l-asparaginase (EC 3.5.1.1) is an enzyme that catalysis mainly the asparagine hydrolysis in l-aspartic acid and ammonium. This enzyme is presented in different organisms, such as microorganisms, vegetal, and some animals, including certain rodent's serum, but not unveiled in humans. It can be used as important chemotherapeutic agent for the treatment of a variety of lymphoproliferative disorders and lymphomas (particularly acute lymphoblastic leukemia (ALL) and Hodgkin's lymphoma), and has been a pivotal agent in chemotherapy protocols from around 30 years. Also, other important application is in food industry, by using the properties of this enzyme to reduce acrylamide levels in commercial fried foods, maintaining their characteristics (color, flavor, texture, security, etc.) Actually, l-asparaginase catalyzes the hydrolysis of l-asparagine, not allowing the reaction of reducing sugars with this aminoacid for the generation of acrylamide. Currently, production of l-asparaginase is mainly based in biotechnological production by using some bacteria. However, industrial production also needs research work aiming to obtain better production yields, as well as novel process by applying different microorganisms to increase the range of applications of the produced enzyme. Within this context, this mini-review presents l-asparaginase applications, production by different microorganisms and some limitations, current investigations, as well as some challenges to be achieved for profitable industrial production.
l-asparaginase aminohydrolase (l-asparaginase, EC 3.5.1.1), has gained attention in recent years due to its important applications, as its use in pharmaceutical industry as an alternative for treatment of different cancers such as acute lymphoblastic leukemia, malignant diseases of the lymphoid system and Hodgkin's lymphomas.1 Also, this enzyme is used in food industry to prevent the acrylamide formation when foods are processed in high temperatures.2 This use is important because acrylamide is a neurotoxin classified as potentially carcinogenic to humans.3
Industrial l-asparaginase production presents some challenges, such as the search for new microorganisms able to produce it with less adverse effects. Nowadays, industrial production is carried out using bacteria such as Escherichia coli and Erwinia chrysanthemi.4 However, the enzyme obtained from prokaryotic microorganisms usually presents some problems such as hypersensitivity and immune inactivation.5 Within this context, eukaryotic microorganisms such as filamentous fungi6 and yeasts7 have been investigated for this enzymes production, due to better compatibility with the human system.
Currently, new studies have been carried out aiming to enhance production process and establish new ways for enzyme synthesis. Thus, some of these aspects are discussed, besides some generalities regarding l-asparaginase applications in pharmaceutical and food industries.
