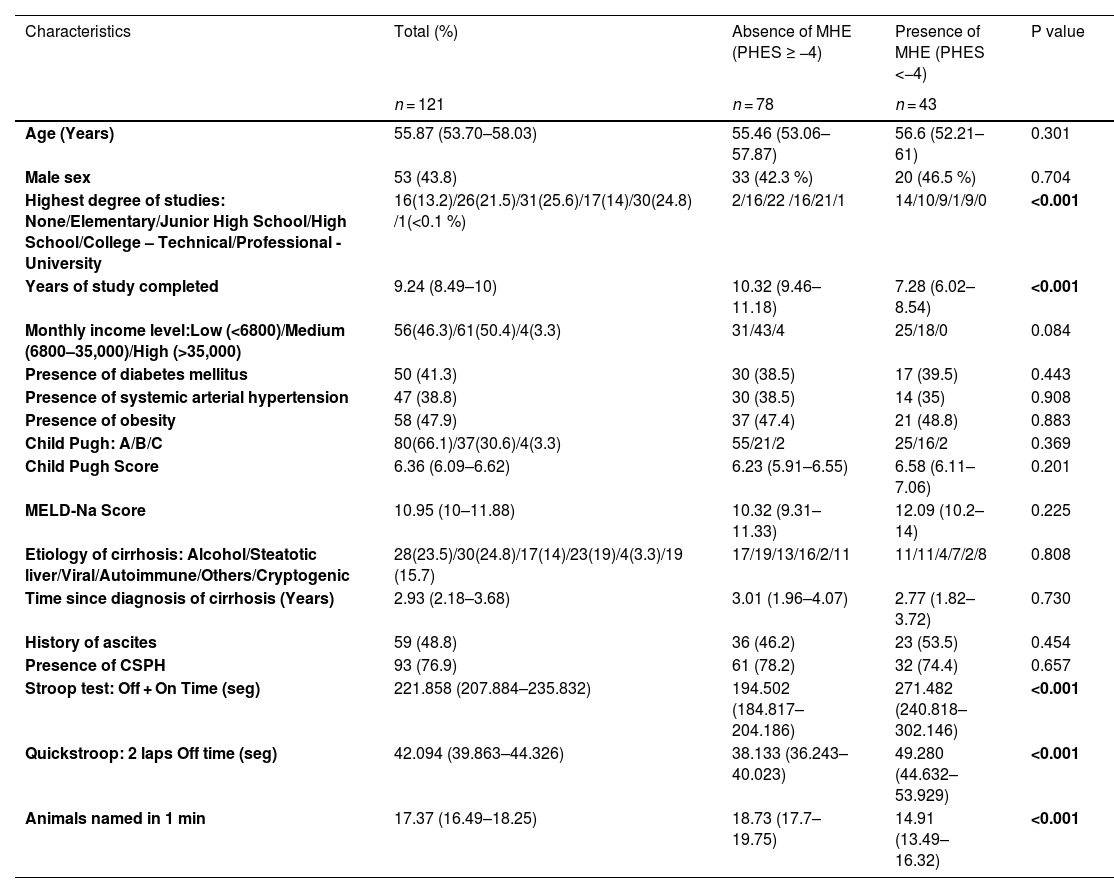
3ResultsA total of 121 patients were analyzed, including seven inpatients and 114 outpatients. Table 1 shows the characteristics of the study participants. Sixty percent of the patients had a maximum grade of junior high school or lower, and 95.7 % had a low or medium monthly income level. Sixty-seven percent of the patients had compensated cirrhosis (Child-Pugh class A), and 33 % had decompensated cirrhosis (Child-Pugh class B or C). The most frequent etiology of cirrhosis was steatotic liver disease, followed by alcohol liver disease and viral etiology.
Baseline characteristics of patients with or without MHE.
Note: values are presented as number (percentage) or as mean (interquartile range with confidence interval >95 %). Fisher's exact test for qualitative variables or the Mann-Whitney U test for quantitative variables was used to compare clinical characteristics between groups with or without MHE. CSPH: clinically significant portal hypertension.
MHE was diagnosed in 43 of the 121 patients, with a prevalence of 35.5 %. Age, sex, monthly income level, etiology of cirrhosis, time since cirrhosis diagnosis, presence of diabetes, hypertension, obesity, clinically significant portal hypertension, history of ascites, and Child-Pugh/MELD-Na scores did not differ between patients with and without MHE.
