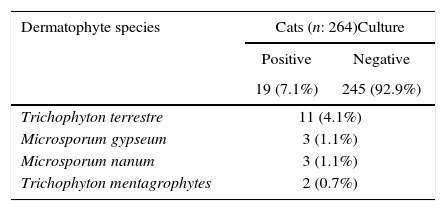
The Van cat is a domestic landrace found in the Van province of eastern Turkey. In this study, we aimed to determine the seasonal carriage of dermatophytes in Van cats without clinical lesions. A total of 264 hair specimens were collected from clinically healthy cats in and around the Van Province. Of these samples, 30.3% were obtained in spring, 30.6% in summer, 16.6% in autumn, and 22.3% in winter; 45.1% of samples were from male cats and the rest from female ones. Of the studied cats, 118 were younger than 1 year, 78 were 1–3 years old, and 68 were older than 3 years. The specimens were subjected to direct microscopic examination with 15% potassium hydroxide and cultured on Sabouraud dextrose agar and dermatophyte test medium supplemented with cycloheximide and chloramphenicol. Dermatophyte identification was carried out based on macroscopic and microscopic colony morphology, urease activities, in vitro hair perforation test, growth at 37°C, and pigmentation on corn meal agar. Dermatophytes were isolated from 19 (7.1%) of the 264 specimens examined. The most frequently isolated fungi were Trichophyton terrestre (4.1%), followed by Microsporum gypseum (1.1%), M. nanum (1.1%), and T. mentagrophytes (0.7%), and these fungi may represent a health risk for humans in contact with clinically healthy Van cats. M. canis was not isolated from any of the specimens. Our results show no significant (p>0.05) association between carriage of dermatophytes and the gender of cats. The carriage rate of dermatophytes was high in spring and winter, and the only possible risk factor for infection was age of the animal.
The Van cat originates from the Eastern Anatolia region of Turkey, and should not be confused with the Turkish Angora cat. One of the characteristic features of the Van cat is eye color and is accordingly classified into three groups: both eyes blue; both eyes amber (yellow and its tones); and one eye blue and the other amber (dischromatopsy). Blue-eyed Van cats usually display turquoise blue, while amber eye color displays tonal variation such as amber, light amber, yellow, and green almond. Although very rare, brown eye color can also be seen. Van cats love to swim and play with water, and are the only cat species to do so. This cat is a semi-long-haired breed that conveniently sheds its hair during the spring and summer only to be replaced by a short velvety coat. Europeans have been introducing the Van cat to the rest of the world since the 1950s.1–3
Dermatophytosis (ringworm) is an infection of keratinized structures, such as nails, hair, and the stratum corneum of the skin, and is the most common fungal disease found in animals.4 Dermatophytes are fungi that cause dermatophytosis and are classified in three genera: Trichophyton (T.), Microsporum (M.), and Epidermophyton (E.). While only a few species belong to the Trichophyton genus, there are approximately 40 accepted species in the genus Epidermophyton; and the Microsporum species usually cause dermatophytosis in domestic animals. Dermatophytes are usually divided into three ecological groups depending on their main natural host or habitat as anthropophilic (humans), zoophilic (animals), or geophilic (soil).5–7
As its name suggests, feline dermatophytosis is an infection of the superficial keratinized tissues in cats, and its most common cause is M. canis fungus. Two other species, M. gypseum and T. mentagrophytes, can also cause dermatophytosis in the cat, albeit with a lower incidence.6,8,9M. canis infections in cats are of major importance as cats serve as a reservoir of this zoonosis, which is considered highly contagious and potentially pathogenic in humans, especially children.10 Dermatophyte transmission occurs upon contact with infected hair or fomites (clippers, brushes), or from the environment (spores in soil), and in rural areas, up to 80% of all human fungal skin infections may be of animal origin.11
It is important to note that canine and feline ringworm infections differ clinically. Canine infections generally produce lesions, whereas clinical signs may not be evident in cats, and it is possible to culture dermatophytes from clinically healthy cats that act as carriers of conidia but are not themselves infected.6,12 While some reports on the prevalence of dermatophytic fungi are based on samples taken only from animals showing ringworm lesions,13,14 others studies using random population samples taken from animals with no lesions reveal different results.15–17
Further, the incidence of dermatophytosis is related to geographic region, climate, and animal husbandry techniques, and is frequently found in young, stray, sick, or otherwise debilitated animals.4 The asymptomatic carrier state is the most important variable in the transmission of the disease among cats and from cats to humans.18 To the best of our knowledge, no previous studies have assessed the asymptomatic carrier state in Van cats. Therefore, the aim of this study was to determine the seasonal carriage of dermatophytes in such pets without overt clinical lesions.
