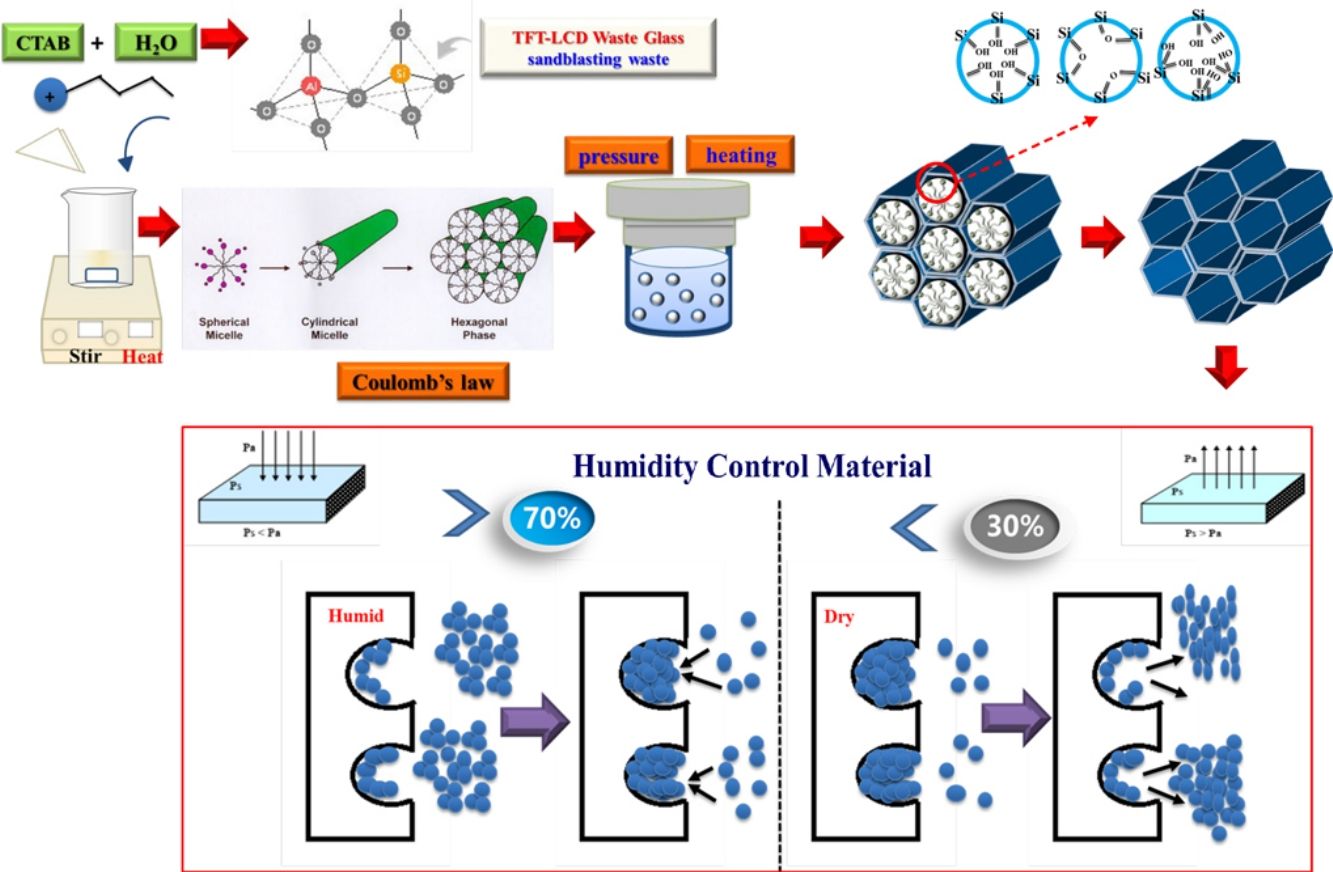
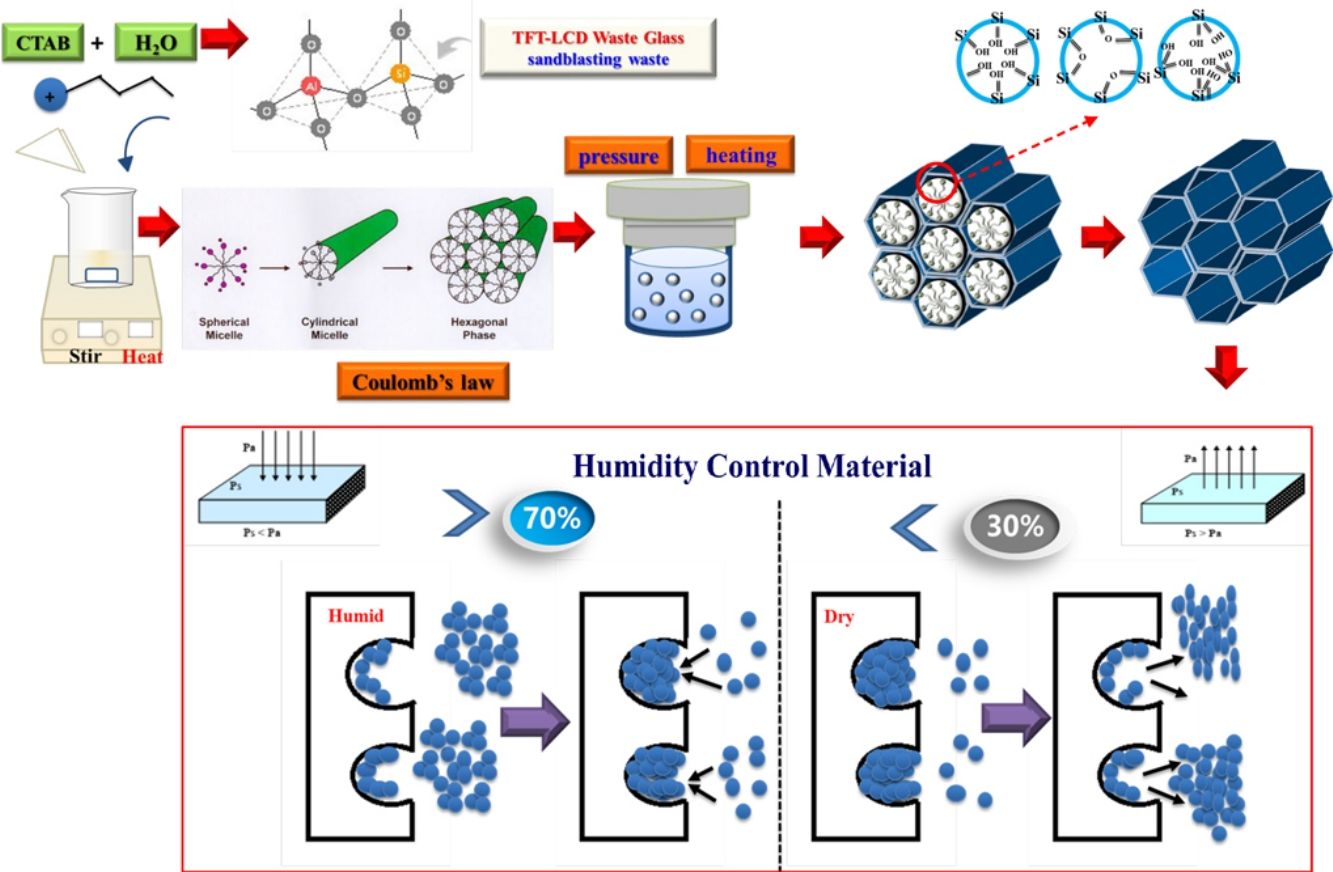
The preparation of an aluminum-mesoporous A1-MCM-41 humidity control material (Al-MHCM) by hydrothermally synthesizing a mixture of thin-film transistor liquid crystal display (TFT-LCD) waste glass and sandblasting (SB) waste was studied. The product has a typical mesoporous structure, with a specific surface area of up to 1013m2/g, the pore size distribution calculated is 3–4nm, and the pore volume of 0.97cm3/g. All the aluminum atoms of the product are in the form of tetrahedral aluminum in the framework, which confirms the successful synthesis of Al-MHCM. Results show that when the hydrothermal synthesis temperature is 105°C, the product synthesized from a mixture with a Si/Al molar ratio of 41.8 exhibits excellent performance (91.45m3/m3) in terms of the equilibrium moisture content and moisture adsorption capacity. The results confirm that the equilibrium moisture content of Al-MHCM is better than the rape straw concrete and hemp concrete (9.8–17.8m3/m3), and that of a diatomite/ground calcium carbonate composite aterial (11.7m3/m3). The research results are expected to provide a new technology for synthesizing TFT-LCD waste glass and SB waste into novel high value-added humidity control materials.
