Over the last years, the susceptibility activity of the most common microorganisms causing community-acquired infections has significantly changed in Spain. Based on the susceptibility rates of Streptococcus pyogenes, Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli, and Klebsiella pneumoniae collected from outpatients aged 15 or older with symptoms of respiratory or urinary tract infections in several Microbiology Departments in Catalonia in 2021, penicillin V should be first choice for most respiratory tract infections, amoxicillin and clavulanate for chronic obstructive pulmonary disease exacerbations and a single dose of fosfomycin or a short-course nitrofurantoin should remain first-line treatments for uncomplicated urinary tract infections. Updated information on antimicrobial resistance for general practitioners is crucial for achieving appropriate empirical management of the most common infections by promoting more rational antibiotic use.
En los últimos años han cambiado significativamente los porcentajes de sensibilidad de los microorganismos más comunes que causan infecciones adquiridas en la comunidad en España. A partir de los porcentajes de sensibilidad de Streptococcus pyogenes, Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli y Klebsiella pneumoniae, recogidas de aislados de pacientes ambulatorios de 15 años o más, con síntomas de infecciones respiratorias o urinarias en servicios de microbiología de Cataluña en 2021, fenoximetilpenicilina debería ser la primera opción en la mayoría de los infecciones respiratorias, amoxicilina y ácido clavulánico en las exacerbaciones de la enfermedad pulmonar obstructiva crónica y la monodosis de fosfomicina o la pauta corta de nitrofurantoína como tratamiento de primera línea en las infecciones urinarias no complicadas. Es importante que los médicos de familia dispongan de información actualizada sobre la resistencia a los antimicrobianos para lograr un manejo empírico adecuado de las infecciones más frecuentes al promover un uso más racional de los antibióticos.
Antimicrobial resistance (AMR) is one of the main public health challenges worldwide.1 Ensuring prudent antimicrobial utilisation is key to an effective response to this huge problem, mainly in primary care, in which nearly 80% of all antibiotics are issued.2 Reducing the emergence and spread of antibiotic resistance, i.e. prioritising antibiotics that are less likely to lead to antibiotic resistance at the societal and the individual level, is a task that all clinicians should and must implement. The main driver for resistance is antibiotic overprescribing.3 Antimicrobial stewardship programmes in primary care should primarily be focused on prudent use of antibiotics and only when the suspicion of a bacterial aetiology is very likely.4 Most of the respiratory tract infections in the community are viral and most bacterial infections are mild and self-limiting. According to the Centers for Disease Control and Prevention about one half of all antibiotics prescribed for community-acquired respiratory tract infections are unnecessary.5
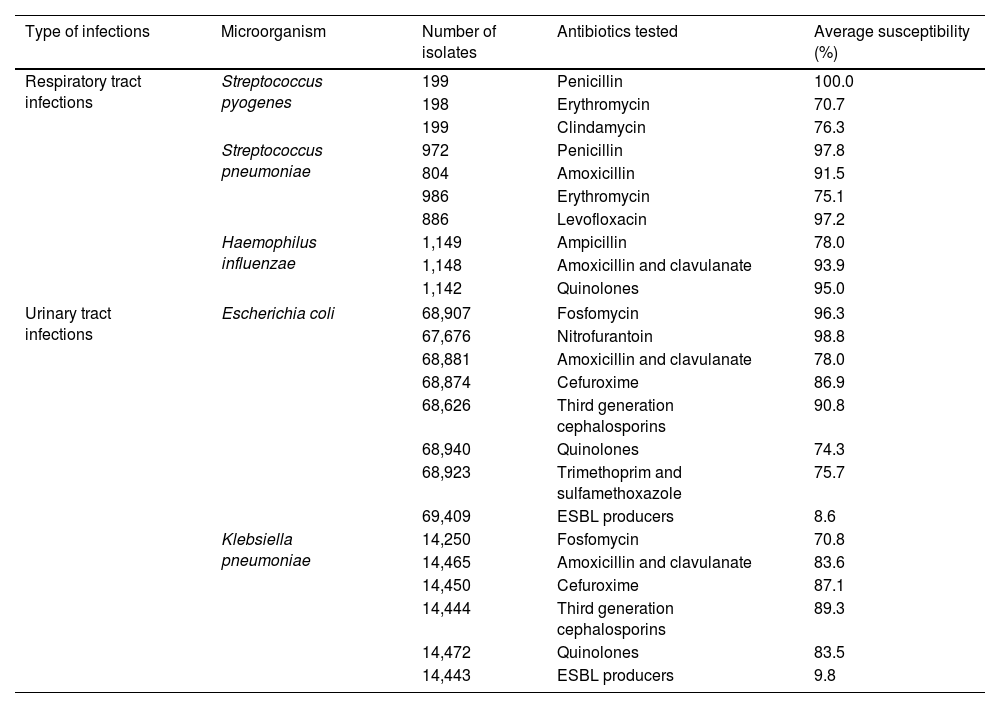
When antibiotic therapy is warranted, the World Health Organization recommends making use of the WHO Access, Watch, Reserve (AWaRe) classification and selecting an antimicrobial drug with a low impact on AMR (ref www.who.int/publications/i/item/2021-aware-classification). AWaRe encourages the so-called ‘Access antibiotics’ as empirical treatment in primary care because they have a narrow antimicrobial spectrum, low cost, good safety profile and are less likely to select for AMR.6 The category ‘Watch and Reserve Antibiotics’ are broader-spectrum antibiotics and more likely to select for antimicrobial resistance. They are more expensive and should primarily be reserved for patients with severe clinical infections