The kinetics of an extracellular β-d-fructofuranosidase fructohydrolase production by Saccharomyces cerevisiae in a chemically defined medium, i.e., sucrose peptone agar yeast extract at pH 6, was investigated. The wild-type was treated with a chemical mutagen, methyl methane sulfonate. Among the six mutants isolated, methyl methane sulfonate-V was found to be a better enzyme producing strain (52±2.4aU/mL). The maximum production (74±3.1aU/mL) was accomplished after at 48h (68±2.7amg/mL protein). The mutants were stabilized at low levels of 5-fluoro-cytocine and the viable ones were further processed for optimization of cultural conditions and nutritional requirements. The sucrose concentration, incubation period and pH were optimized to be 30g/L, 28°C, and 6.5, respectively. The methyl methane sulfonate-V exhibited an improvement of over 10 folds in enzyme production when 5g/L ammonium sulfate was used as a nitrogen source. Thin layer chromatography and high-performance liquid chromatography analysis illustrated the optimal enzyme activity supported by the higher rate of hydrolysis of sucrose into monosaccharides, particularly α-d-glucose and β-d-fructose. The values for Qp (2±0.12cU/mL/h) and Yp/s (4±1.24bU/g) of the mutant were considerably increased in comparison with other yeast strains (both isolates and viable mutants). The mutant could be exploited for enzyme production over a wider temperature range (26–34°C), with significantly high enzyme activity (LSD 0.048, HS) at the optimal temperature.
The enzyme β-d-fructofuranosidase fructohydrolase (FFH, EC 3.2.1.26) cleaves α-1,4 glycosidic linkage between α-d-glucose and β-d-fructose moiety in sucrose to release monosaccharide units by hydrolysis. It has industrial applications particularly soft-centre confectionary production and the fermentation of cane molasses into ethanol.1 Although Saccharomyces cerevisiae, because of its typical higher sucrose fermenting ability, has been a preferred organism for synthesis of FFH, the pursuit for future strain selection is based on factors including strain stability, yield, initial pH, and fermentation period. Other factors notably temperature tolerance, oxygen supply, and shear stress are also worth-mentioning.2,3 Sucrose has been a useful carbon source for enzyme synthesis. Glucose availability for under-cultivation yeast cultures is largely dependent on efficient hydrolysis of sucrose. The optimization of nature and type of potential sources could provide in-house basis for an efficient batch-culture process.4 The fermentation efficiency of yeast strains at relatively higher incubation temperature (>35°C) remains low because of the increased membrane fluidity that changes the type, chain length and composition of fatty acids. Growth temperature rise generally supports the biosynthesis of some heat-shock proteins that are implicated in thermal cross tolerance of different organisms, prominently non-filamentous fungi like yeasts.5,6
In the past, only few studies have revealed the mechanics of catabolic repression. Although enzyme expression is regulated in various yeast cultures, a hyper-producing viable strain with consistent activity is not developed.7 The classical strain improvement methods like ultraviolet (UV) irradiation and use of alkylating agents such as N-nitroso guanidine (NG) to obtain positive mutants with high enzyme productivity have demonstrated some success. The selection and screening of better survivors have also been found critical.8 More work is required to increase enzyme activity by an economical batch process. The use of mild chemical mutagens like methyl methane sulfonate (MMS) to increase frequency of mutation has not been studied in detail. Therefore, efforts are still needed to develop a yeast mutant with improved substrate utilization and rate of enzyme biosynthesis. In this study, we assessed a wild-type of S. cerevisiae IIB-IX for improved FFH enzyme production after randomly induced mutagenesis. The activation enthalpy and entropy for FFH activity and its inactivation were analyzed for understanding of the growth kinetics. The idea involved in enzyme production and subsequent thermal activation of the system was also focussed.
Materials and methodsOrganism, chemically-induced mutagenesis and culture maintenanceS. cerevisiae strain, IIB-IX, isolated from soil was treated with MMS to induce mutagenesis. Five millilitre of the mutagen (concentration: 50–300μg/mL) was added to the centrifuge tubes containing 5mL of the yeast cell suspension. The tubes were placed at an ambient temperature (25°C) for different intervals (ranging from 15 to 90min). This suspension was centrifuged at 5500×g for 20min. The supernatant containing mutagen was carefully decanted and then 6mL of sterile saline water containing NaCl 0.085%, yeast extract 0.05%, and polypeptone 0.025% was dispensed in all tubes containing cell pellet as reported by Das and Nandi.9 The suspension (0.1mL) was spread on the petri-plates containing 10g/L sucrose, 15g/L agar, 2.5g/L peptone, and 2g/L yeast extract with pH 6 (also known as SAPY medium). Bromocresol green dye (40mL/L of 3% dye in 70% ethanol) was then added and kept at 30°C. The yeast colonies, bearing pink colour zones, appeared in 24–36h, exhibited sucrose hydrolysis. These were compared with the control plates containing wild-type S. cerevisiae prepared in parallel. Later, the colonies were aseptically picked and inoculated to the agar slopes of the same medium. The chemicals and reagents used were procured from Sigma Chemicals, Inc. (USA).
Induction of 5-fluoro-cytocine resistance in viable mutant strainsThe treated cells of selected mutant were collected during the log phase. The cells were washed twice with sterile distilled water before plating on the SAPY medium. Different amounts of 5-fluoro-cytocine (0.01–0.1mg/mL) were added before the incubation. However, sucrose was replaced with fructose.10 The plates were incubated for 16–24h, and frequently sub-cultured. The vigorous growth of the yeast mutants was checked for stability during batch culture. The samples were collected periodically during inoculation and plated on the medium containing 5-fluoro-cytocine to select resistant strains. The resistant mutant cultures were screened and preserved in paraffin oil at 4°C.