Se estudió la preparación de un material de control de humedad de aluminio-mesoporoso A1-MCM-41 (Al-MHCM) mediante la síntesis hidrotermal de una mezcla de vidrio de desecho de pantalla de cristal líquido de transistor de película delgada (TFT-LCD) y desechos de arenado (SB). El producto tiene una estructura mesoporosa típica, con una superficie específica de hasta 1013m2/g, la distribución del tamaño de poro calculada es de 3-4nm y el volumen de poro de 0,97cm3/g. Todos los átomos de aluminio del producto están en forma de aluminio tetraédrico en la estructura, lo que confirma la síntesis exitosa de Al-MHCM. Los resultados muestran que cuando la temperatura de síntesis hidrotermal es de 105°C, el producto sintetizado a partir de una mezcla con una relación molar Si/Al de 41,8 presenta un excelente rendimiento (91,45m3/m3) en términos de contenido de humedad de equilibrio y capacidad de adsorción de humedad. Los resultados confirman que el contenido de humedad de equilibrio de Al-MHCM es mejor que el hormigón de paja de colza y el hormigón de cáñamo (9,8–17,8m3/m3), y que el de un material compuesto de diatomita/carbonato de calcio molido (11,7m3/m3). Se espera que los resultados de la investigación proporcionen una nueva tecnología para sintetizar residuos de vidrio TFT-LCD y residuos SB en nuevos materiales de control de humedad de alto valor añadido.
In the optoelectronic industry, a large amount of thin film transistor liquid crystal display (TFT-LCD) waste glass is generated during panel manufacturing, assembly and cutting. TFT-LCD waste contains potentially carcinogenic compounds, which are likely to have a negative impact on the environment and human health [1]. In addition, sandblasting (SB) waste is the surface etching process after the silicon wafer is cut, and the surface of the silicon material is optimized through the use of abrasive sand, thereby mass production of SB waste in the upstream factory of the solar cell module. In the past, most of these wastes were treated by incineration or landfill, thus wasting valuable materials. Afroz et al. [2] pointed out that waste electrical and electronic equipment (WEEE) is currently considered to be one of the fastest growing waste streams in the world (3–5%) [2]. In addition, despite this plethora, reports on MCM-41mesoporous material synthesis from TFT-LCD waste glass and SB waste are still scarce in literature. Therefore, recycling WEEE products can reduce the use of original resources in the manufacturing process, thereby helping to reduce environmental pollution. Therefore, from an environmental and economic point of view, a cleaner process to add value and provide resource reuse and safe disposal research for solid waste is of great significance.
In the early 1990s, scientists at Mobil Petroleum Corporation discovered an ordered mesoporous silicate/aluminosilicate material, which they called the M41S series [3–5]. Since then, a series of studies have been conducted, in which the influence of the concentration of the surface active agent on the prepared mesoscopic particles was studied, and three structural arrangements were determined: a hexagonal structure - Mobil Composition of Matter No. 41 (MCM-41), a cubic structure – Mobil Composition of Matter No. 48 (MCM-48) and a layered structure – Mobil Composition of Matter No. 50 (MCM-50). Among them, MCM-41 is the most studied mesoporous material because of its good thermal, hydrothermal and hydrolytic stability along with its high surface area and pore volume [6–9]. In addition, MCM-41 can prevent the agglomeration of a supported metal oxide by forming a bond between the surface silicon hydroxyl bridge and the deposited oxide [10]. It has been reported that the incorporation of Al into the MCM-41 framework can provide the resulting Al-MCM-41 material with Bronsted acid sites while exhibiting good adsorption affinity, thereby broadening its potential application [11,12]. The mesoporous material of MCM-41 can be prepared by a variety of synthetic methods, such as a hydrothermal treatment [13], a sol–gel method [14], co-condensation method [15], and an impregnation method [16]. The hydrothermal treatment method is the preferred method because it is simple and the operational complexity is low; furthermore, it has been reported that the hydrothermal treatment can increase the structural stability, acidity, activity and surface area of the mesoporous material [17]. The usual synthesis procedure of is to use tetraethylorthosilicate (TEOS) or tetramethylorthosilicate (TMOS) as silica source [18]. However, the process suffered from the drawback of the expensive and toxic silica source. The cost of silicon dioxide precursors commonly used in the synthesis process represents a major problem in the use of such materials in regard to economic and environmental considerations [19,20]. The economic and environmental considerations have attracted an interest in the use of inexpensive inorganic silicate as a starting material. Cazula et al. [21] described the synthesis of Si-MCM-41 molecular sieves using TEOS and rice husk silica as silica sources. The results indicated that despite using different silica sources, the two materials presented very similar characteristics, evidencing the feasibility of using an inexpensive waste material as silica source in the synthesis of these molecular sieves, once the synthesis conditions have been satisfactorily adjusted [21]. Therefore, some research has been conducted to develop mesoporous silica using low-cost silica resources, including fly ash [22], wheat stem ash [23], bentonite [24], Diatomaceous marl [25], palm kernel shell ash [26] and packaging resin waste [27].
Taiwan has a subtropical island climate, with warm and humid weather conditions year round; thus, the hot climate and high humidity of Taiwan provide ideal conditions for mold. According to the literature, the most comfortable range of relative humidity for the human body is 40–70% [28]; additionally, excessively dry or humid environments are not conducive to human health and life [29]. Therefore, to achieve effective humidity control while also avoiding the use of energy-consuming methods, such as mechanical air conditioning, the development of new and energy-free indoor humidity control technologies has been explored to save energy and provide healthy living environments [30,31]. If low-cost silicon dioxide resources (TFT-LCD waste glass and SB waste) can be used to produce aluminum-mesoporous A1-MCM-41 humidity control material (Al-MHCM), then the humidity of the environment can be adjusted while decreasing the amount of pollution in the environment. There is no need to consume energy or use other equipment, as long as the original capillary phenomenon of the mesoporous material is used, the designed Al-MHCM exhibits moisture adsorption and desorption characteristics. Therefore, the purpose of this study is to alkali fusion and hydrothermally synthesize TFT-LCD waste glass and SB as silicon and aluminum sources. This strategy can determine the ideal conditions for obtaining a material with suitable performance in the field of water vapor adsorption-desorption, which uses WEEE as a source of silicon and aluminum instead of commercial TEOS. This study is the first attempt to measure the influence of various temperatures and Si/Al molar ratios on the pore structure and surface characteristics of Al-MHCM produced from waste materials and its performance toward environmental humidity control.
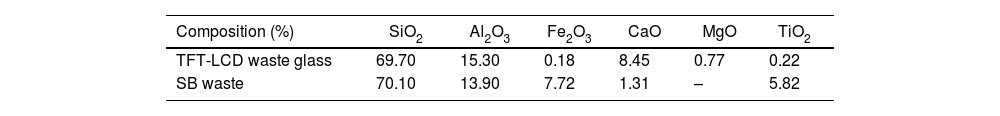
Materials and methodsMaterialsThe TFT-LCD waste glass and SB waste were provided by the Central Taiwan Resource Recycling Plant and Solar Energy Technology Company, respectively. These wastes were dried, ground and sieved to achieve a particle size≤74μm. As shown in Table 1, its chemical composition was measured and analyzed by X-ray fluorescence (XRF) spectroscopy. The main components of the TFT-LCD waste glass were SiO2, Al2O3 and CaO, which accounted for 69.70%, 15.30% and 8.45%, respectively, and the SiO2/Al2O3 ratio was approximately 4.5. The main components of the SB waste were SiO2 and Al2O3, which accounted for 70.10% and 13.90%, respectively. The SiO2/Al2O3 ratio was calculated to be approximately 5.0. Among them, TFT-LCD waste glass mainly came from material cut from glass substrates, and SiO2 and Al2O3 were the main composition of the glass network structure, thereby providing an abundance of SiO2 and Al2O3.
