Hepatitis C virus (HCV) belongs to the Flaviviridae family, and is a single-stranded RNA virus with positive polarity. It is the primary cause of hepatocellular carcinoma (HCC) worldwide. The treatment of HCV has entered a new era with the advent of direct-acting antiviral drugs (DAAs) and is associated with cure rates of more than 95 %, making HCV the only curable viral disease. The successful treatment of chronic hepatitis C has greatly reduced, but not eliminated, the risk of HCC. Certain individuals, especially those with cirrhosis already present, remain vulnerable to HCC after achieving a sustained virological response (SVR). This article systematically reviews the recent studies on the risk and mechanisms of HCC development after HCV viral cure, the screening and predictive value of biological markers, and patient surveillance. Factors such as older age, diabetes, hepatic fat accumulation, alcohol use, and lack of fibrosis reversal are linked to increased HCC risk after HCV cure. The mechanism of HCC development after DAAs treatment remains unclear, but the possible mechanisms include immune cell dysfunction during HCV infection, cytokine network imbalance, epigenetic alterations, and host factors. Several biological markers and risk prediction models have been used to monitor the risk of HCC in CHC patients who have achieved SVR, but most still require validation and standardization. The implementation of risk-stratified surveillance programs is becoming urgent from a cost-effective point of view, but the availability of validated biomarkers to predict HCC in cured patients remains an unmet clinical need. Additionally, managing CHC patients who achieve SVR is becoming a growing challenge as an increasing number of HCV patients are cured.
Hepatitis C virus (HCV) is a hepatophilic, single-stranded, positive-stranded RNA virus belonging to the Flaviviridae family. It can lead to both acute and chronic hepatitis, causing continuous liver damage that may progress to cirrhosis, severe liver dysfunction, and hepatocellular carcinoma (HCC). HCV is a primary contributor to liver-related mortality on a global scale [1]. In 2019, the World Health Organization estimated that there were 58 million individuals with chronic HCV infection globally, with approximately 1.5 million new HCV infections occurring annually, leading to 290,000 deaths each year from cirrhosis or HCC caused by the virus [2]. Research indicates that approximately 20 % of patients with HCV develop cirrhosis within 20–30 years [3,4], and 1–4 % develop HCC [3]. Therefore, HCV continues to pose a significant global health threat and contributes to a substantial disease burden.
In recent years, with the advent of direct antivirals (DAAs), sustained virological response (SVR) has been achieved in approximately 95 % of patients, and pan-genotypic DAA regimens can be safely used in almost all patients with HCV, including elderly patients, patients with decompensated liver disease and those with end-stage renal disease who are not suitable for IFN therapy [5]. Achieving an SVR improves the outlook for patients with HCV, attenuates hepatic fibrosis to some extent, and reduces the incidence and recurrence of HCV-related HCC. However, a subset of patients may still develop HCC, necessitating ongoing monitoring of HCC development [6]. Current international guidelines recommend a largely "one-size-fits-all" HCC monitoring strategy, whereby monitoring is recommended on the basis of the presumed stage of fibrosis, and the same relatively ineffective strategy (ultrasound ± serum AFP) is recommended for all patients, regardless of their potential risk of HCC [7-9]. Biannual HCC monitoring is cost-effective for cirrhotic patients up to the age of 70 years in patients cured of hepatitis C, and up to the age of 60 years in patients with stable advanced fibrosis [10]. Nevertheless, the research does present certain constraints, such as the restricted data regarding the occurrence of HCC in individuals with progressed fibrosis and the call for additional research to establish the length of surveillance in various patient categories. The precise point at which the monitoring of HCC can be cautiously halted remains unspecified.
Currently, the pathogenesis of HCV-associated HCC and the mechanism by which HCC occurs in patients after they achieve SVR are unclear. Whether we can identify predictive factors from mechanistic studies that simplify the surveillance of HCC and improve the survival prognosis of HCV patients requires further research and refinement. As the number of new infections continues to increase, priority should be given to reducing HCC-associated mortality through early detection, ongoing prevention and management of HCV transmission, and treatment of HCV with safe and effective direct antiviral agents (DAAs). The implementation of risk-stratified surveillance programs has become urgent from a cost-effective perspective, but the availability of validated biomarkers to predict HCC in cured patients remains an unmet clinical need. Identifying those at risk for HCC and their ongoing surveillance and management remains an important clinical issue in the DAAs era. In this paper, we first review the risk of hepatocellular carcinoma after HCV eradication and its influencing factors. Second, we summarize the mechanisms of hepatocellular carcinoma development. Third, we summarize the tools for the surveillance of HCC and risk stratification of HCC in patients with CHC who have acquired SVR after antiviral therapy, in terms of both biomarkers and predictive models. Finally, we discuss the management of patients who have acquired SVR with the goal of obtaining the maximum clinical benefit and reducing the global burden of disease.
