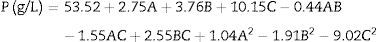The application of high-potential thermotolerant yeasts is a key factor for successful ethanol production at high temperatures. Two hundred and thirty-four yeast isolates from Greater Mekong Subregion (GMS) countries, i.e., Thailand, The Lao People's Democratic Republic (Lao PDR) and Vietnam were obtained. Five thermotolerant yeasts, designated Saccharomyces cerevisiae KKU-VN8, KKU-VN20, and KKU-VN27, Pichia kudriavzevii KKU-TH33 and P. kudriavzevii KKU-TH43, demonstrated high temperature and ethanol tolerance levels up to 45°C and 13% (v/v), respectively. All five strains produced higher ethanol concentrations and exhibited greater productivities and yields than the industrial strain S. cerevisiae TISTR5606 during high-temperature fermentation at 40°C and 43°C. S. cerevisiae KKU-VN8 demonstrated the best performance for ethanol production from glucose at 37°C with an ethanol concentration of 72.69g/L, a productivity of 1.59g/L/h and a theoretical ethanol yield of 86.27%. The optimal conditions for ethanol production of S. cerevisiae KKU-VN8 from sweet sorghum juice (SSJ) at 40°C were achieved using the Box–Behnken experimental design (BBD). The maximal ethanol concentration obtained during fermentation was 89.32g/L, with a productivity of 2.48g/L/h and a theoretical ethanol yield of 96.32%. Thus, the newly isolated thermotolerant S. cerevisiae KKU-VN8 exhibits a great potential for commercial-scale ethanol production in the future.
At present, the world is facing an energy crisis caused by the continuous use of fossil oil. As a result, petroleum oil use has dropped sharply.1 Bioethanol is an alternative energy source of particular interest whose production by microbial fermentation is increasing to replace gasoline.2–4 Yeasts, particularly Saccharomyces spp., are the most common ethanol producers employed in industry.5 Yeast has proven to be more effective for ethanol production than bacteria due to its good fermentative capacity and its ability to tolerate high concentrations of ethanol and the by-products formed during pretreatment and fermentation. S. cerevisiae is capable of fermenting different types of sugars, such as glucose, fructose and sucrose to ethanol via the glycolysis pathway under anaerobic conditions.6,7 The optimal growth temperatures of yeasts range from 25 to 35°C. However, heat stress as well as other stresses such as ethanol and osmotic pressure which are generated during fermentation process, greatly affect ethanol production and decrease the specific growth rate of yeast strains.8,9
Many types of raw materials can be used as potential substrates for ethanol production. They are classified into the following three major groups: (1) sugars such as sugarcane, sugar beet, sweet sorghum, whey and molasses3,4,10,11; (2) starches such as corn, wheat, cassava and potato12–15; and (3) lignocellulosic feedstock such as woody materials, agricultural wastes and crop residues.16–18 In recent years, interest in the utilization of sweet sorghum juice (SSJ) for ethanol production has increased in the southern United States, India, China and other countries including Thailand. The advantageous attributes of SSJ include a shorter growing period for sweet sorghum, lower requirements for fertilizers and water, and lower cultivation costs compared to sugarcane.10,11,19,20
Ethanol production at high temperatures has garnered much interest due to several advantages, including reduced cooling costs and a reduced risk of contaminations.21 To achieve high-temperature fermentation, the fermentation capability of yeasts and its ability to grow and produce ethanol under a variety of inhibitory conditions must be considered, particularly at elevated temperatures and the accumulation of high concentrations of ethanol. Many studies have examined various thermotolerant yeasts and raw materials for use in ethanol production under optimized conditions. Pichia kudriavzevii grows at 42°C and produces ethanol concentrations in the range of 29–78.6g/L from sugarcane and cassava starch hydrolysate at 40°C.4,15,17,22 Other yeasts, such as S. cerevisiae IR2 and IR2*, S. cerevisiae VS1, VS3 and Kluyveromyces marxianus DMKU 3-1042, grow at higher temperatures between 42°C and 45°C and achieve ethanol concentrations at 40°C varying from 28 to 67.8g/L when sugarcane and lignocellulosic biomass are used as substrates.3,5,8 Although yeasts can grow at elevated temperatures above 40°C, the optimal temperature for ethanol production is approximately 30°C.6,10,12,23