and infections in which the causative pathogens are more likely to be resistant.7 Continuing surveillance for the antibiotic resistance of common pathogens is therefore a recognised public health need, particularly in countries with long-standing high resistance rates, such as Spain, where initial antimicrobial treatment of assumed bacterial respiratory and urinary tract infections is always selected empirically. Antimicrobials should therefore provide appropriate coverage against the most common causative microorganisms.8,9 Despite this, updated information about susceptibility patterns have been a matter of course at the hospital level and general practitioners have rarely had updated information about the susceptibility patterns of isolates collected in the community so far.10 Based on the Infection Control and Antimicrobial Stewardship Catalonian Program (VINCat), antimicrobial susceptibilities of common bacterial pathogens causing respiratory and urinary tract infections collected in 2021 from outpatients aged 15 or more (summarised in Table 1),11 a new paradigm in the use of first-line antibiotic therapies should be considered.
In vitro antibiotic susceptibility of common antibiotics against microorganisms causing community-acquired infections in adults in the community collected in 2021 in Catalonia (taken from Ref. 11).
| Type of infections | Microorganism | Number of isolates | Antibiotics tested | Average susceptibility (%) |
|---|---|---|---|---|
| Respiratory tract infections | Streptococcus pyogenes | 199 | Penicillin | 100.0 |
| 198 | Erythromycin | 70.7 | ||
| 199 | Clindamycin | 76.3 | ||
| Streptococcus pneumoniae | 972 | Penicillin | 97.8 | |
| 804 | Amoxicillin | 91.5 | ||
| 986 | Erythromycin | 75.1 | ||
| 886 | Levofloxacin | 97.2 | ||
| Haemophilus influenzae | 1,149 | Ampicillin | 78.0 | |
| 1,148 | Amoxicillin and clavulanate | 93.9 | ||
| 1,142 | Quinolones | 95.0 | ||
| Urinary tract infections | Escherichia coli | 68,907 | Fosfomycin | 96.3 |
| 67,676 | Nitrofurantoin | 98.8 | ||
| 68,881 | Amoxicillin and clavulanate | 78.0 | ||
| 68,874 | Cefuroxime | 86.9 | ||
| 68,626 | Third generation cephalosporins | 90.8 | ||
| 68,940 | Quinolones | 74.3 | ||
| 68,923 | Trimethoprim and sulfamethoxazole | 75.7 | ||
| 69,409 | ESBL producers | 8.6 | ||
| Klebsiella pneumoniae | 14,250 | Fosfomycin | 70.8 | |
| 14,465 | Amoxicillin and clavulanate | 83.6 | ||
| 14,450 | Cefuroxime | 87.1 | ||
| 14,444 | Third generation cephalosporins | 89.3 | ||
| 14,472 | Quinolones | 83.5 | ||
| 14,443 | ESBL producers | 9.8 | ||
ESBL: extended spectrum beta-lactamase.
The most common bacterium causing pharyngotonsillitis in Western countries is group A β-haemolytic Streptococcus. According to the microbiological analysis of strains collected from throat swabs from patients with acute pharyngitis, in 2021 S. pyogenes continues being 100% susceptible towards penicillin – resistant strains have never been isolated against this antibiotic –, indicating that phenoxymethylpenicillin should remain as the first choice for patients with a positive rapid antigen detection test. Despite being recommended for patients with streptococcal pharyngitis for more than one decade, the use of penicillin V continues to be scarce in Spain.12
Hence, given its narrow spectrum of activity, low cost, efficacy in preventing strep throat complications, and benign side-effect profile, high-dose phenoxymethylpenicillin should be the drug of choice for treating streptococcal pharyngitis, with amoxicillin, an antibiotic with a broader spectrum compared to phenoxymethylpenicillin, being reserved as second choice alternative. Although macrolides and lincosamides are recommended as alternative antibiotics in streptococcal infected patients who are allergic to β-lactams, approximately 20% of the strains of S. pyogenes are now resistant.