Inoculum preparationThe inoculum was prepared with 50mL of SAPY medium (without agar) using 250mL sterile Erlenmeyer flasks. The medium was sterilized at 15psi (121°C) for 15min and seeded with a loopful of the yeast cells. The culture medium was incubated in a rotary shaking incubator (4043-Gallenkamp-JB, London, UK) at 28°C for 16–24h (160rpm). The suspension was containing 1.25×107CFU/mL after hemocytometer count (OD590nm∼1).
Fermentation procedureThe FFH enzyme production was carried out under shaking culture using 250mL Erlenmeyer flasks. The SAPY medium (without agar) at pH 6 was added to the sterile, cotton-plugged flasks and sterilized in an autoclave. After cooling to about 20°C, 1mL of the cell suspension was aseptically seeded into each flask containing the sterile medium. The flasks were incubated in a rotary shaker at 200rpm at 30°C for 48h. The batch-cultures were grown parallel in triplicates.
Analysis of biomass development and sugar consumptionThe dry biomass of selected yeast culture was determined after centrifugation at 6250×g, and then by transferring the broth in pre-weighed tubes at 20°C. The tubes were oven dried at 105°C for 2h. The residual sugar was assayed by dinitrosalicylic acid (DNS) method11 at 546nm wavelength using a UV/Vis scanning spectrophotometer (MVP153-CECIL-700, London, England).
FFH assayThe enzyme activity was determined following the procedure described by Myers et al.12 “One FFH unit is defined as the amount of enzyme, which releases 1μmol of inverted sugar in 1min at 20°C, and pH 4.5″. The tubes having 2.5mL of acetate buffer at pH 5.5 (50mM) along with 0.1mL of sucrose (300mM) were allowed to pre-incubate at 32°C for a period of 5min. Then 0.1mL of an appropriately diluted enzyme mixture was added and incubated for another 5min. Later, the assay mixture was kept in a water bath at 90°C for 5min and cooled at room temperature. The tube containing similar ingredients plus distilled water without enzyme was used as control. One millilitre of DNS reagent was dispensed into 1mL of the mixture. The tubes were again placed in boiling water for 5min. After cooling the reaction mixture to about 25°C, the final volume was adjusted to 10mL with distilled water. The percentage transmittance was measured at 546nm. The total protein content was measured using Bradford method.13
Chromatographic analysis of enzyme preparationThin layer chromatography (TLC) was employed for the qualitative analysis of FFH hydrolysis products. The pre-coated TLC plates (E-Merck) were allowed to spot the enzyme samples; n=2 per sample. The plates were developed in acetone: diethyl ether: water (7:3:1 by volume). The plates were sprayed thoroughly with 0.2% ninhydrin and allowed to dry at 70°C for 20min.14 The resulting sugars were quantitatively analyzed by a double beam HPLC (Perkins Elmer, USA). The universal column (C18, HPX-87H ion exchange, 400mm×118mm) was maintained at 25°C. The mobile phase (ethanol–butanol, 2:1) was set at a flow-rate of 0.42mL/min. The samples were identified using a refractive index detector (Turbochron-4 software).
Kinetic studyThe kinetic parameters were computed following the procedures described by Pirt.15 The volumetric rate for FFH production (Qp) was calculated from the plot between enzyme (U/mL) and also calculated ΔT (h), the product yield coefficient (Yp/s) from dP/dS, the specific product yield coefficient (Yp/x, U/g cells) from dP/dX and the specific rate for product formation (qP) from Yp/x×μ. The specific growth rate (μ) was determined from the slope between lnX/X0 (X=cell mass, g/L) and ΔT (h). The growth yield coefficient (Yx/s) was calculated as g cells formed/g [S] utilized. The cell biomass formation (Qx, g X/l/h), and substrate consumption rates were determined from g dry weight of yeast cells or [S]/L vs. ΔT (h).
Statistical analysisThe treatment effects were compared following the procedure of Snedecor and Cochran.16 The significance is presented as Duncan's multiples as probability (≤p>) values of the process parameters.