SSJ contains an abundant carbon source and certain minerals essential for yeast growth and ethanol production10 but lacks certain other inorganic constituents, vitamins and biogenic elements, potentially limiting ethanol fermentation under stresses such as high-temperature conditions.24 Several researchers have reported the addition of exogenous nutrients, such as ammonium sulfate, magnesium sulfate, potassium dihydrogen phosphate, manganese sulfate or yeast extract to increase fermentation efficiency and ethanol yield.3,15,24–28 Recently, statistically designed experiments have been widely employed to optimize ethanol production conditions.24,26,28 These techniques provide several advantages, such as reductions in consumption time and operating costs due to the use of fewer experimental units and the evaluation of interactions between independent variables. Furthermore, optimal conditions are determined with a second-order polynomial equation.29 Many factors, including incubation temperature, initial yeast cell concentration, initial pH and sugar concentration, affect the growth and ethanol production of yeast cells.3,11,26,30
Given the benefits of yeast thermotolerance, which can significantly reduce ethanol production costs, the search for thermotolerant yeasts is a major challenge in achieving high ethanol production levels. There is little information regarding thermotolerant yeast species and their distribution in the three countries of the Greater Mekong Subregion (GMS) (Thailand, Lao PDR and Vietnam) as well as their potential in ethanol production from SSJ at high temperatures. In addition, microbial populations from different geographic locations likely exhibit differential thermotolerance and fermentation capabilities. Therefore, the aims of this study were to isolate and identify thermotolerant and ethanol-tolerant yeast strains from specific regions of Thailand, Lao PDR and Vietnam. We identified five thermotolerant yeast strains, of which two were P. kudriavzevii and three were S. cerevisiae, exhibiting unique characteristics in ethanol production experiments employing YM medium and SSJ as substrates. The optimal conditions for ethanol production from SSJ by one of the newly isolated thermotolerant yeast strains were also investigated using the Box–Behnken experimental design (BBD). Finally, we described the ethanol production potential of the yeast strain using SSJ under high-temperature fermentation conditions.
Materials and methodsSample collectionThe samples used in this study were collected from fruits, flowers and other sources, such as banana, papaya, grape, orange, apple, mango, Vietnamese apple flowers, longan flowers, papaya flowers, alcoholic beverages, soil and sawdust, from Thailand, Lao PDR and Vietnam. One gram (or 1mL if the sample was a liquid) of a sample was added to 100mL of yeast extract-malt extract (YM) medium (3g/L yeast extract, 3g/L malt extract, 5g/L peptone, 20g/L glucose) and incubated at 35°C for 24h. One percent (v/v) inoculum was added into YM liquid medium containing 4% (v/v) ethanol and incubated at 35°C, which were employed as selective pressures for obtaining thermotolerant yeasts, for 24h.3 Then, a spread plate technique was used to obtain isolated colonies on YM agar containing 4% (v/v) ethanol at 35°C incubation temperature for 24h. A single colony of each isolate was selected and streaked on YM agar containing an ethanol concentration of 4% (v/v) and incubated at 35°C for 24h. Samples were kept at −20°C for further analysis.