Extraction of SiO2 and Al2O3 in an alkaline fusion processTFT-LCD waste glass and SB waste were mixed together, and silicate and aluminosilicate were extracted through an alkali fusion temperature of 450°C with the addition of a 1.5 proportion of NaOH (mixed powder:NaOH=1:1.5). The powder was mixed with distilled water (liquid/solid ratio, L/S=5, 10, 20) at different ratios to dissolve the aqueous solution containing SiO2 and Al2O3 and then filtered to obtain sodium aluminosilicate solutions with SiO2/Al2O3 molar ratios of 26, 41.8 and 56.3.
Synthesis of aluminum-mesoporous A1-MCM-41 humidity control material (Al-MHCM)Al-MHCM was synthesized by using the hydrothermal method. First, the required amount of CTAB was dissolved in 30mL of deionized water and ammonia to form a template solution. Sodium aluminosilicate solutions with the different SiO2/Al2O3 molar ratios stated above were slowly added to the CTAB template solution while stirring. Sulfuric acid (1M) was used to adjust the pH of the dispersion, and then the dispersion was stirred continuously for 2h. The mixed solution was then transferred to a stainless steel autoclave (200mL) lined with Teflon. The mixed solution was heated at different hydrothermal temperatures (90, 105, 120°C) for 48h. Next, the obtained product was washed with distilled water, filtered, and then dried in an oven at 105°C overnight. Finally, the obtained product was calcined up to 550°C at a heating rate of 5°C/min in air to burn off the surfactant from the matrix. The obtained mesoporous material was called Al-MHCM.
Test and methodsThe used TFT-LCD waste glass and SB waste were analyzed by an XRF fluorescence analyzer (RIX 2000) to obtain the chemical element composition of the raw materials. The pore structure of Al-MHCM was determined by X-ray powder diffraction (D8A XRD) to identify the crystalline phases in the material. Diffraction data were collected between 2θ=1–8° using the Ni-filtered CuKα radiation (λ=0.1542nm). Scanning electron microscopy (SEM; American FEI company (Nova Nanosem 230) was used to characterize the surface morphology and structural changes of the sample. High-resolution solid state 27Al MAS NMR spectra was recorded on a Bruker AVANCE III 400MHz NMR spectrometer at 104.26MHz, with a pulse width of 1.00μs, a pulse delay of 1s, a spinning rate of 15kHz and 1000 scans. Specific surface area, pore volume, and average pore size of materials were determined at 77K on a nitrogen adsorption–desorption apparatus (TriStar 3000) and calculated by the Brunaer–Emmett–Teller (BET) method. Samples were degassed at 250°C for 2h before performing the adsorption-desorption experiment. Pore-size distributions were created using the adsorption branch of the isotherm via the Barrett–Joyner–Halenda (BJH) model.
Moisture adsorption/desorption testThe moisture adsorption/desorption test was performed in accordance with the method for measuring the equilibrium moisture content in Japanese Industrial Standards JIS A 1475. First, the test body was dried in an oven at 105°C for 24h, and its constant weight was recorded. Next, the sample was placed in a constant temperature and humidity controller, and the relative humidity was changed to 10, 33, 55, 75, 85, and 95% at a fixed temperature of 23°C. The constant weight of adsorption (from low to high) and desorption (from high to low) of the sample was recorded at this relative humidity. Finally, the moisture adsorption per unit area of mesoporous Al-MHCM was obtained at different times. The standard of the Japanese Industrial Regulations (JIS A 1470) humidity control building materials regulations was verified, and the standard value, based on the average equilibrium moisture content (>5kg/m3), was evaluated.
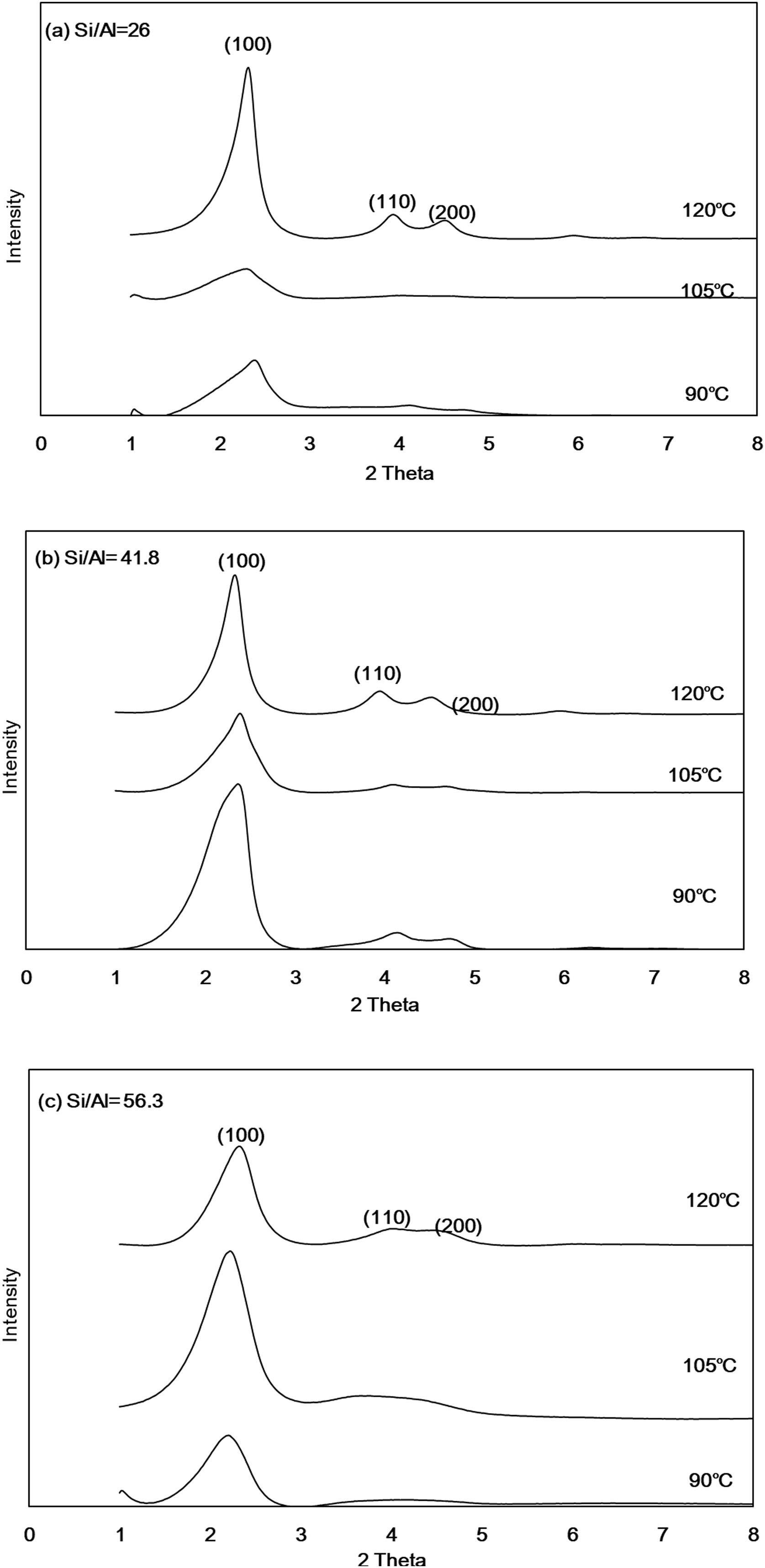
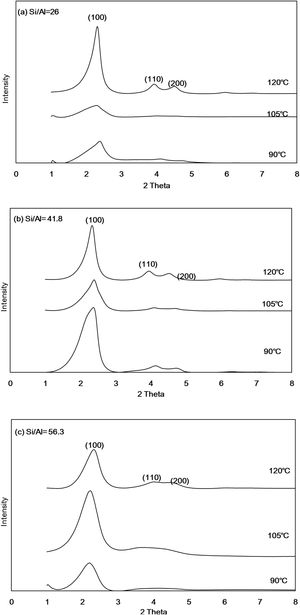
Results and discussionCrystal phase analysis of Al-MHCMFig. 1 shows the XRD diffraction patterns of Al-MHCM synthesized at different temperatures and different Si/Al molar ratios. The XRD pattern of Al-MHCM shows that the main diffraction peaks are at 2.34 and 2.42° along with 3.90 and 4.50°, which correspond to the characteristic d(100), d(110) and d(200) peaks of MCM-41, respectively [32]. At lower Si/Al ratios, the main characteristic peaks are slightly shifted, which is mainly due to the 2θ angle shift caused by the high aluminum content in the source material; this result means that Al atom doping in the crystal lattice changes the crystallinity of the product and causes poor sorting. As the Al content increases, Al-MHCM exhibits a decrease in lattice parameters, which is related to the difference in the ionic radius of Al and Si ions [33]. Therefore, when the Si/Al molar ratio from 41.8 increase to 56.9 (temperature is 90°C), the intensity of the diffraction peak gradually decreases, indicating that the order of the structure decreases [34,35]. In addition, it can be found from the change in the hydrothermal temperature that when the temperature increased from 90 to 120°C, the main characteristic peak d(100) has relatively stable crystallinity. The characteristic peaks (d110 and d200) representing the hexagonal structure also have a tendency to gradually form as the hydrothermal temperature increased because when the hydrothermal temperature increased, the balance between the solid and liquid phases of the initial gel is destroyed; this imbalance accelerates the aggregation of silicate species on the micelle surfaces. Therefore, a high hydrothermal temperature is conducive to the formation of a well-ordered MCM-41 structure because the high temperature will accelerate the condensation rate of silicate on the silicon dioxide wall [36,37].