Materials and methodsGeographical characteristicsVan Province is the 18th largest province of Turkey and lies in the Eastern Anatolia region with a population of over 1 million. It is located between northern latitudes of 38°28′ and eastern longitudes of 43°20′, is 19,069km2 in area, and lies between Lake Van and the Iranian border. This area faces extremes of temperature during summer and winter,19,20 has a dry continental climate with cool snowy winters and warm dry summers. Rainfall primarily occurs during spring and autumn, and annual average rainfall is 384mm. The region's average maximum temperature during winter and summer are −2.2°C and 20.4°C, respectively, and the relative humidity is approximately 59%.19
SamplesA total of 264 Van cats from different parts of the Van Province, all of which were owned and fed at home, were selected for inclusion in the study. Complete body surface of the cats was thoroughly examined for dermatological lesions, and only those animals that were free of cutaneous lesions (Fig. 1) and had not received antifungal therapy in the preceding 3 months were included in the study. All cats were domesticated and housed indoor or in cages. After cleaning the selected area with 70% alcohol, a sterile brush was used to take hair samples from the head, neck, trunk, and tail. Each sample was placed in a plastic storage tube and appropriately labeled. The samples were then transferred to the Department of Microbiology, Faculty of Veterinary Medicine, Yuzuncu Yıl University, Van, Turkey.
Of these 264 samples, 80 (30.3%) were obtained in spring, 81 (30.6%) in summer, 44 (16.6%) in autumn, and 59 (22.3%) in winter; 119 (45.1%) were from male cats and 145 (54.9%) were from female cats. A total of 118 (44.7%) cats were younger than 1 year, 78 (29.5%) were 1–3 years old, and 68 (25.7%) were older than 3 years.