2Risk of HCC after SVR with DAAsWith the advent of DAAs, which can lead to SVR rates of more than 95 % and are short, effective and well tolerated, they have become the current treatment of choice for anti-HCV. However, obtaining SVR does not mean that HCC can be completely avoided. Numerous studies have shown that HCV clearance can greatly improve the clinical regression of patients, and even though the risk of HCC can be reduced by 3- to 4-fold in patients with a SVR in the setting of advanced liver disease, it is still insufficient to eliminate the risk of hepatocellular carcinoma, especially for patients who already have cirrhosis at baseline, and antiviral therapy alone cannot completely reverse cirrhosis; therefore, the possibility of hepatocellular carcinoma still exists [11-13].
However, the risk of HCC occurrence or recurrence after SVR with antiviral drug therapy is still unclear. In 2016, a number of reports reported that DAAs therapy may lead to an increase in the incidence or recurrence of HCC, which has caused widespread concern among scholars [14-16]. Nevertheless, subsequent studies have reported no associations between HCC incidence/recurrence and DAAs treatment. Ioannou et al. retrospectively studied 62,354 HCV patients and reported that achieving an SVR reduced the risk of new HCC by 71 %, regardless of the type of treatment (DAAs alone, DAAs in combination with IFN therapy, or IFN therapy alone) [17]. In addition, this study revealed that patients with cirrhosis had a greater risk of developing HCC than did those without cirrhosis [17]. In a study conducted in the UK by Cheung et al., which included 406 patients with decompensated cirrhosis who were already at higher risk of developing HCC than others, for the majority of patients, anti-HCV therapy was beneficial, even in those with advanced liver disease. They also reported that the cumulative incidence of HCC was 5.4 % in patients who achieved an SVR after treatment with DAAs, whereas the incidence of HCC was greater in patients who did not achieve an SVR (11.3 %), suggesting a protective effect of viral clearance [18]. In a large prospective study conducted by Cabibbo et al. in Italy, which included 143 patients with a median follow-up period of 9 months, the HCC recurrence rates at 6 months and 1 year after DAAs treatment were 12 % and 26.6 %, respectively. They also reported that only a history of previous HCC recurrence and tumor size were independent risk factors for early HCC recurrence [19]. A systematic review and meta-analysis conducted by Waziry et al. analyzed 26 studies and reported that cirrhotic patients were not at increased risk of developing HCC after DAAs treatment [6]. They also compared the incidence of HCC and recurrence rates in patients treated with DAAs or IFN and reported no difference in the incidence of HCC or recurrence rates in patients treated with DAAs or IFN [6]. A study of US veterans that enrolled a total of 48,135 patients who achieved an SVR reported that a decrease in the FIB-4 score from ≥3.25 before SVR to <3.25 after SVR was associated with an approximately 50 % reduction in HCC risk; in addition, the absolute risk of HCC remained higher than 2 % per year. Importantly, the risk of HCC persisted for at least 10 years in the patients who achieved an SVR [11]. The results of a recent systematic review and meta-analysis incorporating the results of 44 studies demonstrated that in patients with cirrhosis after HCV cure, the incidence of HCC decreased over time and was lower in younger and less fibrotic patients with no prior decompensation, in addition to a significantly lower incidence of F3 fibrosis, below the recommended cost-effectiveness screening threshold [20].
However, several risk factors directly or indirectly contribute to the development of HCC after antiviral therapy, of which advanced age and ≥F3/F4 fibrosis are the most important in most studies. Other risk factors for the development of HCC, such as male sex, alcohol intake, hepatic steatosis, HCV genotype 3 infection, diabetes, high GGT and AFP levels, and increased genetic risk scores, have also been reported [21-23].
In conclusion, even when HCV is effectively eradicated, patients at high risk of HCC need to be closely monitored for the development of HCC.