Screening and isolation of thermotolerant and ethanol-tolerant yeastsThermotolerant yeast isolates were selected based on their growth performance at 37°C, 40°C and 45°C on YM agar. Isolated yeasts capable of growing at 40°C or higher were selected for further screening and identification. To screen for ethanol-tolerant yeasts, the thermotolerant yeast isolates were inoculated into YM medium at 35°C for 24h. An initial cell concentration of 1×106 cells/mL was inoculated into YM medium containing different ethanol concentrations of 7, 10 and 13% (v/v). Then, the cultures were incubated at 35°C for 48h. Viable yeast cell numbers were determined by the direct counting method using a haemacytometer and the methylene blue staining technique.31 Ethanol tolerance was determined based on the percentage of survival, which could be divided into three categories: highly tolerant (>50% survival), moderately tolerant (25–50% survival) and slightly tolerant (<25% survival).32
Identification of selected thermotolerant yeastsIdentification of the selected thermotolerant yeasts was performed using morphological characterization and molecular identification. For morphological characterization, the physical appearance of the selected thermotolerant yeasts was observed according to the classical methods described by Barnnett et al.33 The cell morphology was examined under bright field microscopy (Carl Zeiss Primo star microscope, Carl Zeiss microscopy, Germany). Molecular identification was carried out by performing sequencing analysis of the D1/D2 domain and the internal transcribed spacer (ITS) 1 and 2 regions. DNA was extracted from yeast cells using a protocol described by Harju et al.34
The D1/D2 domain of the 26S rDNA was amplified by polymerase chain reaction (PCR) using the primer pairs NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′).35 The internal transcribed spacer (ITS) 1 and 2 regions were also amplified by PCR using the primer pairs ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′).36 The amplification conditions consisted of initial denaturation at 95°C for 5min; 35 cycles of denaturation at 95°C for 1min, annealing at 53°C for 1min, and extension at 72°C for 1min; and a final extension at 72°C for 7min. The PCR products were analyzed by agarose gel electrophoresis and sequenced at First Base Laboratories (First BASE Laboratories Sdn Bhd, Malaysia). The amplified D1/D2 and ITS sequences of the yeast isolates were compared to the sequences of related species retrieved from the National Center for Biotechnology Information (NCBI) database using BLASTN. Phylogenetic analysis was performed using the neighbor-joining method with the Molecular Evolutionary Genetics Analysis (MEGA) program version 5.2. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were also determined.37
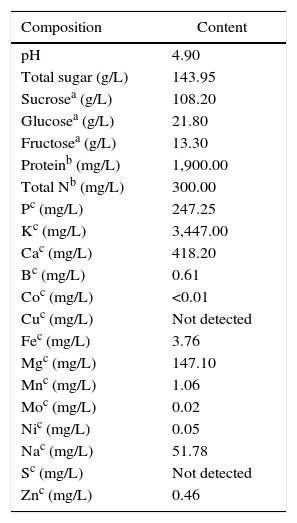
Preparation of raw material for ethanol productionSweet sorghum cv. KKU40 was kindly provided by the Faculty of Agriculture, Khon Kaen University, Thailand, and was used as a substrate for ethanol production in this study. The fresh juice was extracted from sorghum stalks by squeezing in a juice extractor; then, the juice was concentrated to 65°Bx via evaporation and maintained at −20°C prior to use. The composition of SSJ was analyzed at the Central Laboratory (Thailand) Co., Ltd. in Thailand (Table 1).
The composition of sweet sorghum juice cv. KKU40.
| Composition | Content |
|---|---|
| pH | 4.90 |
| Total sugar (g/L) | 143.95 |
| Sucrosea (g/L) | 108.20 |
| Glucosea (g/L) | 21.80 |
| Fructosea (g/L) | 13.30 |
| Proteinb (mg/L) | 1,900.00 |
| Total Nb (mg/L) | 300.00 |
| Pc (mg/L) | 247.25 |
| Kc (mg/L) | 3,447.00 |
| Cac (mg/L) | 418.20 |
| Bc (mg/L) | 0.61 |
| Coc (mg/L) | <0.01 |
| Cuc (mg/L) | Not detected |
| Fec (mg/L) | 3.76 |
| Mgc (mg/L) | 147.10 |
| Mnc (mg/L) | 1.06 |
| Moc (mg/L) | 0.02 |
| Nic (mg/L) | 0.05 |
| Nac (mg/L) | 51.78 |
| Sc (mg/L) | Not detected |
| Znc (mg/L) | 0.46 |
The composition of SSJ was analyzed at the Central Laboratory, Co., Ltd., Thailand.