In the group of patients with MHE, a statistically significant relationship was found with the highest grade of education, years of study completed, and the scores obtained in the EncephalApp-Stroop test, Quickstroop test, and ANT-1.
ROC curves were created for the EncephalApp-Stroop test, Quickstroop test, and ANT-1 (Fig. 1). The areas under ROC curves (AUROCs) obtained were 77.9 %, 74.6 %, and 72.7 % for the EncephalApp-Stroop test, Quickstroop test, and ANT-1, respectively. Moreover, the cut-off values according to Youden's index were 218.398, 40.535, and 16, respectively.
ROC Curves from the EncephalApp Stroop Test, Quickstroop Test and the 1 Min Animal Naming Test.
Notably, hepatic encephalopathy affects multiple components of mental function to different degrees and at different moments in time [5], and each of the diagnostic tests evaluates some of these mental functions. While the Stroop and Quickstroop tests evaluate the psychomotor speed and inhibitory response [7], the ANT-1 evaluates verbal fluency, in addition to aspects of cognitive self-control such as the inhibitory response [15]. Thus, patients with MHE may not have abnormal results in all the performed tests.
PHES is a more complex test that evaluates a greater number of mental functions, including attention, visuospatial perception, visuospatial construction, psychomotor speed, and motor accuracy [16]. Currently, with globalization and access to smartphones, The EncephalApp-Stroop test, Quickstroop test, and ANT-1 have become more accessible to any doctor than pencil and paper tests such as the PHES. The fact that these tests are time-saving would translate into greater use in clinical practice and a timely diagnosis of MHE.
Unlike previous research, where the EncephalApp-Stroop test [7-12] and ANT-1 [15] were validated in other countries, in our study, patients with a history of previous overt hepatic encephalopathy were not included, nor was a control group of patients without cirrhosis.
The latter was unnecessary because our objective was to validate these tests as a diagnostic method for MHE in patients with cirrhosis without a history of episodes of previous overt hepatic encephalopathy.
Using PHES as the gold standard, the prevalence found in our study was slightly higher than that previously reported in Mexico by Garza [3], who reported a prevalence of 32.7 % in a population with similar characteristics to our population.
The presence of MHE, defined by PHES, had a significant relationship with the patient´s education and years of study, which is in agreement with the results of previous research [3,16]. Interestingly, in our study, no relationship was found between the Child-Pugh and MELD-Na scores and the presence of MHE, which could be explained by a low variability in the sample, with most patients corresponding to Child-Pugh class A and with MELD-Na <15.
The presence of MHE was significantly correlated with the scores obtained in the three evaluated tests. The AUROCs showed that the EncephalApp-Stroop test, Quickstroop, and ANT-1 had good diagnostic efficacy. These data indicate that these tests are a good alternative for diagnosing MHE, being more accessible and easier to apply than other diagnostic tests. However, the ANT-1 showed lower sensitivity (58 %) for diagnosing MHE than what has been reported in previous studies [13,17-18]. This could be explained by the socioeconomic characteristics of our population, with more daily exposure to a vast kind of animals, in contrast with centers in which most of the patients live in urban environments.
The EncephalApp-Stroop and Quickstroop tests had the best sensitivity, while the ANT-1 had the best specificity. With these findings, the utility of combining these tests, one as screening and the other as confirmatory, could be considered for future research. Another issue that deserves further investigation is the clinical usefulness of these tests in assessing response to MHE treatment with ammonium-lowering strategies.
5ConclusionsOur study showed that the EncephalApp-Stroop test, Quickstroop, and ANT-1 are valid, reliable, and easy-to-apply tests in clinical practice, which could increase the early diagnosis of MHE and prompt primary prophylaxis initiation for overt hepatic encephalopathy.
More studies are needed regarding the best strategy to combine the different tests to increase the sensitivity and specificity of the diagnosis and their use as tools for monitoring response to prophylactic treatment.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributionsJ.F. Ortiz-Treviño: concept and design of the protocol, acquisition of data, analysis and interpretation of the data, drafting of the manuscript. A.L. Kuljacha-Gastélum: acquisition of data, critical revision of the manuscript, study supervision. A. Tovar. Durán: concept and design of protocol, acquisition of data. M.E. Wade Isidro: acquisition of data.