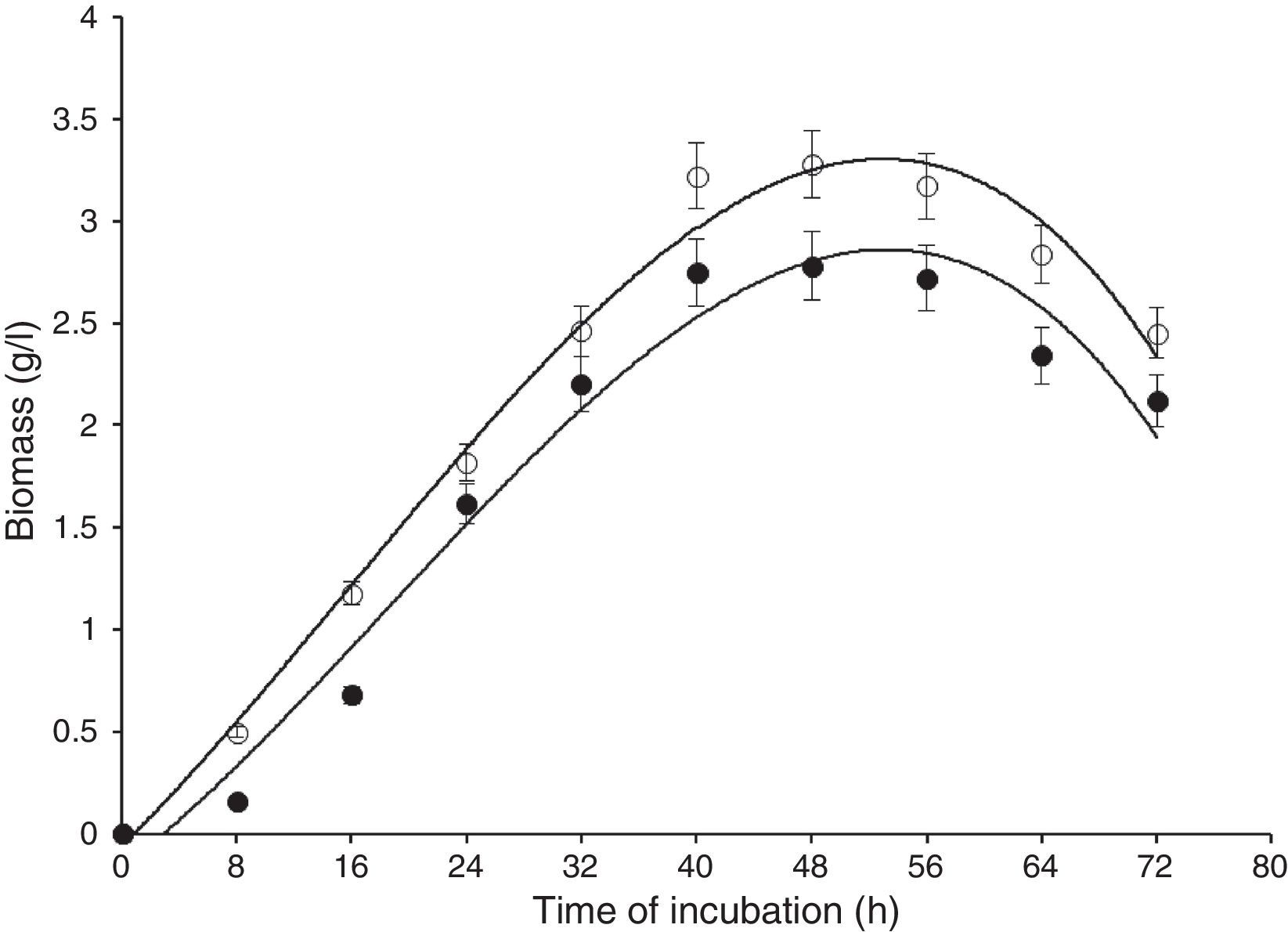
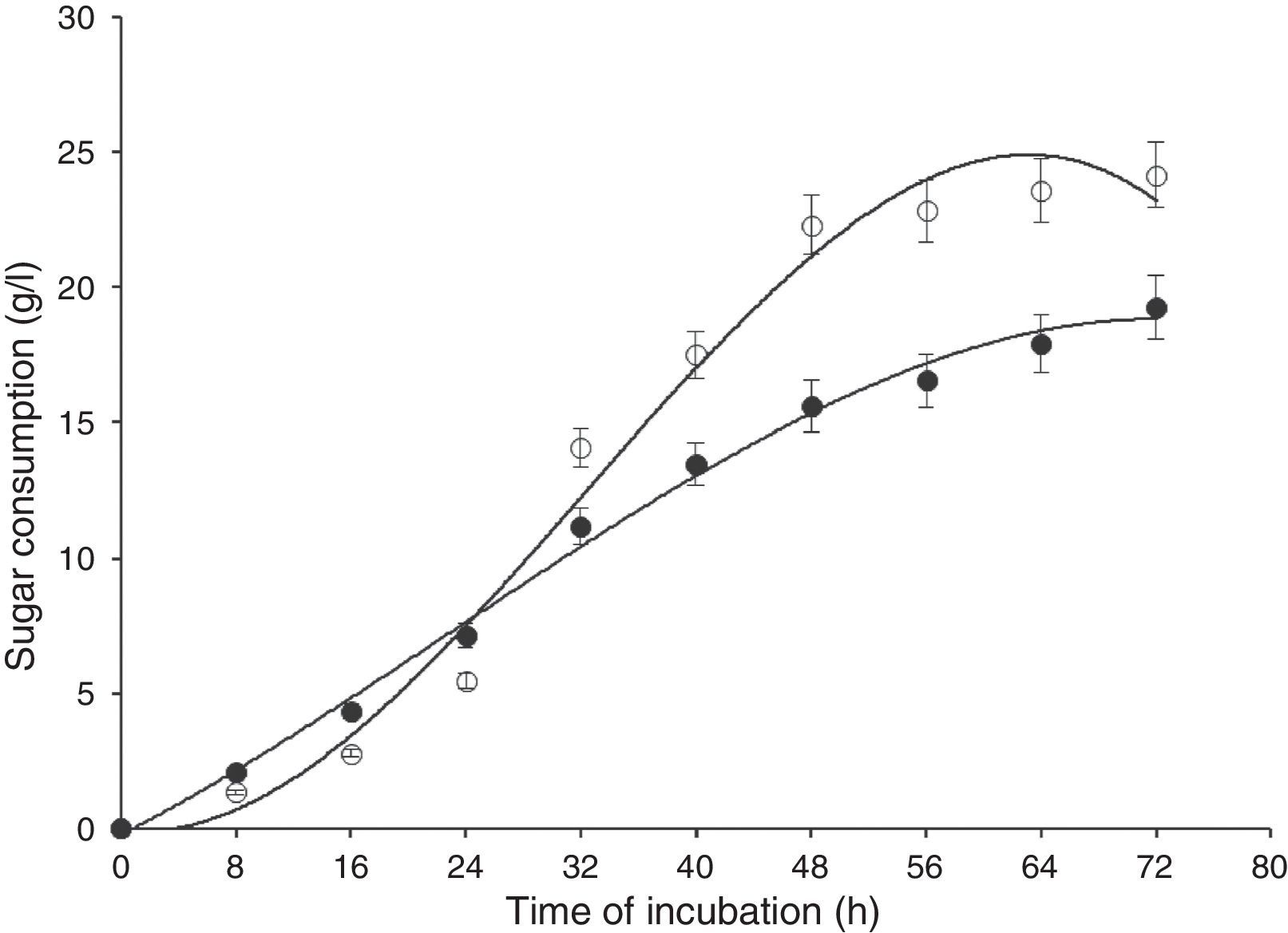
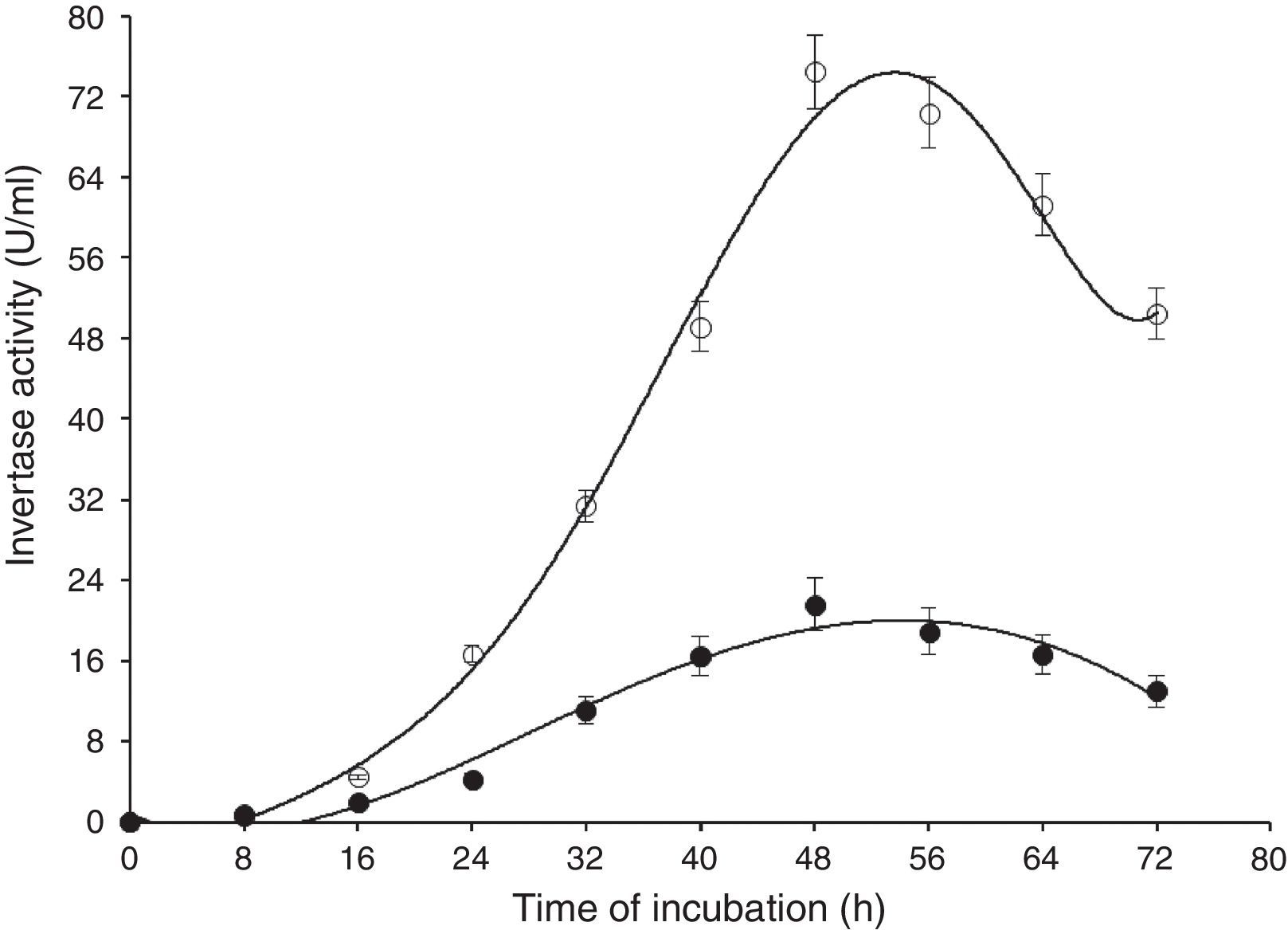
Results and discussionIn this study, wild-type S. cerevisiae IIB-IX was mutated by treating it with a potent chemical mutagen, MMS. The mutant variant MMS-V was found to be a better FFH yielding strain (52±2.4U/mL) when compared with the wild-type (17±1.8U/mL). This mutant was grown on the medium containing low fluorocytocine levels. The stability of FFH production was monitored at various levels. High FFH producing colonies were isolated at 0.02mg/mL of MMS; however, the potential cultures lost their stability after approximately two weeks similar to that report by Fernandez et al.17 This instability may be due to the possible development of resistance in growing yeast cells. Only few unstable mutants thrived for several generations. To eliminate this problem, these mutant strains were cultured in the medium containing optimal concentration as 0.05mg/mL of MMS, in which MMS-V gave consistent FFH production. The selected mutant revealed more than average growth yield coefficients, and lower specific growth rate. IIB-IX (wild-type) and the chemically derepressed mutant (MMS-V) were compared over a period (Figs. 1–3). Both the cultures showed a general growth pattern as revealed by biomass level. MMS-V exhibited much faster growth rate as compared with wild-type IIB-IX. The optimum sugar consumption was recorded at 22.31±1.12g/L by MMS-V in comparison with 16±0.81g/L by wild-type IIB-IX. A notable improvement in FFH production by mutant MMS-V than wild-type and all other mutants was observed. In a similar study by Weber and Roitsch18 yeast mutant showed two fold higher yield of FFH than the wild-type. During the course of incubation, the enzyme production started after a lag phase of approximately 8–12h and peaked at the late log phase prior to the onset of stationary phase. The enzyme biosynthesis was decreased subsequently, probably due to the decreased nutrients availability and the enzyme catabolic repression. The enzyme expression in yeast is adversely affected by the availability of monosaccharides particularly glucose or fructose.19
Comparison of biomass formation by wild-type (IIB-IX) and mutant (MMS-V) strains of S. cerevisiae in batch culture (IIB-IX –●–, MMS-V –○–). Sucrose concentration 30g/L, temperature 28°C, initial pH 6.5, agitation rate 200rpm. Y-error bars indicate standard deviation among the three parallel replicates.