Culture and identificationEach sample was divided into two portions, one portion was used for direct microscopic examination and the other was used for culture. Direct microscopic examination was performed after the sample was placed on a slide and 30μL of 15% potassium hydroxide (KOH) added. After 5min., the wet preparation was carefully examined under both low (10×) and high (40×) power objectives to observe the fungal elements and diagnostic morphology.
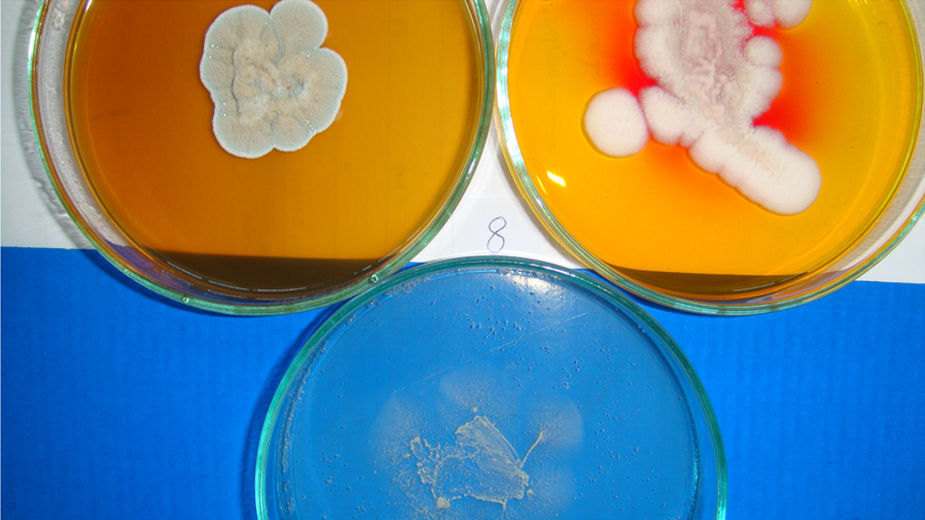
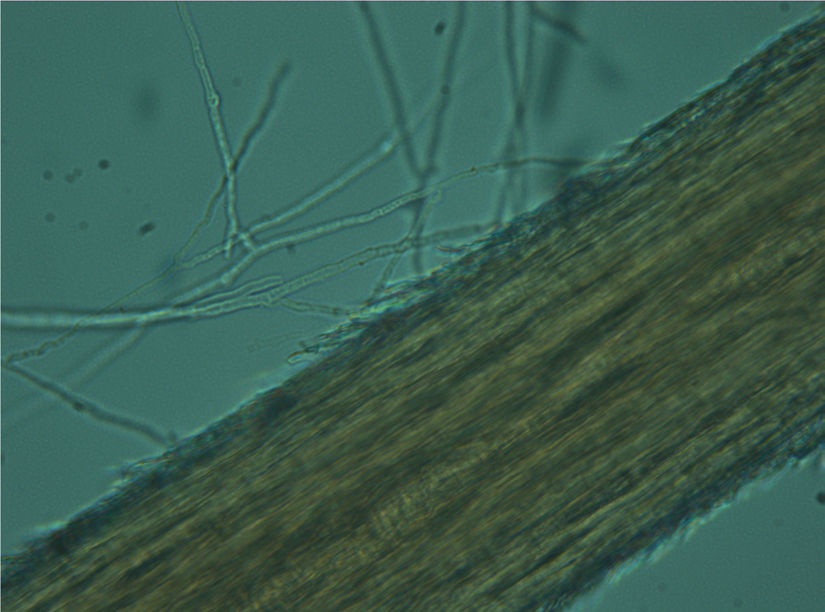
The samples were cultured on Sabouraud dextrose agar (SDA; DM200, Mast Diagnostics, Merseyside, UK) and dermatophyte test medium (DTM; 7265A, Acumedia, Michigan, USA) supplemented with chloramphenicol (0.05mg/mL; 220551, Calbiochem, Darmstadt, Germany) and cycloheximide (0.5mg/mL; C7698, Sigma-Aldrich, Steinheim, Germany). Petri dishes were incubated at 25°C for 5 weeks. The isolates were examined macroscopically and microscopically after staining with lactophenol cotton blue (LFPM; 1.13741, Merck, Germany) wet mount technique. Dermatophyte species were identified based on gross and microscopic morphology (Figs. 2–4). In addition, pigment production on corn meal agar (CMA; 7350A, Acumedia, Michigan, USA), and urease activity on urea agar base (CM52; Oxoid, Basingstoke, UK), and growth at 37°C on SDA were assessed, apart from in vitro hair perforation tests.5,7,21,22
All data were analyzed using the MINITAB ver. 17 demo statistical analysis software for Windows. Results were considered statistically significant at p<0.05 or 0.01.
ResultsDermatophytes were isolated from 19 of the 264 (7.1%) samples. The most frequently isolated fungi were: T. terrestre (4.1%), followed by M. gypseum (1.1%), M. nanum (1.1%), and T. mentagrophytes (0.7%). M. canis was not isolated from clinically healthy Van cats, and T. terrestre was the most commonly (57.8%) isolated species across all seasons (Tables 1 and 2). In addition, some saprophytic fungi (Alternaria spp., Chrysosporium spp., Ulocladium spp., Fusarium spp., Mucor spp., and Penicillium spp.) were also isolated. No statistically significant (p>0.05) association was found between carriage of dermatophytes and gender.