3Mechanisms of HCC after DAAs treatmentDespite years of intensive research, the pathogenesis of HCV-induced HCC remains incompletely understood. The pathogenesis of hepatitis C virus-associated hepatocellular carcinoma is a multifactorial process mediated through a wide range of mechanisms. The possible mechanisms for the development of HCC after DAAs treatment remain unsolved and include the following. (1) Immune cell dysfunction during chronic HCV infection: HCV usually evades innate and adaptive immune responses, as evidenced by decreased secretion of IFN-γ and tumor necrosis factor-α (TNF-α) by natural killer (NK) cells, impaired function of dendritic cells, suppression of HCV-specific CD8+ T cells and an increased proportion of CD4+ regulatory T cells, resulting in impaired tumor surveillance mechanisms [24]. (2) DAAs modulates immune cell responses: NK cell function is partially restored after HCV clearance by DAAs treatment, and reduced expression of natural killer cell surface lectin-like activation receptor (NKG2D) and ligand is associated with HCC recurrence and early onset in HCC patients. In addition, HCV-specific CD8+ T cells proliferate rapidly after HCV clearance, but there is a significant reduction in programmed cell death protein 1 (PD-1) on their surface, suggesting that the depleted state of CD8+ T cells is only partially restored, whereas Treg cells are unaffected by DAAs treatment, leading to an impaired tumor surveillance mechanism [25]. (3) Imbalance of the cytokine network: Altered circulating levels of certain cytokines [especially TNF-α, interleukin (IL)−6, IL-10, IL-13, and tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL)] may contribute to the promotion of tumorigenesis and progression [25,26]. (4) Potential role of DAAs in regulating angiogenic signals: Vascular endothelial growth factor (VEGF) is a growth factor produced mainly by tumor cells, macrophages and platelets and plays a key role in inflammation and tumor neovascularization. Several studies have reported elevated levels of VEGF and angiotensin-2 in HCC patients after DAA treatment, confirming the potential role of VEGF in HCC development [27,28]. (5) Epigenetic alterations: HCV-induced epigenetic alterations remain after SVR, resulting in persistent changes in gene expression. Hamdane et al. [29] reported that chronic HCV infection induced specific genome-wide changes in H3K27ac, which were associated with changes in H3K27ac mRNA and protein expression, and that these changes persisted after SVR was achieved with DAA treatment. Integration pathway analysis of liver tissues from patients and humanized liver model mice revealed that HCV-induced epigenetic alterations are associated with hepatocellular carcinoma risk. (6) Host factors: the expression of interferon genes in the body may be downregulated after DAA treatment, which can promote cell proliferation in the absence of proper examination of the target, which in turn leads to tumor development. Santangelo et al. [30] reported that deletion or a decrease in exosomal miR-122 was also associated with the development of HCC. In addition, it has been suggested that host factors such as obesity, diabetes, cirrhosis and alcohol consumption are associated with persistent elevation of liver enzymes after HCV cure, which may also promote tumor progression [31].
4Surveillance of HCC in CHC patients who achieve SVR after DAAs treatmentIn recent years, the widespread use of DAAs has led to HCV clearance in more than 95 % of patients with hepatitis C and has made hepatitis C a curable disease. As a result, many patients with hepatitis C in current clinical practice will be those who have been virologically cured with DAAs, rather than those with viraemia. However, the risk of HCC may persist for years after SVR, therefore, many professional society guidelines recommend HCC surveillance in post-SVR patients [11]. Guidelines recommend semiannual HCC surveillance in patients with hepatitis C cirrhosis if the incidence of hepatocellular carcinoma (HCC) is greater than 1.5/100 person-years (PY). However, these guidelines are based on older data, and the incidence threshold for surveillance in individuals who have achieved virological cure is unknown. As the number of virologically cured hepatitis C patients increases, there is an urgent need to update the guidelines and clarify who may benefit from surveillance [32]. There is still much controversy regarding strategies for monitoring HCC in HCV patients after they are cured. However, the use of specific biological markers to predict the occurrence of HCC in HCV patients who achieve SVR is certainly advantageous. Predictive biomarkers for HCC in CHC patients who achieve SVR after antiviral therapy and tools for risk stratification are briefly summarized (see Tables 1 and 2) to inform individualized clinical surveillance in the future.
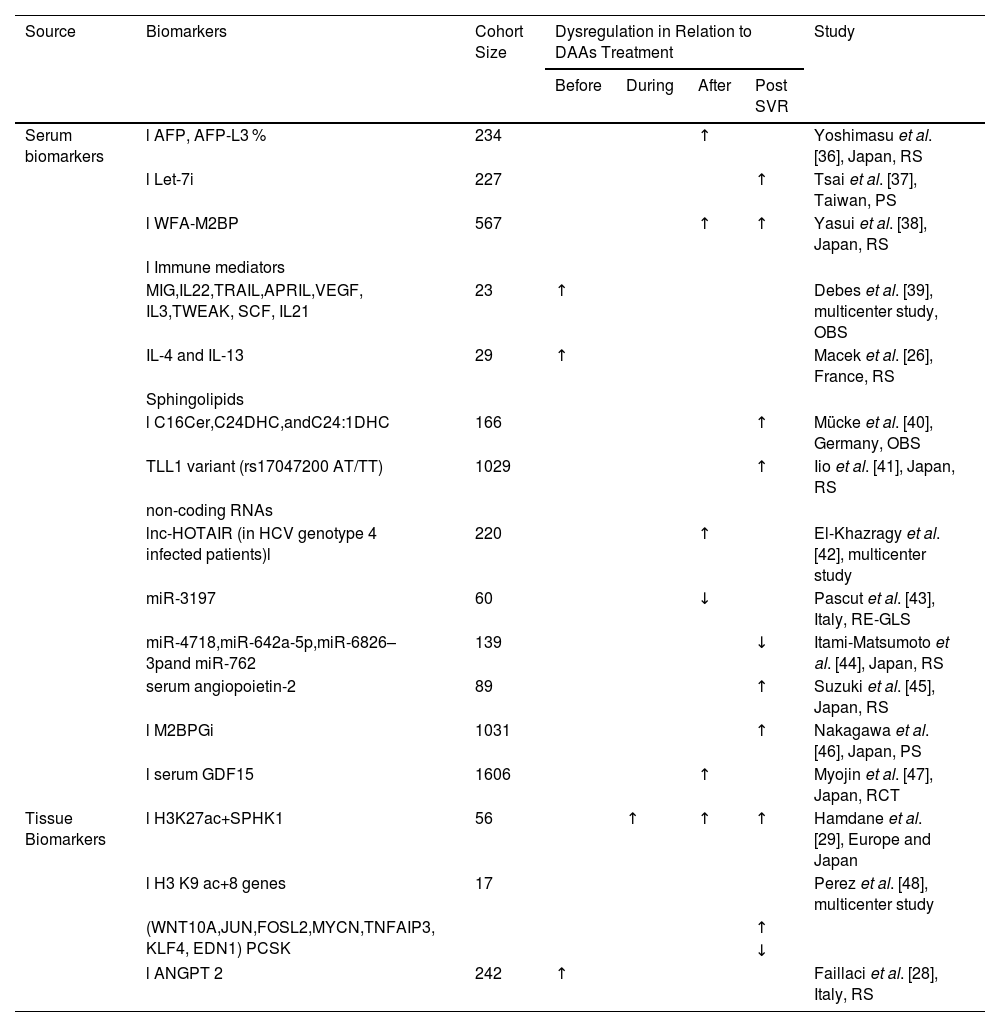
Predictive biomarkers for the development of HCC after treatment with DAAs.