Time of incubation of FFH production by wild-type (IIB-IX) and mutant (MMS-V) strains of S. cerevisiae in batch culture (IIB-IX –○–, MMS-V –○–). Sucrose concentration 30g/L, temperature 28°C, initial pH 6.5, agitation rate 200rpm. Y-error bars indicate standard deviation among the three parallel replicates.
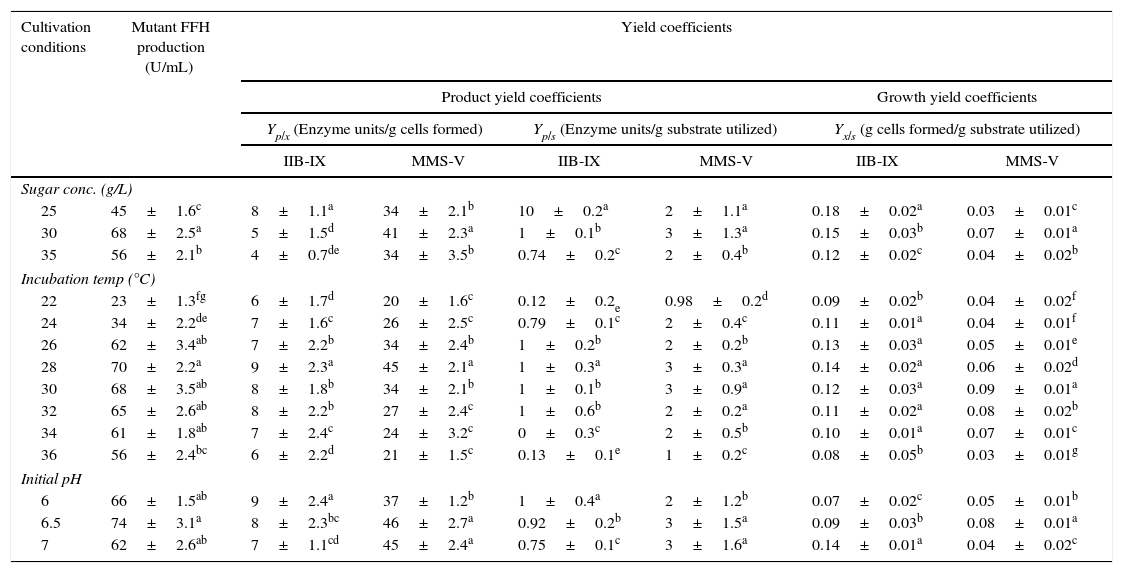
A comparison of FFH yield coefficients and productivity rates of wild-type IIB-IX and the mutant MMS-V during batch culture was made under different cultural conditions. The sugar concentration (25, 30, and 35g/L), thermophilic tendencies (22, 24, 26, 28, 30, 32, 34, and 36°C) and preliminary pH (6, 6.5, and 7) were determined (Table 1). Maximum enzyme production (68±2.5aU/mL) was achieved at 30g/L sucrose in 48h by mutant MMS-V following incubation and subsequent growth. A higher than the optimal sucrose concentration caused an increase in the sugar consumption and so the dry biomass formation rate; however, there was no gross increase in the enzyme productivity. Among the diverse incubation temperatures, the best enzyme activity (70±2.2aU/mL) was observed at 28°C, which was more than 3.5 fold higher compared to the wild-type culture. It was interesting that the mutant strain was quite capable of growing between 34 and 36°C or even higher temperatures; however the enzyme activity decreased abruptly. While comparing pH values, the optimal enzyme synthesis by mutant MMS-V (74±3.1aU/mL) was recorded at an initial pH of 6.5. The wild-type was found to be less thermo-tolerant and it yielded FFH only during a narrow temperature range (30–32°C), revealing no preference for the pH optima.