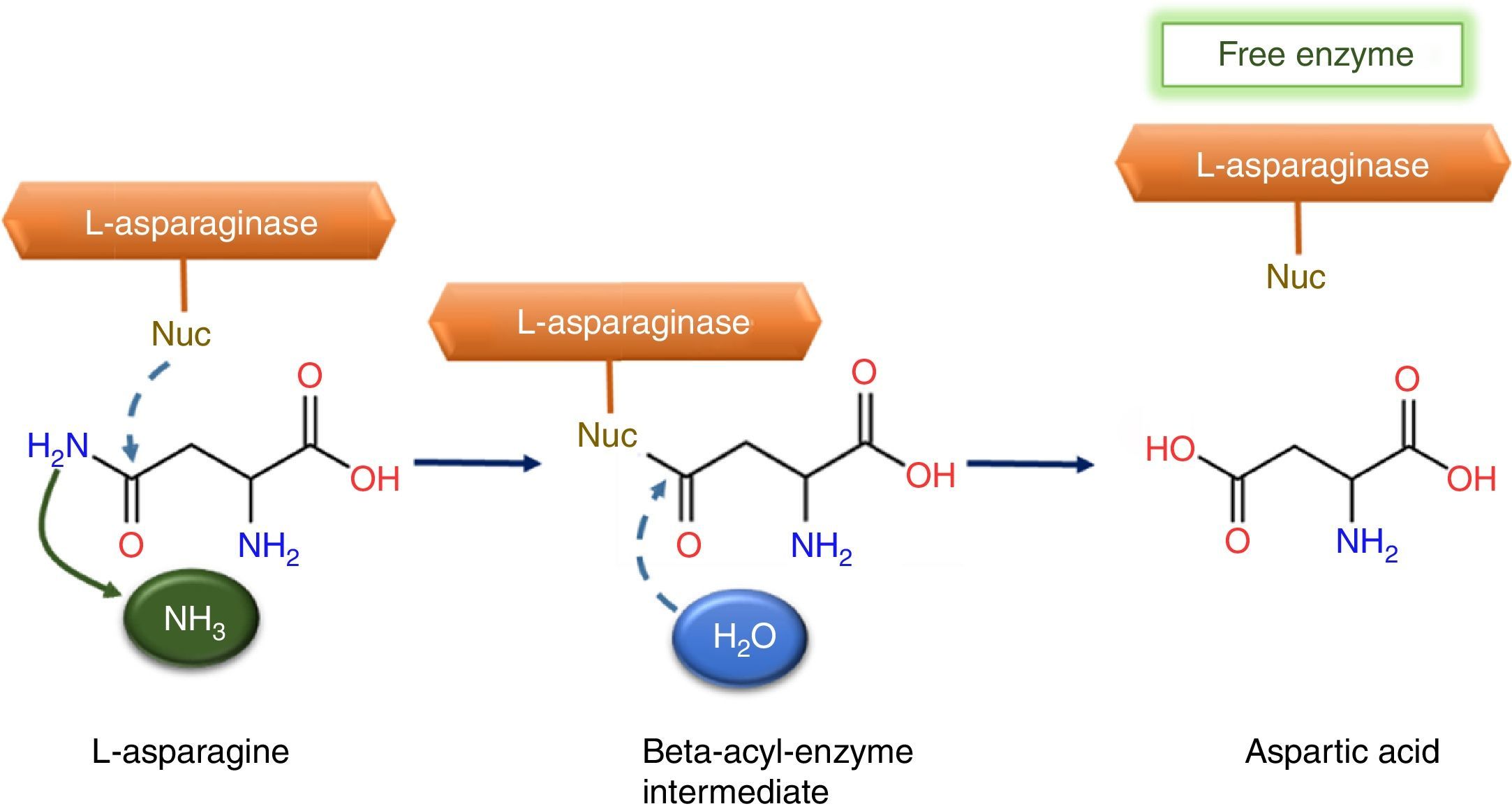
Reactions and mechanismThe hydrolysis process occurs in two steps through an intermediate: beta-acyl-enzyme (Fig. 1). In the first process step, the nucleophilic residue of the enzyme is activated by a strong base and attacks the amide carbon atom of l-asparagine (substrate), generating a product beta-acyl-enzyme intermediate. The second reaction step is an attack on the ester carbon made by a nucleophile activated by a water molecule.8
This mechanism is comparable to serine-proteases classic mechanism, whose activities depends of an amino acid group, classified as catalytic triads. This catalytic triads is composed by one nucleophilic amino acid, serine (Ser), one base, histidine (His) and one amino acid with acid characteristic, aspartic acid (Asp), all connected by hydrogen bonds.8
L-asparaginase has also capacity to catalase other reactions. For example, l-asparaginase produced by Serratia marcescens is able to hydrolase 5% of l-glutamine when compared with l-asparaginase hydrolysis. The same effect occurs to l-asparaginase produced by Escherichia coli and Erwinia chrysanthemi. Other microorganisms, such as Pseudomonas sp. and Acinetobacter glutaminasificans, synthetize l-asparaginase with equal asparaginase and glutaminase activity. In some cases, l-asparaginase starts the l-glutamine hydrolysis only after complete conversion of l-asparaginase in aspartic acid. Actually, l-glutamine is a competitive inhibitor of l-asparagine hydrolysis.12l-glutamine and l-asparagine hydrolysis are similar due to the structural similarity from both amino acids. Therefore, the largest part of microbial l-asparagine presents cross glutaminase activity, with some exceptions such as l-asparaginase produced by Wolinella succinogenes, which do not present l-glutaminase activity.12 Finally, l-asparaginase is also able to hydrolyze β-aspartyl peptide amide, however reaction yield is considerably low.13
l-asparaginase applicationsPharmaceutical industry: antineoplastic actionThe l-asparagine enzymatic hydrolysis in l-aspartate and ammonium was observed in a first time by Lang (1904),14 that detected l-asparaginase activity in bovine's tissues. Results of this researcher were confirmed by Furth and Friedmann (1910),15 that detected l-asparagine hydrolase in horse and pig organs, observing the same amount of l-asparaginase activity in both animals. Also, Clementi (1922)16 related that l-asparaginase in guinea pig serum, although antitumor activity of the enzyme was identified only some years later. In addition, Mashburn and Wriston (1964)17 demonstrated that l-asparaginase of E. coli had inhibitory capacity of tumors in rats. However, the large interest in enzyme started when Broome (1965)18 found that the regression lymphosarcoma transplants in rats treated with guinea pig serum was due to nutritional dependence on malignant cells of exogenous l-asparagine.