Germs causing respiratory tract infections: the dramatic reduction of resistance of Streptococcus pneumoniae to penicillin allows clinicians to consider high-dose penicillin as first-line antibiotic for pneumococcal infectionsStreptococcus pneumoniae and Haemophilus influenzae are the most common bacteria causing respiratory tract infections. S. pneumoniae has traditionally been a pathogen that is highly susceptible to a large number of antimicrobials, with penicillin being its first-choice antibiotic. Infections due to this microorganism were, in general, easily treatable and presented few management difficulties. Although the resistance of S. pneumoniae to penicillin has been known since the 1960s, the extreme rarity of these strains did not present any clinical problems in the past. However, in 1977 an epidemic outbreak caused by S. pneumoniae resistant to penicillin occurred in South Africa.13 Since then there has been a continuous increase in the number of strains of S. pneumoniae resistant to penicillin. In 1979, the first isolates of S. pneumoniae with decreased sensitivity to penicillin were reported in Spain.14 Since the mid-1980s the resistance rate of pneumococci to penicillin has significantly increased, jeopardising the selection of an effective antibiotic therapy.15 At the beginning of this century the percentage of resistance was as high as 40%, as also reported in Spanish news media.16,17
However, during the last two decades the resistance rate of S. pneumoniae towards penicillin has markedly changed.18 Based on isolates from ear and sputum samples in patients with upper respiratory tract infections, the current resistance rate of S. pneumoniae towards penicillin is 2.4%. This finding confirms a rapid decline of the resistance rates to penicillin and other β-lactam antibiotics initiated since the beginning of this century.
The VINCat antimicrobial susceptibility results also confirm the steady resistance pattern of the streptococcal strains to macrolides, which is of concern. Unlike penicillin resistance in S. pneumoniae, there has not been a temporal decreasing trend for macrolides over the last two decades in Spain.18 Awareness of this problem should be considered by clinicians when patients are suspected to have an allergy to β-lactams and, thus, fluoroquinolones must be considered as first choice in this situation.
According to this recent microbiological analysis, the resistance rate of H. influenzae towards ampicillin is also of concern. The prevalence of β-lactamase-producing microorganisms slightly exceeds 20% and, in some areas, it may be even higher. β-Lactamase production is a well-known predictor of treatment failure in respiratory tract infections in hospitalised patients.19 In patients with acute exacerbations of severe chronic obstructive pulmonary disease accompanied by purulent sputum or increased C-reactive protein (≥40mg/l), antibiotic therapy that covers H. influenzae is warranted.20 The Global initiative for Chronic Lung Disease recommends that the choice of antibiotic be based on local resistance patterns, and accordingly, the use of high doses of the association of amoxicillin and clavulanate (875–125mg/8h for 5 days) should, therefore, be recommended in these pacients.21
The current susceptibility pattern of pneumococcal strains suggests penicillin as the first choice agent for the majority of respiratory tract infections. The majority of infections caused by pneumococcal strains are fully susceptible to penicillin. There is a small percentage of pneumococci with intermediate susceptibility (minimum inhibitory concentration between 0.12 and 2μg/l),22 against which a high dose of antibiotic might be required. To encompass the few cases of resistance we suggest high doses of penicillin, either G or V, to be used as first-line therapy for patients with community-acquired pneumonia. Caution is mandatory regarding this infection as this is the leading cause of death due to infectious diseases in adults worldwide. Classic bacteriologic techniques have generally identified pneumococcus as the overwhelmingly common cause, but there has been a great deal of discussion about the role of other microbes in the aetiology of pneumonia, as only half of the episodes of pneumonia show positive cultures in hospitalised patients. With the use of high-quality sputum specimens and modern microbiological techniques, we are able to identify more bacteria and viruses as possible aetiological agents, but there is debate about the true finding when nucleic acid amplification tests detect bacteria, mainly H. influenzae, Staphylococcus aureus, Pseudomonas aeruginosa or Moraxella catarrhalis, which are not later found by culture. This probably reflects bacterial presence in the microbiome and clinicians should not, therefore, treat such cases.23 As Gadbsy et al.24 put it recently, basing antibiotic selection on results of these modern techniques may potentially lead to unnecessary escalation of antibiotic use in some patients, thereby hampering efforts at antibiotic stewardship. The usage of microbiological tests in primary care are not recommended, and although the sensitivity of the pneumococcal antigen in urine ranges from 69% to 81%, with a specificity of 85–98%, the yield of this test in primary care is low as the incidence of pneumonia in the community is much lower than that observed in a hospital. Thus, treatment in primary care must be empirical as soon as severity criteria have been discarded. Doctors are requested to carefully assess if a patient with pneumonia must be referred to the hospital by considering the CRB-65/75 hospitalisation score (confusion, respiratory rate ≥30breaths/min, blood pressure <90/60mmHg, confusion, or older age) and the quick Sequential Organ Failure Assessment (SOFA) sepsis criteria (respiratory rate ≥22breaths/min, systolic blood pressure <100mmHg or altered mental status with a Glasgow score <15).25