Comparisons of yield coefficients for FFH production by wild-type and mutant cultures of S. cerevisiae.
| Cultivation conditions | Mutant FFH production (U/mL) | Yield coefficients | |||||
|---|---|---|---|---|---|---|---|
| Product yield coefficients | Growth yield coefficients | ||||||
| Yp/x (Enzyme units/g cells formed) | Yp/s (Enzyme units/g substrate utilized) | Yx/s (g cells formed/g substrate utilized) | |||||
| IIB-IX | MMS-V | IIB-IX | MMS-V | IIB-IX | MMS-V | ||
| Sugar conc. (g/L) | |||||||
| 25 | 45±1.6c | 8±1.1a | 34±2.1b | 10±0.2a | 2±1.1a | 0.18±0.02a | 0.03±0.01c |
| 30 | 68±2.5a | 5±1.5d | 41±2.3a | 1±0.1b | 3±1.3a | 0.15±0.03b | 0.07±0.01a |
| 35 | 56±2.1b | 4±0.7de | 34±3.5b | 0.74±0.2c | 2±0.4b | 0.12±0.02c | 0.04±0.02b |
| Incubation temp (°C) | |||||||
| 22 | 23±1.3fg | 6±1.7d | 20±1.6c | 0.12±0.2e | 0.98±0.2d | 0.09±0.02b | 0.04±0.02f |
| 24 | 34±2.2de | 7±1.6c | 26±2.5c | 0.79±0.1c | 2±0.4c | 0.11±0.01a | 0.04±0.01f |
| 26 | 62±3.4ab | 7±2.2b | 34±2.4b | 1±0.2b | 2±0.2b | 0.13±0.03a | 0.05±0.01e |
| 28 | 70±2.2a | 9±2.3a | 45±2.1a | 1±0.3a | 3±0.3a | 0.14±0.02a | 0.06±0.02d |
| 30 | 68±3.5ab | 8±1.8b | 34±2.1b | 1±0.1b | 3±0.9a | 0.12±0.03a | 0.09±0.01a |
| 32 | 65±2.6ab | 8±2.2b | 27±2.4c | 1±0.6b | 2±0.2a | 0.11±0.02a | 0.08±0.02b |
| 34 | 61±1.8ab | 7±2.4c | 24±3.2c | 0±0.3c | 2±0.5b | 0.10±0.01a | 0.07±0.01c |
| 36 | 56±2.4bc | 6±2.2d | 21±1.5c | 0.13±0.1e | 1±0.2c | 0.08±0.05b | 0.03±0.01g |
| Initial pH | |||||||
| 6 | 66±1.5ab | 9±2.4a | 37±1.2b | 1±0.4a | 2±1.2b | 0.07±0.02c | 0.05±0.01b |
| 6.5 | 74±3.1a | 8±2.3bc | 46±2.7a | 0.92±0.2b | 3±1.5a | 0.09±0.03b | 0.08±0.01a |
| 7 | 62±2.6ab | 7±1.1cd | 45±2.4a | 0.75±0.1c | 3±1.6a | 0.14±0.01a | 0.04±0.02c |
± Indicates standard deviation among the three parallel replicates. The values designated by different letters in each set differ significantly from each other at p≤0.05.
The sugar concentration, incubation temperature and initial pH directly affect the fungal growth and hence influence the secretion of primary or secondary metabolites. The hyper-secretive mutant MMS-V exhibited lower rates for biomass formation and much higher rates for sucrose fermentation ability compared with the wild-type culture. A greater than the optimal (30g/L) inverted sucrose concentration in the production medium results in a faster glucose-induced repression of FFH.20 The mutant MMS-V was quite capable of growing up to 36°C (pH 6.5), a much higher temperature than its wild-type counter-part. The final pH and the enzyme production rate were found to be proportional. The possible reason for this liaison is that the FFH production complements secretion of various anions along with some basic proteins, or to the selective uptake of certain cations.21,22 Although the wild-type IIB-IX achieved an upper value of Yx/s (0.15±0.03bg yeast cells/g) than mutant MMS-V, the later demonstrated an improvement in the volumetric rate of product development. In addition to these findings, when the cultures were equated for specific rate constant, mutant MMS-V revealed much higher values of qp (>15–20 fold improvement). It is noteworthy that mutant MMS-V showed several fold improved values of Qp, Yp/x, Yp/s and qp when compared with the wild-type culture (LSD 0.048).