| Source | Biomarkers | Cohort Size | Dysregulation in Relation to DAAs Treatment | Study | |||
|---|---|---|---|---|---|---|---|
| Before | During | After | Post SVR | ||||
| Serum biomarkers | l AFP, AFP-L3 % | 234 | ↑ | Yoshimasu et al. [36], Japan, RS | |||
| l Let-7i | 227 | ↑ | Tsai et al. [37], Taiwan, PS | ||||
| l WFA-M2BP | 567 | ↑ | ↑ | Yasui et al. [38], Japan, RS | |||
| l Immune mediators | |||||||
| MIG,IL22,TRAIL,APRIL,VEGF, IL3,TWEAK, SCF, IL21 | 23 | ↑ | Debes et al. [39], multicenter study, OBS | ||||
| IL-4 and IL-13 | 29 | ↑ | Macek et al. [26], France, RS | ||||
| Sphingolipids | |||||||
| l C16Cer,C24DHC,andC24:1DHC | 166 | ↑ | Mücke et al. [40], Germany, OBS | ||||
| TLL1 variant (rs17047200 AT/TT) | 1029 | ↑ | Iio et al. [41], Japan, RS | ||||
| non-coding RNAs | |||||||
| lnc-HOTAIR (in HCV genotype 4 infected patients)l | 220 | ↑ | El-Khazragy et al. [42], multicenter study | ||||
| miR-3197 | 60 | ↓ | Pascut et al. [43], Italy, RE-GLS | ||||
| miR-4718,miR-642a-5p,miR-6826–3pand miR-762 | 139 | ↓ | Itami-Matsumoto et al. [44], Japan, RS | ||||
| serum angiopoietin-2 | 89 | ↑ | Suzuki et al. [45], Japan, RS | ||||
| l M2BPGi | 1031 | ↑ | Nakagawa et al. [46], Japan, PS | ||||
| l serum GDF15 | 1606 | ↑ | Myojin et al. [47], Japan, RCT | ||||
| Tissue Biomarkers | l H3K27ac+SPHK1 | 56 | ↑ | ↑ | ↑ | Hamdane et al. [29], Europe and Japan | |
| l H3 K9 ac+8 genes | 17 | Perez et al. [48], multicenter study | |||||
| (WNT10A,JUN,FOSL2,MYCN,TNFAIP3, KLF4, EDN1) PCSK | ↑ | ||||||
| ↓ | |||||||
| l ANGPT 2 | 242 | ↑ | Faillaci et al. [28], Italy, RS | ||||
RS, retrospective study; PS, prospective study; OBS, observational study; RCT, randomized controlled trial; RE-GLS, random-effects generalized least square regression model;.
↑—upregulated; ↓—downregulated.
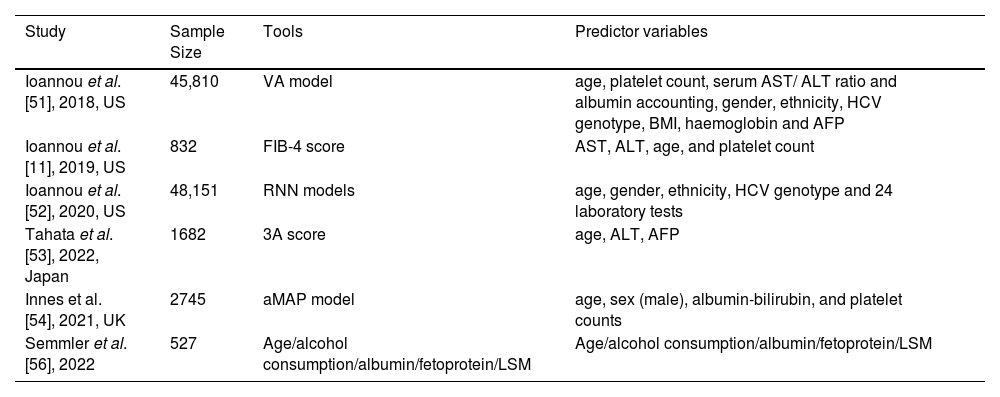
Tools for estimating HCC risk after SVR to inform HCC screening strategies.