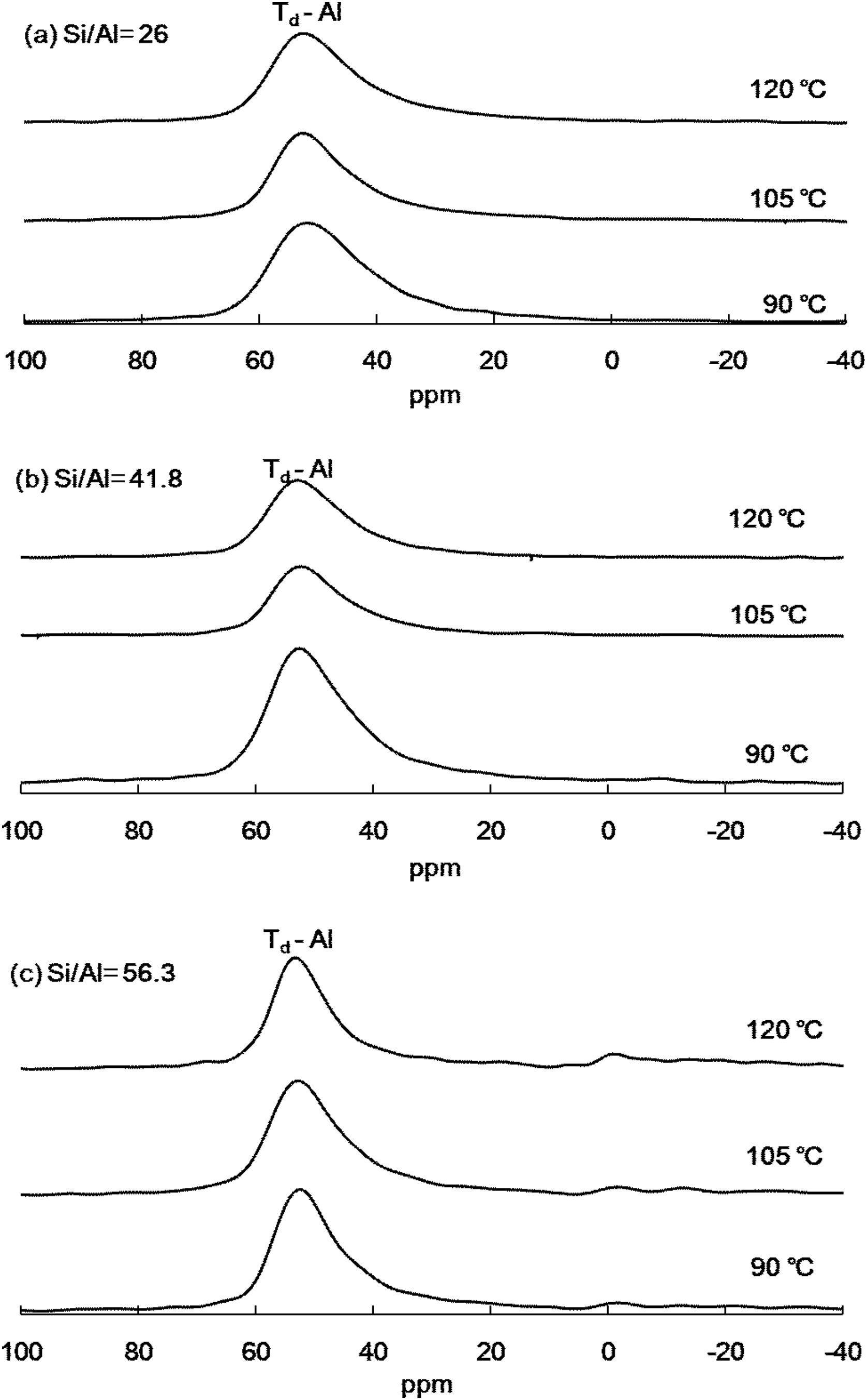
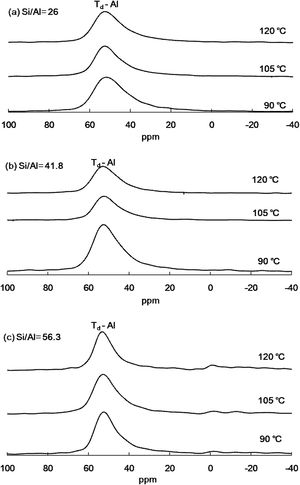
Solid-state 27NMR analysis of Al-MHCMThe 27Al NMR spectrum was used to track the change in the position of Al added to Al-MHCM. All the spectra of the current Al-MHCM material are dominated by the signal of the tetrahedral-coordinated aluminum (AlIV) species with 27Al=53ppm (Fig. 2), confirming that they were related to the Bronsted acid site. If the material can exist in the form of tetrahedrons, the number of Bronsted acid sites on the surface of Al-MHCM will increase, thus increasing the affinity and catalytic performance of the material and simultaneously strengthening the humidity-regulating performance [34]. The weak signal at 27Al=0ppm is due to the octahedral-coordinated aluminum (AlVI) species (Fig. 2(c)) [38], and the appearance of these extra-framework aluminum species indicated that partial dealumination has occurred, which was related to Lewis acid sites [39]. However, when the Si/Al molar ratio is lower (Fig. 2(a), (b)), more Al atoms (AlVI) are introduced into the silicon dioxide framework, thereby generating and enhancing the AlIV signal. It is well known that AlIV can enhance the acid strength of adjacent SiOH groups, thereby forming Brønsted acid sites (BAS) with high catalytic activity [40]. In addition, from the area content of the tetrahedral-coordinated aluminum (AlIV) species, it can be seen that when the hydrothermal temperature is low (90°C), the occupied area is between 38.50 and 52.02%, and when the hydrothermal temperature is increased to 105°C, the area occupied by AlIV species is between 52.67 and 59.61%. In addition, when the hydrothermal temperature is 120°C, the area occupied by species at 53ppm increased to 54.98–67.17%. These results show that as the absence of AlVI sites for the Si/Al=26 or 41.8 means simply that all Al is incorporated as AlIV. However, previous studies have shown that by decreasing the Si/Al ratio, the AlIV signal can be enhanced [41], this led to more Bronsted acid sites on the surface of Al-MHCM.