Scandinavian and Dutch guidelines recommend narrow spectrum penicillin G/V in monotherapy as first-line empirical treatment in patients with non-severe CAP with no routine empirical coverage for H. influenzae or atypical pathogens. An example is the Danish or the Norwegian guideline that recommends a dose of 1,000,000IU of penicillin V every 6h for five days.26,27 Two different Scandinavian studies have recently found that penicillin therapy for pneumonia is as safe as other treatments and is not associated with a greater mortality in mild to moderate CAP.28,29 In addition, the Norwegian study showed that penicillin in monotherapy was associated with a reduced risk of 30-day readmission compared to other antibiotic treatments.29 Based on the results of the current susceptibility patterns of pneumococcal strains in Spain, high-dose penicillin V should be the drug of choice for community-acquired pneumonia. The use of phenoxymethylpenicillin is now only recommended for streptococcal pharyngitis in our country and the recommendation to use this antibiotic for other respiratory tract infections, such as pneumonia, acute bacterial rhinosinusitis, and acute otitis media in children under 2 years of age, will entail an off-label prescription. However, off-label prescription of antibiotics is common in Western countries and should not hinder rational antibiotic usage.30
We are aware of the lack of presentations of phenoxymethylpenicillin in our country, but this should not represent a limitation for prescribing this narrow spectrum antibiotic in Spain. This lack of presentations is now compounded by the fact that there is a shortage of some first-line antibiotics since autumn 2022. The European Medicine Agency, the European Commission and the Heads of Medicines Agencies, through the Executive Steering Group on Shortages and Safety of Medicinal Products are closely monitoring the current shortages of antibiotics affecting Europe.31 This shortage of antibiotics, which has been an ongoing public health concern and the situation in the European Union has been exacerbated by geopolitical events or trends such as the war in Ukraine, the energy crisis, and high inflation rates, mainly affects first-choice antibiotics, and an example is the current lack of oral presentations of 500mg of penicillin V in our country.32
Microorganisms causing urinary tract infections: either the 3g single dose of fosfomycin or the short-course of nitrofurantoin should be prioritisedResistance of uropathogens, mainly Escherichia coli and Klebsiella pneumoniae, to the classical antibiotics has significantly increased in the last years in Spain, mainly due to the high use of antibiotics.33 The resistance of enterobacteria to third generation cephalosporins, mediated by the production of extended-spectrum β-lactamases is a growing problem, with approximately 10% of these strains being producers, reducing the empirical therapeutic options in the community.34 As shown in Table 1, the most active antimicrobials against E. coli are carbapenem, nitrofurantoin and fosfomycin, with susceptibility rates exceeding 95% of the isolates. On the contrary, amoxicillin and clavulanate, quinolones, trimethoprim, and sulfamethoxazole are the least effective antibiotics, with less than 80% of isolates being susceptible. According to the recommendations of the Infectious Diseases Society of America, empirical antibiotic therapy should be substituted when the rates of resistance surpass 20%.35 In our country, current guidelines no longer recommend the former antibiotics for empirical use. Therefore, a single 3g dose of fosfomycin or a short course of nitrofurantoin should be recommended for the treatment of uncomplicated urinary tract infections, as these shorter lengths are equally effective and show fewer adverse reactions compared to antibiotics used for longer durations.36
ConclusionsAppropriate antibiotic prescribing is essential not only for patient safety and outcome, but also for reducing the emergence of antimicrobial resistance. There is a need to avoid the use of broad-spectrum antibiotics to reduce the risk of selecting resistant strains and to cause the least possible side effects and dysregulation in the patient's microbiome. The current susceptibility rates to common antibiotics are closer to the reality of other northern European countries compared to the rates observed in our country two and three decades ago. A ‘scandinavisation’ of first-line antibiotic therapy for common community-acquired infections has been discussed in this special paper, representing a new paradigm that should be endorsed by family doctors and paediatricians in our country and updated in our local clinical guidelines, and could also be considered in other neighbouring countries that are experiencing similar changes in susceptibility rates.
FundingNot funded.
Conflict of interestCL has received research grants from Abbott Diagnostics. The other authors declare no conflicts of interest.