| Study | Sample Size | Tools | Predictor variables |
|---|---|---|---|
| Ioannou et al. [51], 2018, US | 45,810 | VA model | age, platelet count, serum AST/ ALT ratio and albumin accounting, gender, ethnicity, HCV genotype, BMI, haemoglobin and AFP |
| Ioannou et al. [11], 2019, US | 832 | FIB-4 score | AST, ALT, age, and platelet count |
| Ioannou et al. [52], 2020, US | 48,151 | RNN models | age, gender, ethnicity, HCV genotype and 24 laboratory tests |
| Tahata et al. [53], 2022, Japan | 1682 | 3A score | age, ALT, AFP |
| Innes et al. [54], 2021, UK | 2745 | aMAP model | age, sex (male), albumin-bilirubin, and platelet counts |
| Semmler et al. [56], 2022 | 527 | Age/alcohol consumption/albumin/fetoprotein/LSM | Age/alcohol consumption/albumin/fetoprotein/LSM |
SVR, sustained virological response; HCC, hepatocellular carcinoma; VA, Veterans Affairs; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; AFP, alpha fetoprotein; US, United States; FIB-4, fibrosis 4 score; RNN, recurrent neural network; UK, United Kingdom; LSM, liver stiffness measurement.
The level of serum AFP, a widely utilized biomarker for HCC screening and diagnosis, is notably increased in approximately 65 % to 80 % of individuals with primary liver cancer [33]. However, it does not completely indicate the presence of HCC, as elevated AFP levels have also been found in patients with active hepatitis, reproductive tumors, and other gastrointestinal tumors [34]. Although monitoring AFP is not very satisfactory, in a prospective study, Oze et al. found that in patients with chronic HCV infection, AFP levels were reduced during interferon therapy. High post-treatment levels of AFP predict HCC, regardless of whether patients achieve an SVR [35]. Furthermore, Yoshimasu et al. identified AFP and AFP-L3 % as circulating biomarkers to assess the risk of HCC occurrence or recurrence in patients treated with DAAs [36]. In particular, AFP levels are elevated in HCV patients with HCC occurrence or recurrence both before and after DAAs treatment, whereas the expression of AFP-L3 % is only associated with only the occurrence and recurrence of HCC after DAAs treatment [36]. Studies have revealed that in patients with CHC, those who achieve an SVR after antiviral treatment exhibit higher levels of the Let-7 family in their peripheral blood than those who do not achieve an SVR. Additionally, the levels of this molecular family are reduced in CHC patients who develop HCC compared with those who do not develop HCC. Therefore, Let-7i of the Let-7 family could be used as an independent predictor of HCC risk in CHC patients after antiviral therapy. When the Let-7i level of CHC patients was ≥1.696, patients who achieved SVR had a lower incidence of HCC [37]. A retrospective study conducted in Japan on patients who achieved SVR after DAAs treatment found that a Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA-M2BP) ≥ 1.75 critical index (C.O.I., P < 0.001) and an AFP level ≥ 6 ng/mL (P = 0.01) after SVR were significant predictors of HCC development. A WFA-M2BP ≥1.75 C.O.I. after SVR was an independent factor significantly associated with the development of HCC (hazard ratio [HR] 6.0; 95 % confidence interval (CI), 1.8–19.4; P = 0.003). Moreover, an AFP concentration ≥ 6 ng/mL after SVR was the only factor associated with recurrence-free survival (HR 3.1; 95 % CI, 1.3–7.5; P = 0.01) [38]. In addition, the serum levels of immune mediators (including cytokines, growth factors, and apoptotic markers) may also serve as biomarkers for predicting the risk of HCC. Debes et al. compared patients who developed HCC with those who did not develop HCC after DAAs therapy and reported that those who developed new-onset HCC had significantly higher serum levels of 12 immune mediators prior to receiving DAAs therapy. Nine of these genes (MIG, IL22, TRAIL, APRIL, VEGF, IL3, TWEAK, SCF, and IL21) were elevated in the serum of patients who developed HCC after DAAs treatment, suggesting that changes in the levels of inflammatory cytokines during DAAs treatment further influence the development of HCV-associated hepatocellular carcinoma [39]. In addition to this group of potential predictors of HCC in DAAs-treated patients, IL-4 and IL-13 were found to be significantly increased prior to DAAs treatment in patients who subsequently developed HCC [26]. Both markers were consistently higher in the serum of HCV patients at the beginning, during and at the end of treatment, and even 3 months after the end of treatment. IL-4 and IL-13 are commonly associated with oncogenic effects and play key roles in immune-related mechanisms [26], emphasizing that the dysregulation of immune mechanisms induced by HCV infection cannot be reversed by DAAs treatment and can still drive the progression of malignancy. Sphingolipids have been identified as new biomarkers of chronic liver disease and HCC. Mücke et al. reported that a panel of serum sphingolipids (C16 Cer, C24 DHC and C24:1DHC) can be used to predict the risk of HCC in patients with cirrhosis after treatment; in particular, C16 Cer was able to predict, with a high level of diagnostic accuracy, even in AFP-negative patients, the risk of HCC, suggesting that this biomarker may have greater value than AFP [40]. Iio and colleagues reported that the TLL1 variant of rs17047200 was an independent risk factor for HCC after HCV eradication via DAAs therapy. Furthermore, they observed that the cumulative incidence of HCC was significantly higher in patients with rs17047200 AT/TT than in patients with AA (P = 0.006). These findings suggest that genetic testing for the TLL1 genotype could facilitate personalised monitoring of HCC in patients who have achieved a SVR [41]. In addition, non-coding RNAs [42-44] (e.g., lnc-HOTAIR, miR-3197, miR-4718, miR-642a-5p, miR-6826–3, and miR-762), serum angiopoietin-245, high levels of Mac-2-binding protein glycosylated isoform (M2BPGi) [46] and serum GDF1547 are potential biomarkers for predicting the occurrence or recurrence of HCC after treatment with DAAs.