Considering its properties, l-asparaginase has been an important chemotherapeutic agent used for treatment of lymphoproliferative and lymphoma diseases. Particularly, it presents large importance in chemotherapeutic protocols for acute lymphoblastic leukemia (ALL) and Hodgkin's lymphomas.19
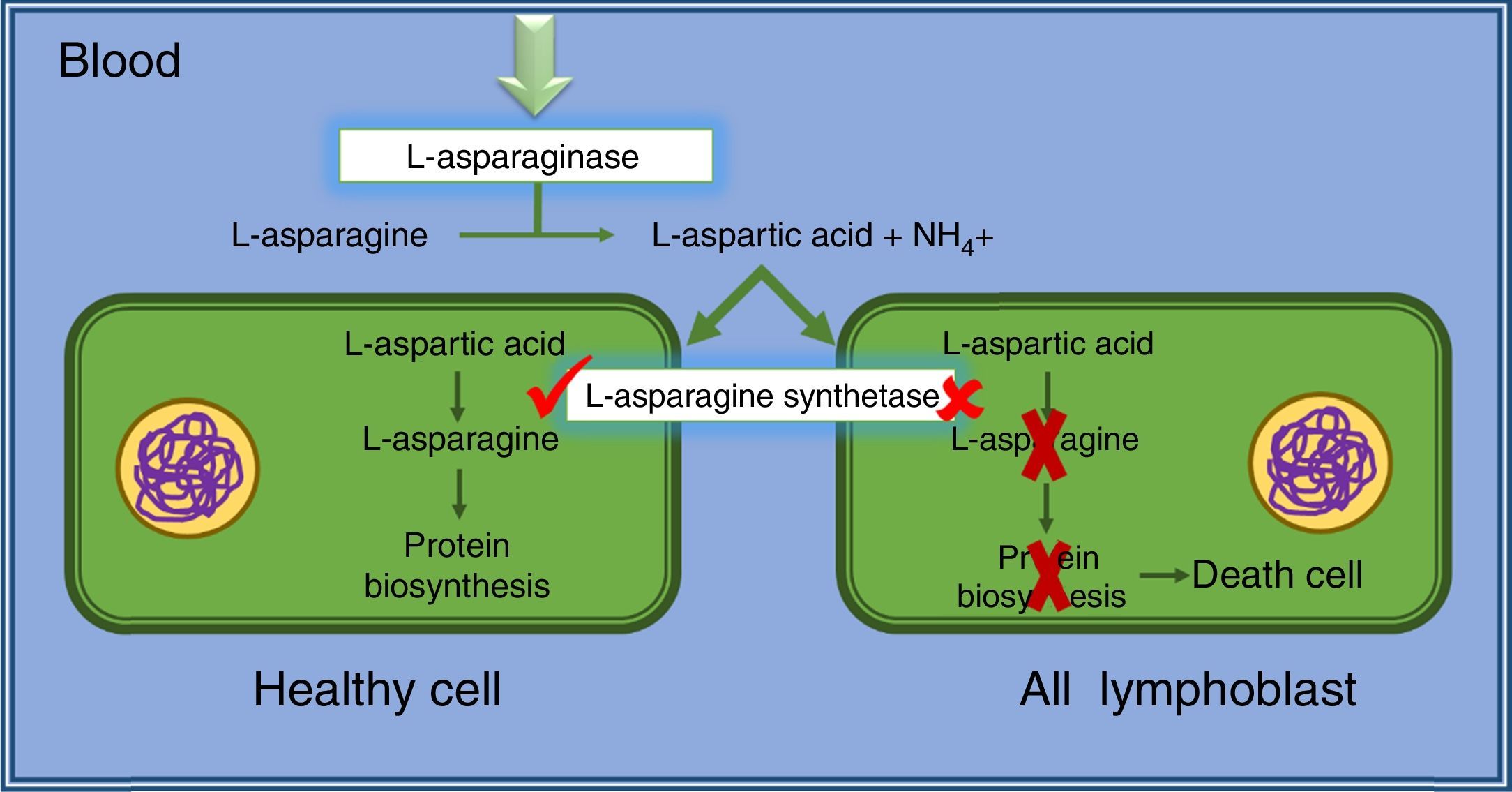
Cancer cells, mainly lymphatic cells, require high amount of asparagine for fast and malignant growth. In this way, cancer cells requires the amino acid from diet (blood serum) as well as amino acids produced by themselves. However, leukemic lymphoblasts and some others tumor cells do not have or present low quantity of l-asparagine synthetase used for l-asparagine syntheses. Thus, these malignant cells are dependent of asparagine from blood serum for their proliferation and survival.20,21
l-asparaginase hydrolyzes asparagine from blood serum, leading tumor cells to death by lacking of an essential factor for protein synthetases (p53-dependent apoptosis). However, healthy cells are not affected, because they are able to produce asparagine using l-asparagine synthetase present in enough quantities (Fig. 2). Considering these concepts, Fig. 2 schematically shows the antineoplastic action of l-asparaginase.