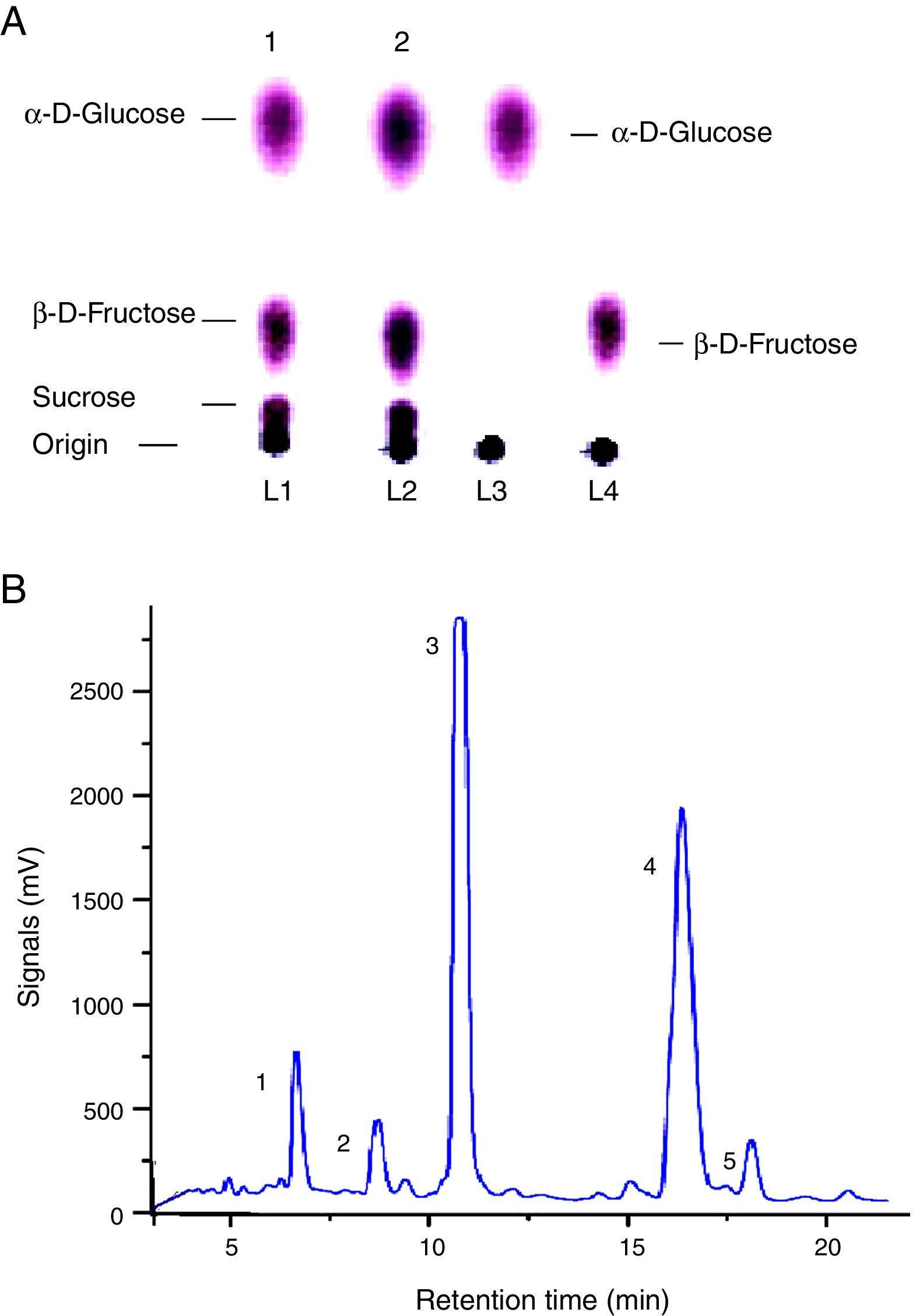
The qualitative analysis through thin layer chromatography (TLC) confirmed production of sugars viz. α-d-glucose, β-d-fructose, and sucrose by FFH hydrolysis. Image 1 is one of the TLC chromatograms carried out to detect sugars (Fig. 4A). The spot analysis and comparison of the Rf values (relative front) confirmed the sugar hydrolysis when compared with their ultrapure sugar standards suggesting the complete (100%) separation as reported by Dastager et al.14 HPLC analysis depicted the separate and well-organized peaks for α-d-glucose, β-d-fructose and sucrose, thus highlighting the product stability in the mixture form (Fig. 4B). Raffinose and Stachyose were also secreted into the reaction broth but their highest productivities remained at 0.0112 and 0.0007mg/mL, respectively.
(A) Thin layer chromatography (TLC) for the separation of FFH hydrolysis sugars (α-d-glucose, β-d-fructose, sucrose) in duplicate samples of the reaction broth at optimal conditions run with the standards. (B) HPLC chromatograph of FFH activity by mutant strain of S. cerevisiae MMS-V at optimal conditions (1. Raffinose, 2. Stachyose, 3. α-d-Glucose, 4. β-d-Fructose, 5. Sucrose).
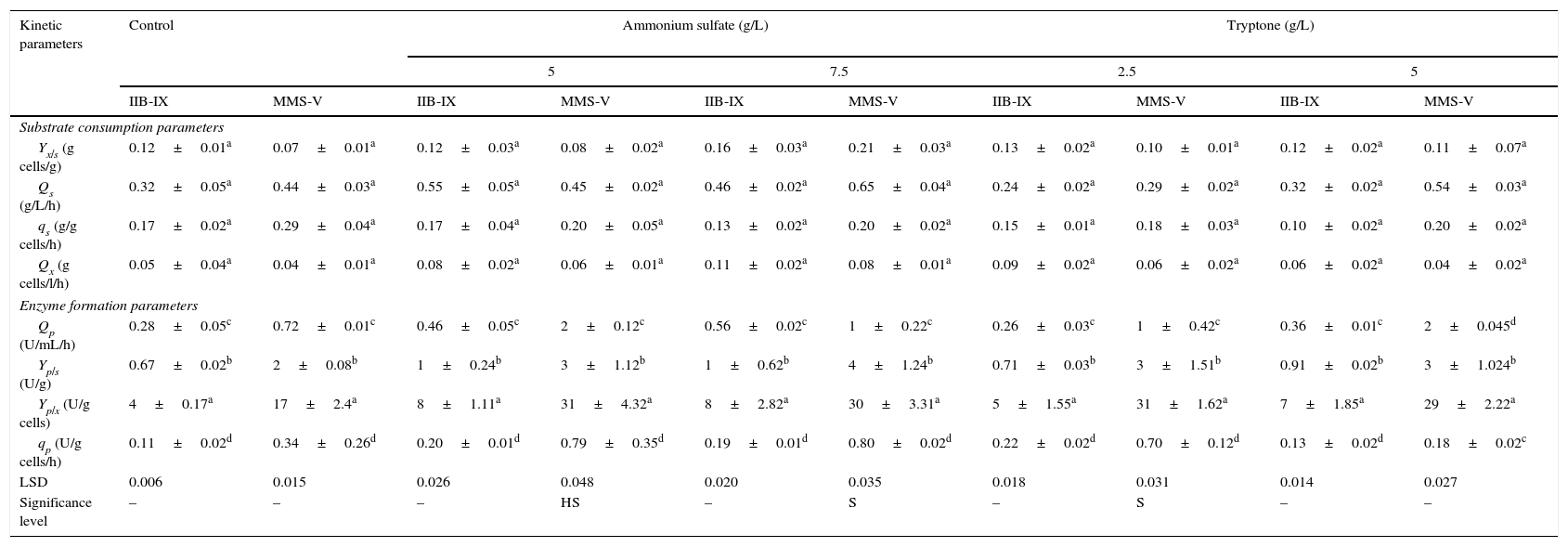
The influence of culture conditions on product formation was computed by biomass formation, specific production rate, substrate conversion rate, synthetic activity and the product breakdown.23,24 The values for Yp/x (enzyme units/g cells formed) and Yp/s (enzyme units/g substrate consumed) were found to be 45.49±2.7aU/g cells and 3±1.5aU/g substrate, respectively. Ammonium sulfate (5, 7.5g/L) and tryptone (2.5, 5g/L) were added solely into the culture medium (Table 2). The values for Yp/s, Yx/s, and Yp/x at various ammonium sulfate concentrations were improved considerably (LSD 0.048, HS, p≤0.05) as compared with the control (tryptone+yeast extract) or sole peptone supplementation. This steady increase could be attributed to ammonium sulfate which facilitates the release of periplasmic enzyme by permeabilization of the yeast cell walls as reported by Pirt.15 The volumetric rate (Qx) was marginally different at 5–7.5g/L ammonium sulfate in 48h. Similar findings were reported by Pirt15 and Gomez et al.25; however, FFH production in this study was more than 4.5 times higher than the previously reported. When the mutant strain MMS-V was compared for Qp and qs, significant enhancement (p≤0.05) was noted at 5g/L ammonium sulfate when compared with other ammonium sulfate concentrations or even other nitrogen sources.