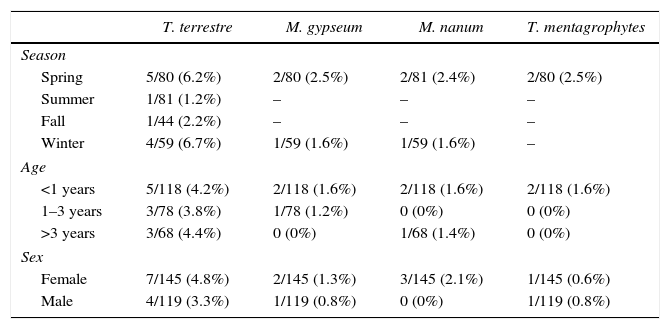
Prevalence of dermatophytes isolated from the hair samples of asymptomatic Van cats according to season, age, and gender.
| T. terrestre | M. gypseum | M. nanum | T. mentagrophytes | |
|---|---|---|---|---|
| Season | ||||
| Spring | 5/80 (6.2%) | 2/80 (2.5%) | 2/81 (2.4%) | 2/80 (2.5%) |
| Summer | 1/81 (1.2%) | – | – | – |
| Fall | 1/44 (2.2%) | – | – | – |
| Winter | 4/59 (6.7%) | 1/59 (1.6%) | 1/59 (1.6%) | – |
| Age | ||||
| <1 years | 5/118 (4.2%) | 2/118 (1.6%) | 2/118 (1.6%) | 2/118 (1.6%) |
| 1–3 years | 3/78 (3.8%) | 1/78 (1.2%) | 0 (0%) | 0 (0%) |
| >3 years | 3/68 (4.4%) | 0 (0%) | 1/68 (1.4%) | 0 (0%) |
| Sex | ||||
| Female | 7/145 (4.8%) | 2/145 (1.3%) | 3/145 (2.1%) | 1/145 (0.6%) |
| Male | 4/119 (3.3%) | 1/119 (0.8%) | 0 (0%) | 1/119 (0.8%) |
Dermatophytosis constitutes a major public health problem in several countries and the most common factors affecting the distribution and transmission of dermatophyte infections are animal contact, general hygiene, and climatic conditions.6,11,14,23 Information on the asymptomatic carriage of dermatophytes in pets is important for reducing human transmission of zoophilic fungal infections. A study to detect dermatophyte carriage in dogs, conducted in Adana, Turkey, stated that a detailed survey of keratinophilic fungi, with particular emphasis on cats, was required to precisely determine dermatophyte microbiota.16 Therefore, the aim of the current study was to determine the prevalence and seasonal carriage of dermatophytes among Van cats without clinical lesions.
The Van cat has a semi-long-haired coat that is conveniently shed during spring and summer, and replaced by a short velvety coat.3 These cats are much loved by the people, especially in the Van Province and other regions of Turkey, and are generally fed in homes by their owners. As these cats live in close contact with humans, they might be a potential hazard for human health if they passively carry dermatophytic fungi. We isolated many species of dermatophytes from asymptomatic cats, and these animals could be considered as sources of some geophilic (T. terrestre and M. gypseum), anthropophilic (T. mentagrophytes), and zoophilic (M. nanum) dermatophytes. These results indicate that an infected cat is probably responsible for the dissemination of fungal material into its environment and its spread to other animals in contact with it.
Epidemiological studies on clinically healthy cats have shown that dermatophyte isolation rates range between 0.5% and 47.3%.15,24,25 In one study, M. gypseum (0.5%), T. mentagrophytes (1.7%), and M. canis (47.3%) were cultured from asymptomatic stray cats.24 In another study, 100 asymptomatic stray cats from the north of Iran were examined to determine cutaneous fungal microbiota and this study isolated M. gypseum (3%) and T. mentagrophytes (1%), but no M. canis was isolated.25 Contrarily, Alpun and Ozgur15 reported that M. canis was cultured in 11% of samples obtained from clinically healthy cats in Istanbul, Turkey. We isolated pathogenic dermatophytes (M. nanum, M. gypseum, and T. mentagrophytes) from clinically healthy Van cats, which concurs with other reports on the prevalence of T. mentagrophytes and M. gypseum.24,25 With respect to the prevalence of M. canis, the results of the present study are in complete agreement with those of Shokohi and Naseri.25 Taken together, these results indicate that the prevalence of dermatophytes varies, and depends on season, temperature, and geographical area.8
M. nanum is a zoophilic species that is more common in pigs and is a rare cause of ringworm in humans. T. mentagrophytes is another zoophilic species that can infect humans. The major animal hosts of M. nanum and T. mentagrophytes are different pets and mammals. In Adana, Turkey, T. mentagrophytes is the most common dermatophyte species isolated from the scalp of asymptomatic children (carrier state) and probably indicates close contact with asymptomatic pets, especially cats and dogs.26 It is also important to add that these dermatophyte species are usually acquired directly from the soil, rather than from another host. However, the isolation of M. nanum and T. mentagrophytes from healthy Van cats is of interest to pet owners who must be informed about potential dermatophyte infections, as well as the risks associated with a carrier state.16
M. gypseum is a geophilic dermatophyte with a variable isolation rate from domestic animals. Ranganathan et al.27 reported a frequency of isolation of 8% from 100 healthy cats in India, while other surveys from Iran reported 7.7% prevalence from different animals, including cats,28 and 3% prevalence in asymptomatic stray cats.29 Of the 130 hair coat samples from healthy wild cats examined in another study, only two (1.5%) were positive for M. gypseum.17 Although M. gypseum is a geophilic dermatophyte, reports from Japan,30 Italy,31 and Egypt,32 reaffirm the existence of this mold in human skin lesions. In the present study, three (1.1%) of the 264 samples showed presence of M. gypseum, which denotes the importance of Van cats as asymptomatic carriers and a possible source of human infection.