Food industry: acrylamide formationAcrylamide (C3H5NO) is also known as 2-propenamide, acrylic amide, ethylene carboxamide, propenamide, propanoic acid amide, monomer of acrylamide or acrylic acid amide, presenting 71.08g/mol of molecular mass.22 Several studies show that l-asparagine is the main amino acid responsible for acrylamide production in fried and baked foods when reducing sugars are condensed with a carbonyl source. This phenomenon does not occur in boiled food.23
Acrylamide formation has been quite studied in the last years. Zyzak et al. (2003)24 detected that the amide chain present in the acrylamide structure is provided from l-asparagine. Reagents (l-asparagine or reducing sugars) reduction or removal is one of the evaluated strategies for decreasing acrylamide quantity in foods. For l-asparagine reduction, several options have been investigated, such as: selection of vegetal species with lower level of l-asparagine in their composition; deletion of important enzymes for l-asparagine biosynthesis control by suppression of specific genes; acid hydrolysis of l-asparagine leading the formation of aspartic acid and ammonia; and acetylation process of l-asparagine to form N-acetyl-l-asparagine, preventing the formation of acrylamide from intermediate N-glycosides.22
In the study of Zyzak et al. (2003),24 authors confirmed that the use of l-asparaginase enzyme before frying or baking food process could reduce more than 99% acrylamide level in the processed final product. This is because the enzyme reduces more than 88% of the l-asparagine concentration from the initial feedstock. In last years, other works have dealt with this application of l-asparaginase, that can decrease the negative effects of acrylamide containing foods without impair their characteristics.3,25–28
Production by different microorganismsl-asparaginase is present in mammals, birds, plants, yeast, and a wide range of bacteria.10,29 Although l-asparaginase production is observed in animals, plants,12,30 the microorganisms are considered mainly source for l-asparagine synthesis.30,31
The production of this enzyme is mainly proceeded by submerged fermentation.30 Several researchers have studied the isolation of microbial strains that produce this important enzyme, such as Pseudomonas fluorescens,32Serratia marcescens,33Escherichia coli,34Erwinia carotovora,35Proteus vulgaris,36Saccharomyces cerevisiae, Karnatakensis Streptomyces, Streptomyces venezuelae and several genres of fungi as Aspergillus, Penicillium and Fusarium.37