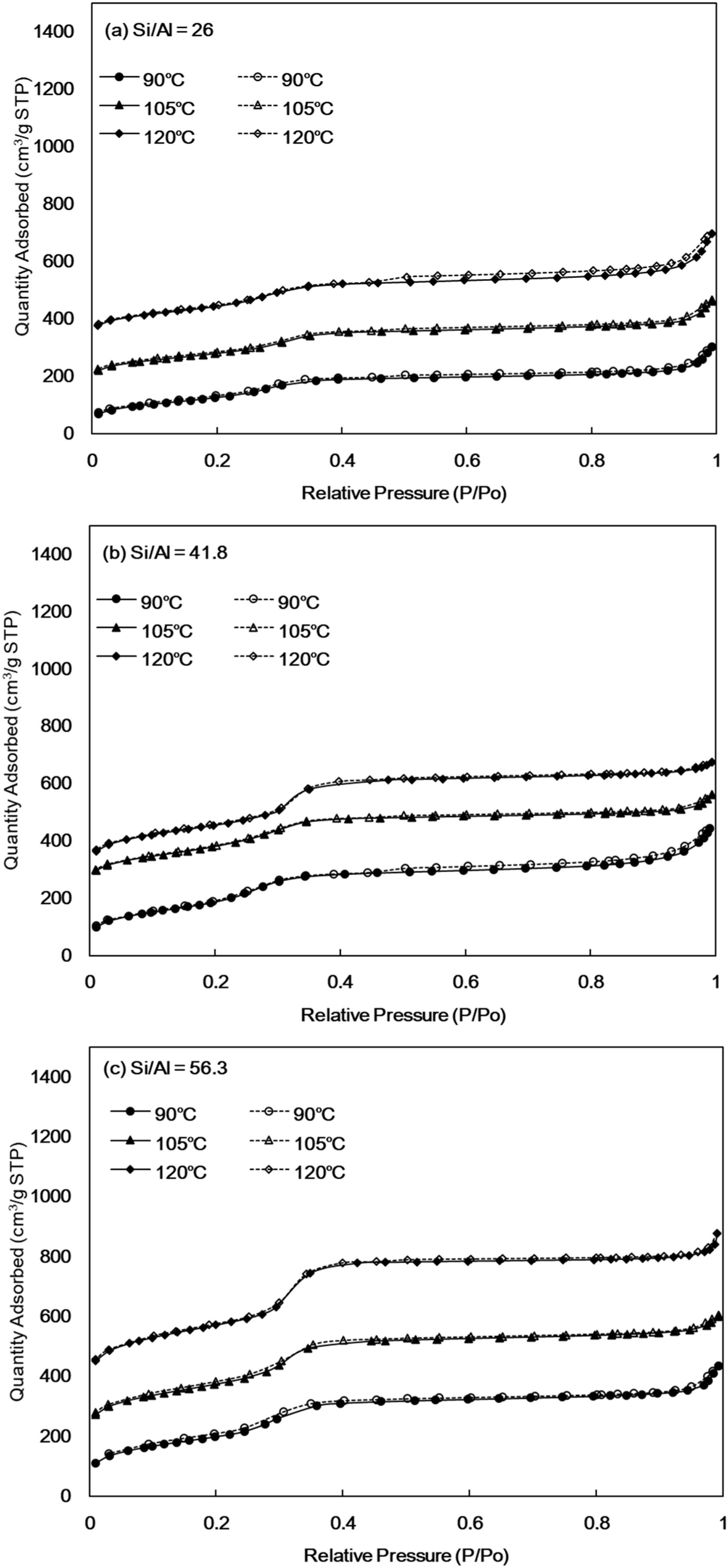
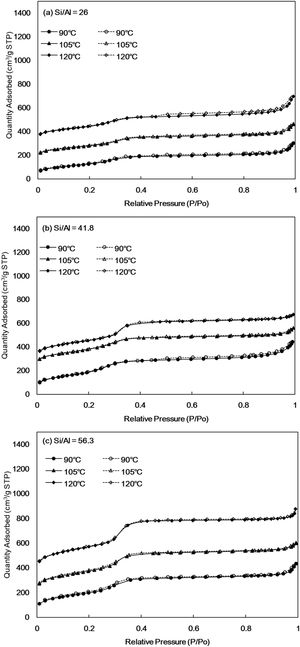
N2 isothermal adsorption-desorption of Al-MHCMThe N2 adsorption–desorption phenomenon provides a technique for determining the surface area, pore volume and pore size distribution. The N2 isotherm adsorption–desorption curve is shown in Fig. 3. All the synthesized Al-MHCM junctions exhibit a type-IV nitrogen adsorption-desorption isotherm. According to the International Union of Pure and Applied Chemistry chemical nomenclature (IUPAC), this is a typical feature of homogeneous mesoporous materials [42], attributed to the retention of the ordered mesoporous structure [43]. The isotherm shows the following three stages: when the low relative pressure is 0.2–0.3, the monolayer adsorption of nitrogen on the mesoporous wall is observed; and when the relative pressure is 0.3–0.4, a sharp increase occurs under the same parameter conditions with different Si/Al ratios. This is characteristic of capillary condensation in mesopores, which shows a narrow hysteresis loop. Finally, when the relative pressure is 0.5–0.9, the synthesized Al-MHCM shows slightly tilted plateau, which is caused by multilayer adsorption on the outer surface of particles [44]. In addition, when the Si/Al ratio is 26, 41.8 and 56.3, the specific surface area ranges from 506–587, 733–795 and 781–1013m2/g, respectively. The adsorption–desorption characteristics for all samples are typical of mesoporous materials, indicating that mesoporous materials have better adsorption performance.