M. canis is the major dermatophyte species that causes Tinea capitis in humans and is prevalent in most of Europe, Australia, South America, and in parts of Africa.33 Animals often serve as the source of human dermatophytic infections, and cats are accepted principal reservoirs of M. canis.8,9,13 Indeed, a study of 111 cases of human dermatophytosis due to M. canis according to their origin of infection showed that it was transmitted by cats in 91 (81.9%) of the cases.34 In a separate study to determine the prevalence and carriage of M. canis in cats, samples from 632 animals were examined by culture and by Wood's light examination which showed a carriage rate of 2.1%.18 In contrast, we did not isolate M. canis from the Van cats included in our study, and this observation concurs with a previous investigation.25 Our failure to isolate M. canis may either be due to small number of samples evaluated or because Van cats are not natural reservoirs of M. canis. To the best of our knowledge, this is the first report describing investigation of dermatophyte carriage of Van cats, but further studies should be conducted to determine other dermatophyte species that could be harbored by asymptomatic Van cats.
A variety of studies have discussed the seasonal distribution of dermatophyte species in cats.4,8,14 We report a relatively high isolation rate in spring and winter; however, no significant (p>0.05) association was found between season and isolation rate.
Some studies have indicated that the age of animals is related to dermatophyte infections, and that young animals, especially those aged less than one year, are more susceptible to dermatophytosis.4,8,14,21 In accordance with these findings, we show an isolation rate of 4.1% (11/264) from animals younger than one year, 1.5% (4/264) from those aged between 1 and 3 years and, 1.5% (4/264) from cats older than 3 years.
Several previous studies have not observed any correlation between gender and dermatophyte infections in cats and dogs,8,14 but Cafarchia et al.4 stated that male dogs were more frequently affected by dermatophytosis. In the present study, no significant association was found between carriage of dermatophytes and the gender of cats, even though females showed a higher isolation rate (4.9%, 13/264), compared to males (2.2%, 6/264). This could be related to the health of the cat, stress factors, number of spores, hygiene, and genetic predisposition.
Of the saprophytic fungi isolated, Alternaria spp., Chrysosporium spp., Ulocladium spp., Fusarium spp., and Mucor spp., are common to many environments; Aspergillus spp. may be found in food, while Penicillium spp. may be found on the ground22,35 and the cats could have acquired these saprophytes from these sources.
In conclusion, dermatophyte species were found in 19 (7.1%) of the 264 Van cats included in the study, but clinically healthy Van cats cannot be considered as a significant reservoir of M. canis. The isolation of T. terrestre, M. gypseum, M. nanum, and T. mentagrophytes clearly indicates that Van cats should be considered as a major source of human pathogenic dermatophytes, even if the animals are clinically asymptomatic. In addition, we observed no significant (p>0.05) association between carriage of dermatophytes and gender of Van cats. The carriage rate of dermatophytes was high in spring and winter, and the age might be a risk factor.
Conflicts of interestThe authors declare no conflicts of interest.