Concerning to bacteria, the best producers of l-asparaginase are members of the Enterobacteriaceae family.30 For example, in pharmaceutical industry, this enzyme is produced mainly from bacteria such as Escherichia coli and Erwinia carotova, (also known as Erwinia chrysanthemi), generally used for leukemia and lymphoma treatment.4 However, most of these treatments can result in immunological sensitization (hypersensitivity) and immune inactivation in patients that receive bacterial enzymes.5 Another issue is that glutaminase activity generated by these enzymes can cause secondary effects such as allergic reaction, nausea, pancreatitis, diabetes and coagulation abnormalities.8,38 Also, most of asparaginases has low stability and catalytic activity, presenting only active in a narrow pH range.39
Currently, l-asparaginases from E. coli and Erwinia chrysanthemi (synonymous of Erwinia carotovora) are the only preparation available for medical use.12l-asparaginase from E. coli produces two types of enzyme, l-asparaginase I (EC1), found in the cytoplasm and l-asparaginase II (EC2), with periplasmic origin.40 However, only the second one has anti-cancer activity.41 Some studies describes EC1 as a constitutive enzyme and EC2 as secreted only as a response to exposure to low concentrations of nitrogen.8 EC2 has an estimated molecular weight of 141kDa and its kM is about 12.5μM, meaning a high affinity for substrate.42 Its half-life is around 1.24±0.17 days and its optimum pH and temperature are 7–8 and 37°C, respectively.12,42
As an alternative for treatment of patients allergic to l-asparaginase from E. coli, l-asparaginase from E. chrysanthemi (ErA) is used. It has half-life of 0.6±0.13 days, a kM of 18μM, molecular mass about 140 and 150kDa, optimal pH 8 and 50°C as optimal temperature.43,44 The difference between its kM and that one from E. coli's l-aspraginase is because glutaminase activity of ErA is higher.42,45
In recent years, different studies were developed aiming to find this enzyme with improved characteristics compared to l-asparaginase from E. coli, with economically viable production as well as causing minimal collateral effects. Searching from different l-asparagine sources, specifically eukaryotic microorganisms, can lead to enzymes with less adverse effects and different features, which are advantageous for its application.46
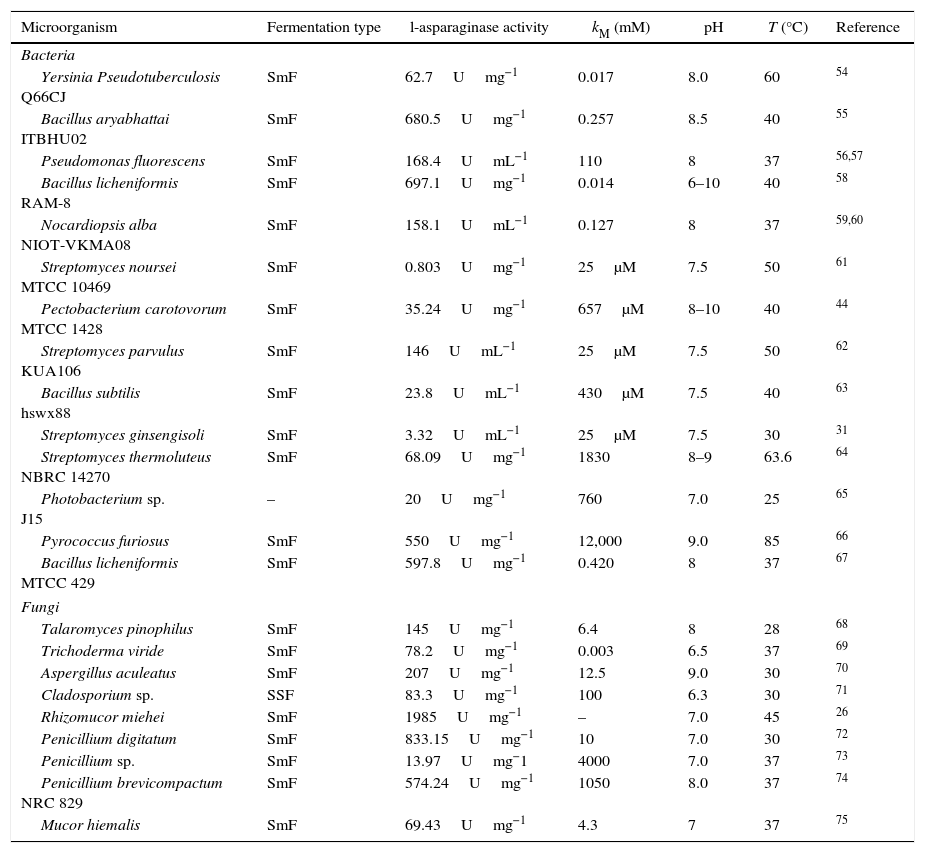
In the last years, eukaryotic fungi have been investigated as l-asparaginase source.47 For l-asparaginase production by fungi, the genera Aspergillus, Penicillium and Fusarium have been studied.6 Currently, fungal recombinant l-asparaginase from Aspergillus oryzae and Aspergillus niger has already been used in food industry for reduction of acrylamide formation in some foods.28 Moreover, authors have reported positive results by using endophytic fungi of the genus Colletotrichum, Eupenicillium, Talaromyces.48,49 Also, positive asparaginolytic activity were also shown by researchers that used fungi isolated from marine environments, endophytes seaweed, of genera Alternaria, Chaetomium, Cladosporium, Colletotrichum, Curvularia, Nigrospora, Paecilomyces, Phaeotrichoconis, Phoma and Pithomyces.50 Within this context, Table 1 present some works related to l-asparaginase production by bacteria and fungi.