4.1.2Tissue biomarkersA previous study revealed that HCV-induced epigenetic changes associated with hepatocellular carcinoma risk persist after a SVR [29,48]. Hamdane et al. reported that chronic HCV infection induced specific genome-wide changes in H3K27ac, which were associated with changes in mRNA and protein expression [29]. The alterations induced by H3K27ac involve the oncogene sphingosine kinase 1 (SPHK1), which is a major regulator of apoptosis inhibition and proliferation promotion [49]. Hamdane et al. also computationally analyzed the strong correlation between high SPHK1 expression and the risk of HCC in patients with HVC-induced cirrhosis, even in patients who achieved SVR [29]. In addition, there was a positive correlation between SPHK1 expression and tumor size, tumor stage and histological differentiation [50]. Thus, elevated SPHK1 expression can be a good predictor of HCC in patients who achieve SVR. Similarly, Perez et al. [48] investigated genome-wide epigenetic changes induced by HCV infection via chromatin immunoprecipitation sequencing (ChIP-seq) and reported that acetylation of histone H3 lysine 9 (H3K9ac) was detected in HCV-infected patients. WNT10A, JUN, FOSL2, MYCN, TNFAIP3, KLF4, EDN1, and PCSK9, are eight genes whose high expression is associated with an increased risk of HCC, whereas low expression of PCSK9 is associated with an increased risk of HCC. By analyzing the expression levels of these genes, researchers have been able to predict the risk of HCC development and thus provide personalized monitoring and treatment strategies for HCV-infected patients. In addition, Faillaci et al. [28] found that ANGPT 2 expression was elevated in cirrhotic and primary tumor liver tissues from susceptible patients with activated neoangiogenesis (patients with severe fibrosis and visceral collateralization). High levels of ANGPT 2 prior to DAAs treatment were independently associated with the risk of HCC recurrence (OR, 1.137; 95 % confidence interval (CI), 1.044–1.137; p = 0.003) and incidence (OR, 1.604; 95 % CI, 1.080–2.382; p = 0.019), suggesting a possible role for ANGPT 2 as a biomarker for the identification of cirrhotic patients at risk of HCC development prior to treatment with DAA.
4.2Risk prediction models4.2.1VA modelIoannou et al. [51] developed and internally validated a model for predicting the risk of HCC via Cox proportional risk regression modeling using the baseline characteristics of patients on antiviral therapy, known as the VA model, which included age, platelet count, the serum AST/ALT ratio and the serum albumin level, accounting for the majority of the predictions, with sex, ethnicity, the HCV genotype, body mass index, hemoglobin and serum alpha-fetoprotein contributing less. The fitted models were well calibrated and very discriminatory, with a greater net benefit of using model-based HCC risk estimates to decide whether to recommend screening. These models can be used as web-based tools to guide risk-based HCC surveillance strategies for individual patients.
4.2.2FIB-4 scoreThe FIB-4 score provides a simplified method for assessing the risk of HCC after SVR. The FIB-4 score is calculated via a simple formula based on the aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, age, and platelet count ([AST × age]/[platelet count × √ALT]) and was initially developed as a noninvasive fibrosis staging biomarker panel. A follow-up study revealed that a FIB-4 score ≥3.25 also appears to be a particularly strong predictor of HCC risk, in patients with and without cirrhosis. Specifically, patients whose FIB-4 score was ≥3.25 before and after SVR had a very high risk (∼2 %/year), whereas patients whose FIB-4 score decreased from ≥3.25 before SVR to <3.25 after SVR had a much lower risk of HCC [11].