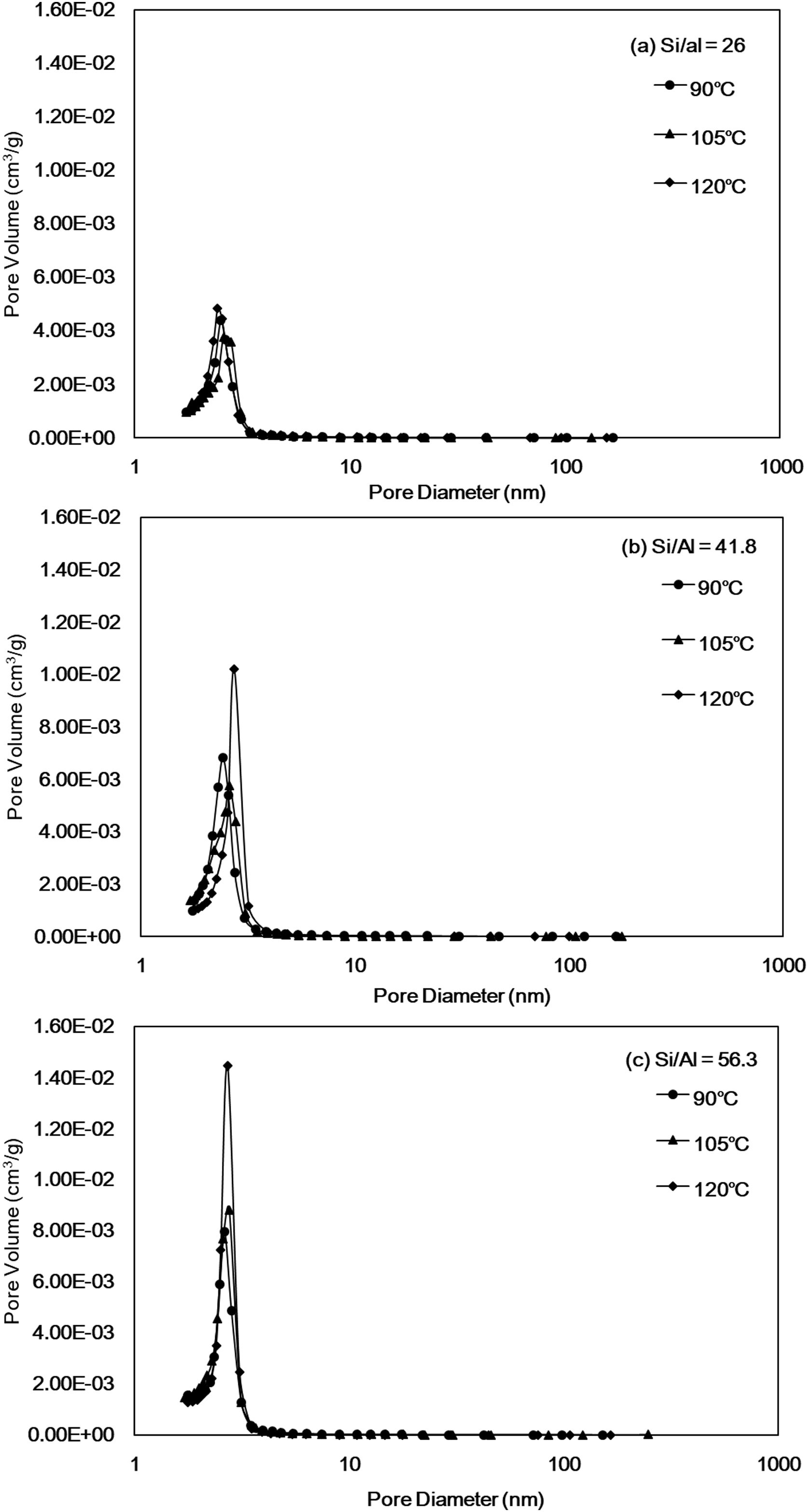
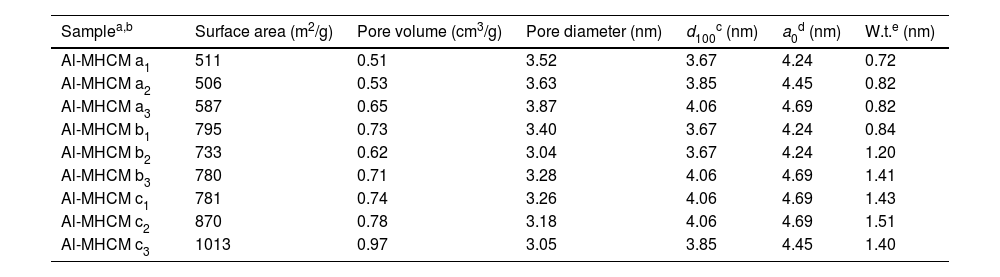
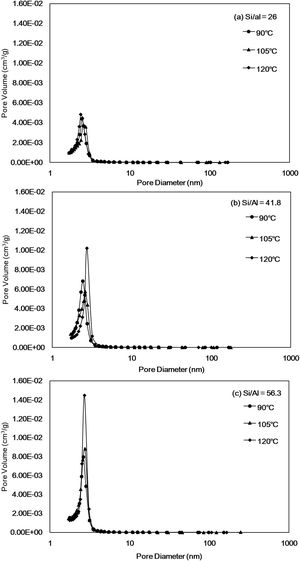
Fig. 4 shows the total pore volume and pore diameter calculated by the N2 adsorption–desorption measurements and the BJH method and are listed in Table 2. The pore size distribution curves of all Al-MHCM shown in Fig. 4 are unimodal and belong to a narrow range of mesopores that are approximately 3–4nm, indicating the existence of mesopores in the particles. Thus, this result confirms the successful synthesis of a uniform mesoporous material, and is consistent with the BJH method for calculating the pore size (Table 2). In addition, Fig. 4(a) shows that when the Si/Al ratio is 26, the pore volume of the synthesized Al-MHCM is 0.4–0.5cm3/g. When the Si/Al ratio is increased to 56.3, the pore volume is increased to 0.9–1.4cm3/g. Based on the above knowledge, the increase in the Si/Al ratio and hydrothermal temperature can form an ordered mesoporous structure. The pore volume and specific surface area of the synthesized Al-MHCM increase accordingly, with the highest pore volume being 0.97cm3/g, and the highest specific surface area being 1013m2/g (Al-MHCMc3). The results of Sohrabnezhad and Mooshangaie show that adding metal (such as Ag/AgBr) to an MCM-41 material will increase the pore size and pore volume, which indicated that some of the metal was dispersed on the inner pore surfaces of the MCM-41 material [45]. The unit cell parameter “a0” can also be determined using the equation a0=(2*d(100)/√3) from the diffraction peak (1 0 0). Table 2 shows the computed d(100) and a0 values. Furthermore, Table 2 shows the surface area and pore volume of three samples. The maximum value of a0 is 4.69nm. The increase of a0 indicates that the pore wall of silica becomes thicker and more orderly with the increase of Si/Al molar ratio and hydrothermal temperature. Additionally, when the hydrothermal temperature is 120°C, the Si/Al molar ratio from 41.8 increase to 56.3, the a0 of Al-MHCMc3 from 4.69nm decreased to 4.45nm, this behavior might possibly be caused by a slight distortion of the mesoporous channels due to the partial polymerization over pore structures induced by superficial reaction. The thickness of the pore wall was calculated to be 0.72–1.51nm, indicating the high hydrothermal stability of the synthesized Al-MHCM.
The pore structure characteristics of Al-MHCM.
| Samplea,b | Surface area (m2/g) | Pore volume (cm3/g) | Pore diameter (nm) | d100c (nm) | a0d (nm) | W.t.e (nm) |
|---|---|---|---|---|---|---|
| Al-MHCM a1 | 511 | 0.51 | 3.52 | 3.67 | 4.24 | 0.72 |
| Al-MHCM a2 | 506 | 0.53 | 3.63 | 3.85 | 4.45 | 0.82 |
| Al-MHCM a3 | 587 | 0.65 | 3.87 | 4.06 | 4.69 | 0.82 |
| Al-MHCM b1 | 795 | 0.73 | 3.40 | 3.67 | 4.24 | 0.84 |
| Al-MHCM b2 | 733 | 0.62 | 3.04 | 3.67 | 4.24 | 1.20 |
| Al-MHCM b3 | 780 | 0.71 | 3.28 | 4.06 | 4.69 | 1.41 |
| Al-MHCM c1 | 781 | 0.74 | 3.26 | 4.06 | 4.69 | 1.43 |
| Al-MHCM c2 | 870 | 0.78 | 3.18 | 4.06 | 4.69 | 1.51 |
| Al-MHCM c3 | 1013 | 0.97 | 3.05 | 3.85 | 4.45 | 1.40 |
aThe Si/Al molar ratio of Al-MHCM. a=5; b=10; c=20.
bThe hydrothermal temperature of Al-MHCM. 1=90°C; 2=105°C; 3=120°C.