Recent studies about l-asparaginase production by bacteria and eukaryotic fungus.
| Microorganism | Fermentation type | l-asparaginase activity | kM (mM) | pH | T (°C) | Reference |
|---|---|---|---|---|---|---|
| Bacteria | ||||||
| Yersinia Pseudotuberculosis Q66CJ | SmF | 62.7Umg−1 | 0.017 | 8.0 | 60 | 54 |
| Bacillus aryabhattai ITBHU02 | SmF | 680.5Umg−1 | 0.257 | 8.5 | 40 | 55 |
| Pseudomonas fluorescens | SmF | 168.4UmL−1 | 110 | 8 | 37 | 56,57 |
| Bacillus licheniformis RAM-8 | SmF | 697.1Umg−1 | 0.014 | 6–10 | 40 | 58 |
| Nocardiopsis alba NIOT-VKMA08 | SmF | 158.1UmL−1 | 0.127 | 8 | 37 | 59,60 |
| Streptomyces noursei MTCC 10469 | SmF | 0.803Umg−1 | 25μM | 7.5 | 50 | 61 |
| Pectobacterium carotovorum MTCC 1428 | SmF | 35.24Umg−1 | 657μM | 8–10 | 40 | 44 |
| Streptomyces parvulus KUA106 | SmF | 146UmL−1 | 25μM | 7.5 | 50 | 62 |
| Bacillus subtilis hswx88 | SmF | 23.8UmL−1 | 430μM | 7.5 | 40 | 63 |
| Streptomyces ginsengisoli | SmF | 3.32UmL−1 | 25μM | 7.5 | 30 | 31 |
| Streptomyces thermoluteus NBRC 14270 | SmF | 68.09Umg−1 | 1830 | 8–9 | 63.6 | 64 |
| Photobacterium sp. J15 | – | 20Umg−1 | 760 | 7.0 | 25 | 65 |
| Pyrococcus furiosus | SmF | 550Umg−1 | 12,000 | 9.0 | 85 | 66 |
| Bacillus licheniformis MTCC 429 | SmF | 597.8Umg−1 | 0.420 | 8 | 37 | 67 |
| Fungi | ||||||
| Talaromyces pinophilus | SmF | 145Umg−1 | 6.4 | 8 | 28 | 68 |
| Trichoderma viride | SmF | 78.2Umg−1 | 0.003 | 6.5 | 37 | 69 |
| Aspergillus aculeatus | SmF | 207Umg−1 | 12.5 | 9.0 | 30 | 70 |
| Cladosporium sp. | SSF | 83.3Umg−1 | 100 | 6.3 | 30 | 71 |
| Rhizomucor miehei | SmF | 1985Umg−1 | – | 7.0 | 45 | 26 |
| Penicillium digitatum | SmF | 833.15Umg−1 | 10 | 7.0 | 30 | 72 |
| Penicillium sp. | SmF | 13.97Umg−1 | 4000 | 7.0 | 37 | 73 |
| Penicillium brevicompactum NRC 829 | SmF | 574.24Umg−1 | 1050 | 8.0 | 37 | 74 |
| Mucor hiemalis | SmF | 69.43Umg−1 | 4.3 | 7 | 37 | 75 |
U, international units for enzyme activity; SmF, submerged fermentation; SSF, solid-state fermentation.
Also, several studies have shown that Aspergillus genus is available to produce significant amounts of l-asparaginase. For example, Sarquis et al. (2004)37 presented filamentous fungi like Aspergillus tamarii and Aspergillus terreus as producers of l-asparaginase by submerged fermentation, resulting in 38U/L and 58.8U/L, respectively. Authors concluded that enzyme production was regulated by the nitrogen source. Moreover, Balasubramanian et al. (2012),51 in a screening study of l-asparaginase producers, reported that Aspergillus terreus was able to produce 9.3U/mL of enzyme. In other study of culture conditions optimization (temperature 35°C, initial pH 6.3, inoculum size 1% (v/v), agitation rate 140rpm, and incubation time 58.5h), Gurunathan and Sahadevan (2012)52 reported l-asparaginase production of Aspergillus terreus by submerged fermentation, reaching production of 44.38U/mL. In another optimization project, but by using Aspergillus niger, Anjum Zia et al. (2013)53 verified a l-asparaginase activity of 2.83U/ml under submerged fermentation. In that work, authors observed that glucose concentrations above 1% inhibited the enzyme production.
Another interesting technique for asparaginase production is the solid-state fermentation, that allows the use of agroindustrial residues as substrate or support.30 Within this context, recently, Dias et al. (2015)76 presented the use of different organic residues (wheat bran, soybean meal, cottonseed meal and orange peel), evaluating the production of l-asparaginase from Aspergillus niger. The maximum enzyme production (94.21U/g) was obtained after 96h of fermentation using mixture of wheat bran (1/3), soybean meal (1/3) and cottonseed meal (1/3).