4.2.3RNN modelsIn a forward-looking study in the United States involving 48,151 individuals with cirrhosis linked to HCV, the research utilized unprocessed longitudinal data from electronic health records (EHRs) and formulated three comparative models [52]. The study found that the deep learning RNN model outperformed the traditional LR model in predicting the risk of developing HCC in patients with HCV-related cirrhosis. These findings suggest that RNN models can be used to identify high-risk patients and may have multiple applications in clinical practice, such as in screening and surveillance strategies for HCC. The variables considered for prediction included age, sex, ethnicity, HCV genotype, and 24 laboratory tests. Although the RNN model has proven to be more precise than conventional regression models, its integration into clinical practice currently presents challenges. Overall, this study highlights the promise of deep learning models for healthcare forecasting and offers innovative tools and approaches for estimating the risk of HCC in patients with cirrhosis in the future.
4.2.43A scoreA prospective multicenter cohort study conducted in Japan [53] on 1682 HCV patients without advanced liver fibrosis (defined as a fibrosis-4 index < 3.25) and without a history of HCC who were treated with DAAs and achieved SVR revealed that baseline age ≥ 65 years (p = 0.030), ALT level ≥ 30 U/L (p = 0.001) and AFP level ≥ 5.0 ng/ml (p = 0.001) were independent predictors of HCC incidence in the study cohort. The study then developed a systematic score (3A score) around these three factors for HCV-infected individuals. The results revealed that the cumulative incidence of HCC at 5 years was 7.1 % when patients with a score of 2 or 3 were scored, and there was no incidence of HCC among patients with a score of 0. Consequently, researchers suggest utilizing the "3A score" as a tool to categorize the risk of HCC among patients who have achieved a SVR. This recommendation extends to patients who do not exhibit advanced liver fibrosis and underscores the necessity for ongoing surveillance for HCC in these individuals.
4.2.5aMAP modelIn a study conducted in the UK [54] based on cirrhotic and cured HCV patients in the Scottish database and the STOP-HCV cohort, the investigators compared six HCC prediction models in these 2 different cohorts and reported that the prediction model consisting of age, sex (male), albumin, bilirubin, and platelet count (aMAP) had the best accuracy and sensitivity.
A study conducted in France [55] included alcoholic cirrhosis patients and/or cured HCV-infected patients (from the prospective CirVir and CIRRAL cohorts) from an HCC surveillance program to investigate how genetic variants can be integrated into clinical models to improve the risk stratification of cirrhotic patients with HCC risk stratification. The prognostic value of these SNPs for HCC occurrence was assessed via the Fine‒Gray model and combined with a 7-SNP genetic risk score (GRS). PNPLA3 and WNT3A-WNT9A variants were found to be independently associated with HCC occurrence. Multivariate modeling identified age, male sex, diabetes status, platelet count, GGT level, albuminemia and GRS as independent risk factors. The performance of the clinical model for 5-year HCC prediction was similar to that of the aMAP score (C-Index 0.769), with a slight improvement in performance with the addition of the GRS (C-Index 0.786 and 0.783). Moreover, the investigators concluded that patients with cirrhosis can be stratified into different HCC risk classes by variants affecting lipid metabolism and the Wnt–β-catenin signaling pathway and that the incorporation of this genetic information into the clinical scoring system could moderately improve its performance for HCC risk stratification.
4.2.6Age/alcohol consumption/albumin/fetoprotein/LSMSemmler et al. [56] conducted a cohort study of 527 HCV patients treated with DAAs who achieved SVR and presented with compensated progressive chronic liver disease (cACLD). This study proposed an algorithm based on age, alcohol intake, albumin and alpha-fetoprotein levels, and the LSM for HCC prediction in patients with cACLD, which is simple and easy to implement and provides a reliable basis for clinical risk monitoring, as the above parameters can be assessed at a single time point after treatment. Moreover, approximately 2/3 of their derivation and validation cohorts were determined to have a risk of HCC <1 %/year and thus were significantly below the cost-effectiveness threshold for HCC surveillance; therefore, these patients may not need to undergo liver ultrasound every 6 months.
Current international guidelines for HCC recommend lifelong surveillance of HCV-cured cirrhotic patients. However, this universal surveillance strategy may lead to an increased health cost burden, so many current efforts aimed at identifying HCC predictors, developing predictive models, estimating the residual risk of hepatocellular carcinoma to improve individual risk stratification, and ultimately developing personalized surveillance are necessary.