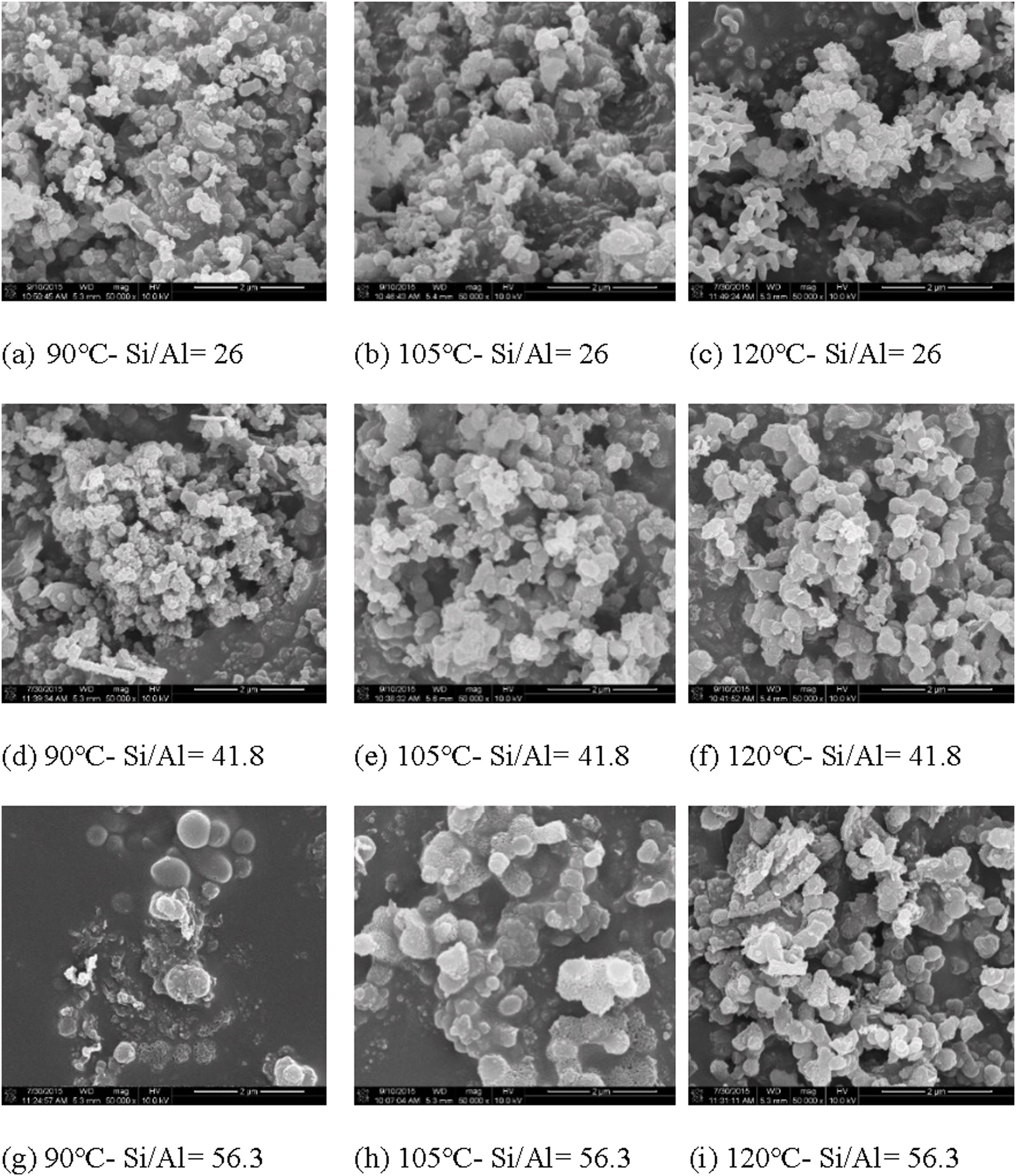
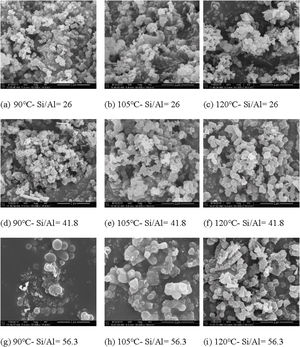
In this study, the microstructure changes of Al-MHCM were observed through FE-SEM. The morphology of the Al-MHCM material at a 50,000× magnification is shown in Fig. 5. During the hydrothermal treatment under alkaline conditions (pH=10), silicate and aluminosilicate are extracted from the TFT-LCD waste glass and SB waste. Then, the extracted silicate and aluminosilicate are mixed with the CTAB surfactant to form a mesoporous silica material similar to MCM-41 [42]. In addition, it can be seen that even after the calcination process (550°C), the macrostructure of Al-MHCM remains intact, thus confirming the high thermal stability of Al-MHCM [46]. The morphology of Al-MHCM is clearly observed as round nanoparticles, and the appearance of the particles is mainly spherical. From the observations in Fig. 5(a)–(c), when the Si/Al ratio is 26 and the hydrothermal temperature is 90–120°C, the size distribution of Al-MHCM crystals is approximately 0.13–0.22μm. When the Si/Al ratio is increased to 41.8, the crystal size is approximately 0.11–0.40μm. At the highest Si/Al ratio (56.3), the Al-MHCM crystal size distribution is approximately 0.22–0.60μm. This phenomenon shows that the increase in hydrothermal temperature causes the crystal size to increase significantly. According to the study by Tian et al. [47] this increase in crystal size was mainly due to the increased synthesis temperature and rapid hydrolysis reaction that affected the uniformity of the generated particles [47]. In addition, Xie et al. [48] stated that the difference between products obtained by the same synthesis method was due to the formation of delayed structures due to changes in the reaction medium pH or the occurrence of uneven precipitation [48].
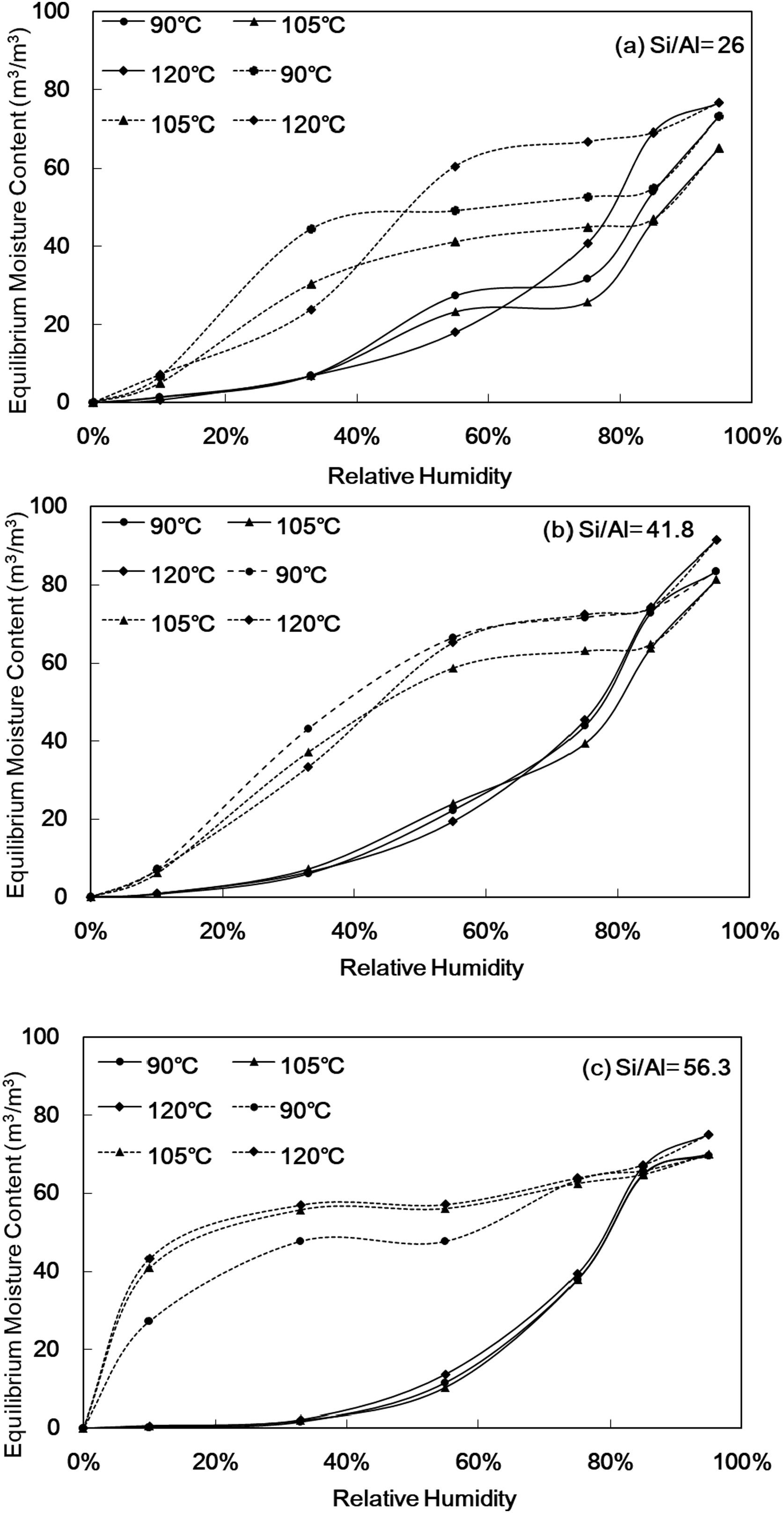
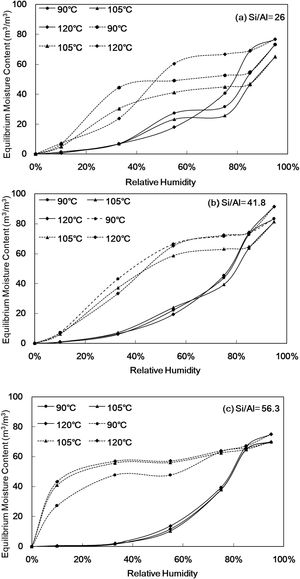
The 24h equilibrium moisture content curve of Al-MHCMFig. 6 shows the equilibrium moisture content of Al-MHCM at different relative humidities (RH%). The solid line in Fig. 6 shows the process of water vapor adsorption, and the dotted line is the process of water vapor desorption. The results show that the equilibrium water contents of all samples increase with increasing RH%. However, there is a significant difference in the equilibrium moisture content in the material, especially when the RH% is high (RH%=75–95). Fig. 6 shows that when the Si/Al ratio is 26, the RH% of the synthesized Al-MHCM is 95%, the equilibrium moisture content is in the range of 65.20–76.60m3/m3 (Fig. 6(a)). When the Si/Al ratio is 41.8, the highest equilibrium moisture content is shown to be in the range of 81.34–91.45m3/m3 (Fig. 6(b)). In addition, when the Si/Al ratio is 56.3 and the RH% of the synthesized Al-MHCM is 95%, the equilibrium moisture content is 69.63–75.15m3/m3 (Fig. 6(c)). Rahim et al. [49] noted that the equilibrium moisture content of rape straw concrete and hemp concrete ranged from 17.8 to 9.8m3/m3 at a high RH%=95 [49]. Hu et al. [17] confirmed that more mesopores can make the material more conducive to capillary condensation and improve the moisture adsorption capacity, and the equilibrium moisture content of diatomite/ground calcium carbonate composite material was 11.7m3/m3 at RH%=98 [17]. The results confirm that the equilibrium moisture content of Al-MHCM is better than the rape straw concrete and hemp concrete (9.8–17.8m3/m3) [49], and that of a diatomite/ground calcium carbonate composite aterial (11.7m3/m3) [17]. These results show that the adsorption characteristics are closely related to the specific surface area and pore size. In the process of water vapor adsorption and desorption, the pore size of Al-MHCM is significantly related to capillary condensation [50]. According to the study of Xie et al. [48] when the effective pore size for capillary condensation was 2.93–9.09nm when temperature was 23°C and relative humidity was 33–75% [48]. This theory corresponds to the result that the pore diameters of all synthesized materials in this study were between 3 and 4nm. Therefore, the pore size distribution of the synthesized Al-MHCM was in the mesopore range, so that it can perform the most effective humidity control function. In addition, Jansen et al. [51] observed that water saturation increased with relative humidity in a relatively nonlinear manner. Furthermore, they found that the diffusion coefficient did not depend on the water concentration itself because there was no difference in the diffusion rate between adsorption and desorption [51]. It can be clearly observed that when the RH% gradually increased, the larger pores also can be filled up [48]. This result showed that the larger slopes and peaks in the isothermal equilibrium moisture content curve indicated higher