In addition, yeasts have been becoming an interesting alternative for l-asparaginase production. Some investigations have reported, e.g., the use of the yeasts Pichia polymorpha and Candida utilis, for this enzyme production. l-asparaginase of P. polymorpha showed a kM value of 13.7mM and optimum pH 6.7.77 On the other hand, the enzyme produced by C. utilis has kM value of 77μM.78 In a recent study, Soler et al. (2015)7 tested 43 different strains of yeasts, verifying that only strains of Issatchenkia orientalis and Rhodotorula glutinis showed periplasmic l-asparaginase activity when growth in liquid CD-m. Also, Sajitha et al. (2015)79 presented an investigation by using an expression study of gene ansB of E. coli, which encodes l-asparaginase enzyme, in yeast. This study was developed on a new protein expression system based on the yeast Pichia pastoris. The resulting enzyme was extracellular and showed activity of 2.5U/mL at optimum temperature of 37°C. By these results, authors concluded that this new system of expression could be effective for production of humanized enzyme by glycosylation patterns similar to mammals.79
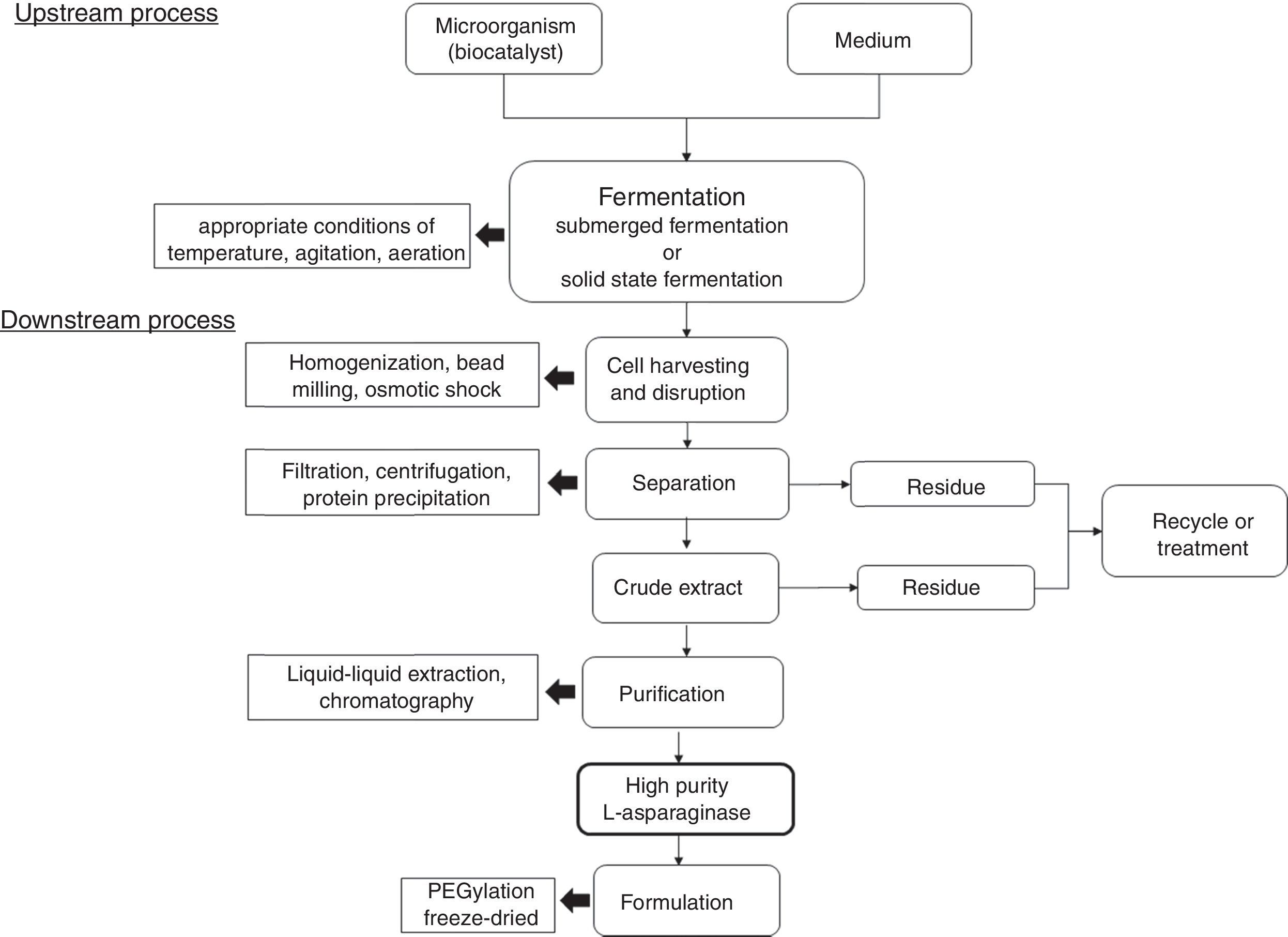
Industrial production of l-asparaginaseFor industrial production of l-asparaginase, many factors need to be taken into account aiming to a process with higher yield and economic viability. For example, type and concentration of carbon and nitrogen sources, pH, aeration, temperature, fermentation time, and, mainly, the microbial agent, have great influence in the process.52,80 As previously reported, several microorganisms are presented as l-asparaginase producers; however, bacteria E. coli and E. chrysanthemi are the current main microbial agents for industrial-scale production in pharmaceutical area, while the fungus Aspergillus oryzae is the most used in food industry.30,81Fig. 3 shows an schematic representation for an industrial process for l-asparaginase production.