5Management of CHC patients who achieve SVR after DAAs treatmentThe management of CHC patients who have achieved SVR has become a growing challenge as an increasing number of HCV patients are cured. A recent international, multicenter cohort study analyzed the clinical features and outcomes of HCC patients with a post SVR across various geographic locations. The findings revealed notable disparities in the clinical manifestations and prognoses of HCC patients following SVR in different regions. Differences in prognosis may be related to changes in HCC monitoring procedures for patients after SVR and treatment practice patterns for patients with HCC. Efforts to improve monitoring and treatment practices for HCC patients after SVR are necessary to improve the survival benefit for patients with hepatitis C virus eradication [57]. Despite the possibility of fibrosis regression after SVR, methods to assess subsequent fibrosis progression or regression have not been clearly defined. Serial assessment of liver disease severity via noninvasive tools (e.g., FIB-4 and Fibroscan) or, in some cases, liver biopsy may be helpful in making management decisions. In addition, several models that have been validated (e.g., the aMAP model) or are being developed for risk prediction may play important roles in stratifying risk and moderating the intensity of HCC monitoring. Although a number of signals for reduced HCC risk have been identified, including younger age, lower fibrosis, and the absence of previous decompensation [20], the time to safely discontinue HCC monitoring has not yet been clarified, especially as advanced HCC development can still be observed after SVR. Currently, in patients with SVR, routine HCC monitoring should be continued in patients with cured HCV and advanced (F3‒F4) fibrosis, as the risk of HCC in the setting of advanced fibrosis and cirrhosis is reduced but not eliminated, and the signal for safe cessation of monitoring has not been defined [8]. In these patients, every effort should be made to minimize coexisting risks, including steatosis (if present) and alcohol consumption, optimize glycemic control in diabetic patients, reduce iron levels, and identify and treat co-infections, such as HBV, at the appropriate time [58]. Moreover, to improve the prognosis of life in patients with hepatitis C, complications, including extrahepatic lesions, should be assessed before and after treatment, and the use of biomarkers such as M2BPGi and Ang-2 for customized monitoring can be used to identify patients at elevated risk of extra-hepatic manifestations (EHM) and treat these patients with early prevention or therapy to improve morbidity, mortality and quality of life [59]. In the future, we should strive to develop validated biomarkers and predictive models for better and highly selective identification of individuals at high risk of HCC in patients with SVR, develop new guidelines for surveillance, clarify when and in which populations HCC surveillance can be safely discontinued, enable personalized follow-up and management, and reduce the global burden of disease.
6Summary and outlookIn the era of DAAs, 95 % of patients with hepatitis C achieve a clinically cured state. However, even after achieving a clinical cure, hepatitis C patients remain at risk of progression to HCC, particularly in patients with progressive liver fibrosis and cirrhosis. For such patients, we propose an increased frequency of follow-up, including monitoring liver function, performing imaging tests, and measuring tumor markers (e.g., AFP) every six months to facilitate the early detection and treatment of potential HCC. For patients with comorbid risk factors, such as advanced age, obesity, diabetes mellitus, hepatic steatosis, alcohol consumption, and genetic risk scores, it is recommended that measures be taken to control blood glucose, reduce weight, and abstain from alcohol consumption in order to promote a healthier life after SVR and reduce the risk of hepatocellular carcinoma in patients with hepatitis C, even in the presence of factors that cannot be modified, such as advanced age and genetic risk scores. Systematic management of post-SVR hepatitis C patients through regular monitoring and intervention with external measures, leading to the early identification and diagnosis of HCC and increasing the likelihood of a liver cancer cure, is an effective way to reduce costs and improve efficiency.
In all patients who achieve SVR, the key question is how we can reliably estimate HCC risk and changes in HCC risk over time to determine whether patients are likely to benefit from HCC surveillance, as HCC risk is a key determinant of the cost-effectiveness of screening. Promising biomarkers and risk prediction models for HCC are under development, and for risk-based surveillance to become a reality, it needs to be improved and validated in different populations so that we can reliably estimate HCC risk. Currently, the pathogenesis of HCC after HCV cure is still poorly understood, and an understanding of the molecular mechanisms underlying the development of HCV-associated HCC could contribute to the discovery of new biomarkers for the early detection of hepatocellular carcinoma. Efforts have been made to develop hepatocellular carcinoma predictors and prediction models for the occurrence of hepatocellular carcinoma after SVR for hepatitis C patients. However, most existing predictors and prediction models are based on routine clinical indicators, rely on simple noninvasive indicators, or lack further validation, which is less accurate and personalized for the prediction of hepatocellular carcinoma risk, and complex programming models are difficult to carry out in the clinic. In summary, currently developed liver cancer prediction models and predictors still face insufficient specificity and accuracy for widespread use. Therefore, it is necessary to further develop simple, accurate, and clinically easy-to-apply factors and models based on risk factors, clinical indicators, and the mechanism of hepatocellular carcinoma to identify high-risk groups for hepatocellular carcinoma early and accurately, risk stratify patients after SVR for hepatitis C, and provide a basis for the development of hepatocellular carcinoma screening strategies. As an increasing number of HCV patients are cured, the management of CHC patients who have achieved SVR is also a great challenge, and efforts should be made to optimize care management and follow-up strategies to obtain maximum cost benefits and thus reduce the global burden of disease.
Current and future research frontiers are how HCC risk decreases (or not) with the number of years since HCV eradication, how to estimate HCC risk in individual patients and how to incorporate HCC risk estimates into individualized risk-based surveillance strategies, and the molecular mechanisms underlying the onset of HCC after HCV cure to better guide surveillance. Patients and healthcare systems also urgently need guidance on how to achieve individualized follow-up and management, as well as clarity on when HCC surveillance can be safely discontinued in patients who have achieved SVR.
Author contributionsThe authors have contributed equally to this work.
FundingKey Research and Development Program of Shaanxi2023ZDLSF-34; Clinical Research Project of Air Force Military Medical University (2021LC2105, 2023LC2311) .