moisture storage capacity, and the smallest hysteresis loop between the moisture adsorption and desorption curves indicated that the water desorption capacity was similar to the water adsorption process [52]. In addition, when the low hydrothermal synthesis temperature is 90°C, the equilibrium moisture content of Al-MHCM at RH%=95 is 73.19m3/m3, and when the hydrothermal temperature is increased to 120°C, the equilibrium moisture content increased significantly to 76.60m3/m3 (Fig. 6(a)). The adsorption-desorption curve shows a hysteresis loop. Since the inner wall of the pore is in a wet state after the loss of water during the dehumidification process, the contact angle of the water molecules on the inner wall of the pore is small, thereby delaying the desorption effect. In contrast, in the process of moisture adsorption, the inner wall of the dry pore has a better moisture adsorption effect with its large water contact angle. The above results show that the change in the Si/Al ratio exhibits no obvious difference in regard to the change in equilibrium moisture content, while an increase in the hydrothermal temperature can destroy the balance between the solid and liquid phases of the initial gel; thus, the concentration of silicate and aluminosilicate in the liquid phase results in the aggregation of silicate species on the microcell surface. Aggregation promotes the crystallization process and increased the pore structure and specific surface area of Al-MHCM. Therefore, the equilibrium moisture content gradually increased.
ConclusionThis study uses TFT-LCD waste glass and SB waste to produce Al-MHCM. The microscopic characteristics of the synthesized mesoporous Al-MHCM are established by changing the Si/Al ratio and hydrothermal temperature and analyzing the moisture adsorption performance of each sample. The XRD analysis shows that a high hydrothermal temperature is conducive to the formation of a well-ordered structure. The 27Al-NMR analysis shows that AlVI can be converted more effectively to a tetrahedron (Td-Al), which is the form that enters the skeleton. However, the crystal size increased as the Si/Al ratio increased, and the pore volume and specific surface area of the synthesized Al-MHCM increase accordingly; the highest pore volume is 0.97cm3/g, and the highest specific surface area is 1013m2/g (Al-MHCMc3). These results show that the addition of Al metal to Al-MHCM will increase the pore size and pore volume; therefore, some of the metal is dispersed on the inner pore surface of the material. The pore size of Al-MHCM is significantly related to the capillary condensation, and it is expected that the adsorption characteristics are closely related to the specific surface area and pore size. Finally, when the Si/Al ratio is 41.8, the highest equilibrium moisture content is shown to be 91.45m3/m3. The results confirm that the equilibrium moisture content of Al-MHCM is better than the rape straw concrete and hemp concrete (9.8–17.8m3/m3), and that of a diatomite/ground calcium carbonate composite aterial (11.7m3/m3). In this study, it is only used as a test for the adsorption and desorption performance of water vapor, and the results confirm that the sample can be reused by drying the sample at 105°C, which is the simplest. In addition, during the preparation of Al-MHCM, Al-MHCM also needs to be obtained by calcination at 550°C. Therefore, the results confirm that the physicochemical properties of Al-MHCM will not be changed in the equilibrium moisture content test. In future, this study will focus more on the stability test of the research samples, and further study the physical and chemical properties of the samples after humidity test. Therefore, Al-MHCM's products as humidity control building materials exhibit excellent equilibrium moisture content, have excellent pore characteristics, and can be used in various construction applications at low cost.
Authors’ contributionsYa-Wen Lin: Writing – original draft. Methodology. onceptualization. Wei-Hao Lee: Supervision. Chiao-Ying Chen: Validation, Investigation, Methodology.Yan-Jun Liu: Validation. Wei-Qing Zhang: Investigation. Mei-Yu Lin: Validation. Kae-Long Lin: Resources, writing-commenting and editing. All authors reviewed and approved the final manuscript.
Availability of data and materialsAll data generated or analyzed during this study are available from the corresponding author upon request.
FundingThis work was supported by Taiwan Ministry of Science and Technology, for supporting this research financially (Contract No. MOST-107-2221-E-197-002-MY3).
Conflict of interestsThe authors declare they have no competing interests.
This work was supported by Ministry of Science and Technology of Taiwan, for supporting this research financially (Contract No. MOST-107-2221-E-197-002-MY3).