Different types of culture medium have been explored for l-asparaginase production. However, carbon source and inductor (nitrogen source) are the more influencing components in the medium. For example, several studies have demonstrated that best inductors for reaching high yields are l-asparagina; 82,83L-glutamine83 and L-proline,37,84–86 and the most common carbon source is glucose, in addition to alternative sources such as starch87 and maltose.82,88
l-asparaginase extraction and purification are other pivotal steps for the production of this enzyme. For example, for pharmaceutical application, high level of purification is needed. Other important concern is that most of microorganisms produce intracellular l-asparaginases, with few exceptions.
Different methods for downstream process are reported such as centrifugation, filtration, liquid–liquid extraction, chromatography and protein precipitation. Regarding industrial production, protein precipitation is an advantageous technique due to features such as ease scale up, with simple equipment requirements, low costs and possibility to use large number of precipitants. Additionally, the precipitant agent can be recycled in the final process, reducing the environmental impact associated to its disposal. Actually, precipitation is one of the first steps in the downstream process and it is usually combined with traditional techniques to enhance biomolecules purification and process yield.30 Also, other highlighted step used for high degree of enzyme purity is chromatography, such as ionic exchange, affinity chromatography, size exclusion, and gel filtration.71,89 For example, Lopes et al. (2015)30 reported that the most used purification steps are gel filtration and ion exchange chromatography, which often are preceded by precipitation with (NH4)2SO4. According to authors, considering 50–80% of the total production costs of proteins are provided by extraction and purification steps, optimized downstream can result in significant economic viability.
For pharmaceutical applications, a step of conjugation with polyethylene glycol (PEG), or PEGylation, has been used to improve the compound biostability and bioavailability, influencing in pharmacokinetics and pharmacodynamics properties of the enzyme and reducing the immunological response against this biomolecule.90 However, this step of PEGylation can also result in loss of biological activity of the conjugate compared with the native enzyme.91 On the other hand, this step is not required in food industry application.
Freeze-drying is other important step to improve the long and short-term storage of the enzyme l-asparaginase formulation. It can prevent most water-related reactions by sublimating water from the frozen product under vacuum, also allowing sterile drying without heating or chemical sterilization. However, problems related to cold denaturation, freeze denaturation and osmotic pressure increase due to dehydration and cryoconcentration.92
Conclusion and future recommendationsl-asparaginase is an interesting enzyme with important applications in pharmaceutical and food industry. However, its use in these industrial sectors requires some specific properties, as security for use by humans. As chemotherapeutic agent, an efficient action is required, in addition to reduced adverse effects, such as hypersensitivity and immune inactivation. In food, this enzyme helps to decrease the concentrations of acrylamide (carcinogenic compound for humans) formed in the process, maintaining their nutritional and sensory properties. Thus, research work seeking for new l-asparaginases, mainly produced by eukaryotic microorganisms, instead of bacterial enzymes currently used, has potential to obtain new enzymes with desirable properties. These discoveries have to be followed by an intensive work aiming to increase the process productivity to enable and extend the use of this enzyme, mainly in food industries. Taking this into account, tools of molecular biology are useful, although even a more traditional work of biochemical engineering have not been extensively related in literature, indicating needs of further works such as different process configuration evaluation, as well as use of bioreactors options.
The authors would like to thank FAPESP (Process 2014/27055-2), CNPq, CAPES and Programa Estudantes-Convênio de Pós-Graduação – PEC-PG, da CAPES/CNPq – Brazil for financial support, and the Coleção de Culturas Tropical Fundação André Tosello.