Comparison of kinetic parameters for FFH production by wild-type and mutant cultures of S. cerevisiae.
| Kinetic parameters | Control | Ammonium sulfate (g/L) | Tryptone (g/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 7.5 | 2.5 | 5 | |||||||
| IIB-IX | MMS-V | IIB-IX | MMS-V | IIB-IX | MMS-V | IIB-IX | MMS-V | IIB-IX | MMS-V | |
| Substrate consumption parameters | ||||||||||
| Yx/s (g cells/g) | 0.12±0.01a | 0.07±0.01a | 0.12±0.03a | 0.08±0.02a | 0.16±0.03a | 0.21±0.03a | 0.13±0.02a | 0.10±0.01a | 0.12±0.02a | 0.11±0.07a |
| Qs (g/L/h) | 0.32±0.05a | 0.44±0.03a | 0.55±0.05a | 0.45±0.02a | 0.46±0.02a | 0.65±0.04a | 0.24±0.02a | 0.29±0.02a | 0.32±0.02a | 0.54±0.03a |
| qs (g/g cells/h) | 0.17±0.02a | 0.29±0.04a | 0.17±0.04a | 0.20±0.05a | 0.13±0.02a | 0.20±0.02a | 0.15±0.01a | 0.18±0.03a | 0.10±0.02a | 0.20±0.02a |
| Qx (g cells/l/h) | 0.05±0.04a | 0.04±0.01a | 0.08±0.02a | 0.06±0.01a | 0.11±0.02a | 0.08±0.01a | 0.09±0.02a | 0.06±0.02a | 0.06±0.02a | 0.04±0.02a |
| Enzyme formation parameters | ||||||||||
| Qp (U/mL/h) | 0.28±0.05c | 0.72±0.01c | 0.46±0.05c | 2±0.12c | 0.56±0.02c | 1±0.22c | 0.26±0.03c | 1±0.42c | 0.36±0.01c | 2±0.045d |
| Yp/s (U/g) | 0.67±0.02b | 2±0.08b | 1±0.24b | 3±1.12b | 1±0.62b | 4±1.24b | 0.71±0.03b | 3±1.51b | 0.91±0.02b | 3±1.024b |
| Yp/x (U/g cells) | 4±0.17a | 17±2.4a | 8±1.11a | 31±4.32a | 8±2.82a | 30±3.31a | 5±1.55a | 31±1.62a | 7±1.85a | 29±2.22a |
| qp (U/g cells/h) | 0.11±0.02d | 0.34±0.26d | 0.20±0.01d | 0.79±0.35d | 0.19±0.01d | 0.80±0.02d | 0.22±0.02d | 0.70±0.12d | 0.13±0.02d | 0.18±0.02c |
| LSD | 0.006 | 0.015 | 0.026 | 0.048 | 0.020 | 0.035 | 0.018 | 0.031 | 0.014 | 0.027 |
| Significance level | – | – | – | HS | – | S | – | S | – | – |
± Indicates standard deviation among replicates. LSD for least significant difference, HS denotes highly significant and S for significant values. The values designated by different letters in each set differ significantly from each other at p≤0.05.
In the present study, a locally developed S. cerevisiae mutant (MMS-V) exhibited a substantial improvement (p≤0.05) in FFH production when 5g/L ammonium sulfate was added as an organic nitrogen ingredient directly into the SAPY medium at pH 6.5. Several folds improvement in volumetric enzyme productivity was recorded by the mutant at all the rates examined. The TLC and HPLC studies revealed that the enzyme produced by the mutant is active and efficient in hydrolysis of sucrose, which may be highly useful for inverted-syrup production at a larger-scale. The potential mutant could be exploited for enzyme production at a wider temperature range (26–34°C).
Conflict of interestThe authors have no conflict of interest to declare.
Vice Chancellor, GC University Lahore is gratefully acknowledged for his excellent services to promote research culture at IIB and of the whole University.