The ethanol production efficiency of the thermotolerant yeast strains was tested in 250-mL Erlenmeyer flasks containing 100mL of YM broth and 200g/L of glucose. The YM medium was inoculated with thermotolerant yeast strains at an initial yeast cell concentration of approximately 1×107cells/mL and incubated at 37°C or 40°C for 48h. Viable yeast cell numbers were determined by the direct counting method using a haemacytometer and the methylene blue staining technique.31 The fermentation broth was centrifuged at 13,000rpm for 10min, and the total residual sugar concentration of the supernatant was tested using the phenol sulfuric acid method.38 Ethanol production was analyzed by gas chromatography as described by Laopaiboon et al.11 The ethanol yield (Yp/s) was calculated based on the actual ethanol produced and expressed as g of ethanol per g of sugar utilized (g/g). The ethanol productivity (QP, g/L/h) was calculated from the final ethanol concentration per the fermentation time at the highest ethanol concentration. The isolated yeast strains with ethanol concentrations greater than 35g/L were chosen for further study.8
Ethanol production by selected thermotolerant yeasts using SSJ as a substrateSterile SSJ containing 200g/L of total sugar was inoculated with the selected thermotolerant yeast strains at an initial yeast cell concentration of approximately 1×107 cells/mL. S. cerevisiae TISTR 5606, an industrial strain used for commercial-scale ethanol production in Thailand, was used as a reference strain. The culture was incubated with shaking at 150rpm at 37°C, 40°C or 43°C for 48h. After 48h of incubation, viable yeast cell numbers, the total residual sugar concentration and ethanol production were analyzed as previously mentioned. Thermotolerant yeast strains exhibiting ethanol yields similar to that of S. cerevisiae TISTR 5606 were selected for ethanol production at 37°C, 40°C and 43°C for 72h. During ethanol fermentation, samples were collected every 6h, and viable yeast cell numbers, the total residual sugar concentrations and ethanol production were analyzed according to previously described protocols.
Optimization of ethanol fermentation conditions using statistical experimental designTo identify the significant variables affecting ethanol production from SSJ at a high temperature (40°C) by the newly isolated thermotolerant S. cerevisiae KKU-VN8 strain, various fermentation factors which were selected based on a literature review, including initial sugar concentration, initial pH, ammonium sulfate ((NH4)2SO4) and trace elements such as magnesium sulfate (MgSO4·7H2O) and manganese sulfate (MnSO4·H2O), were evaluated using the Plackett–Burman design. The following two levels (low and high) for each experimental variable were employed: A (initial sugar concentration, g/L): 180 and 250; B (initial pH): 4 and 6; C ((NH4)2SO4, g/L): 1 and 5; D (MgSO4·7H2O, g/L): 0.1 and 0.5; and E (MnSO4·H2O, g/L): 0.02 and 0.1.
Fermentation factors exerting a significant effect on ethanol production efficiency at 40°C by S. cerevisiae KKU-VN8 were evaluated based on the Plackett–Burman design. These significant factors were then selected for optimization using the Box–Behnken design (BBD). In this study, three variables, specifically initial sugar concentration, initial pH and (NH4)2SO4 concentration, were chosen, and ethanol concentration was selected as a response variable. According to the BBD, three levels corresponding to the low, middle and high values for each experimental variable were employed: A (initial sugar concentration, g/L): 150, 195 and 240; B (initial pH): 4, 5 and 6; and C ((NH4)2SO4, g/L): 0.1, 1.55 and 3. The Design-Expert 7.0 demo version (STAT EASE Inc., Minneapolis, USA) was used to generate the experimental designs and perform regression analysis. Analysis of Variance (ANOVA) was carried out to estimate statistically significant parameters. The quality of the quadratic model equation was expressed as the coefficient of determination (R2). A validation experiment was conducted using the optimized conditions determined from the response surface plots. The experiments were carried out in 500-mL Erlenmeyer flasks incubated on a rotary shaker at 150rpm and 40°C for 72h. During ethanol fermentation, samples were withdrawn, and total residual sugars and ethanol production were analyzed according to previously described protocols.
Effects of the initial cell concentration of S. cerevisiae KKU-VN8 on ethanol production efficiency using SSJ as a substrateEffects of the initial cell concentration of S. cerevisiae KKU-VN8 on ethanol production from SSJ were investigated. One hundred milliliters of SSJ containing the optimized factors, i.e., 238.52g/L of initial total sugars, 2.04g/L of ammonium sulfate and the pH value of 5.82 was inoculated with different initial yeast cell concentrations of 1×107, 1×108 and 3×108 cells/mL, and incubated on a rotating shaker at 150rpm at 40°C for 48h. Samples were taken out every 12h. The viable yeast cell number, the total residual sugar concentration and the ethanol concentration were analyzed according to previously described protocols. Acetic acid and glycerol concentrations were analyzed by high performance liquid chromatography (HPLC) using an Aminex HPX-87H column (Bio-Rad Laboratories, CA, USA) and refractometer detection at 40°C. The samples were eluted with 5mM sulfuric acid at a flow rate of 0.6mL/min.
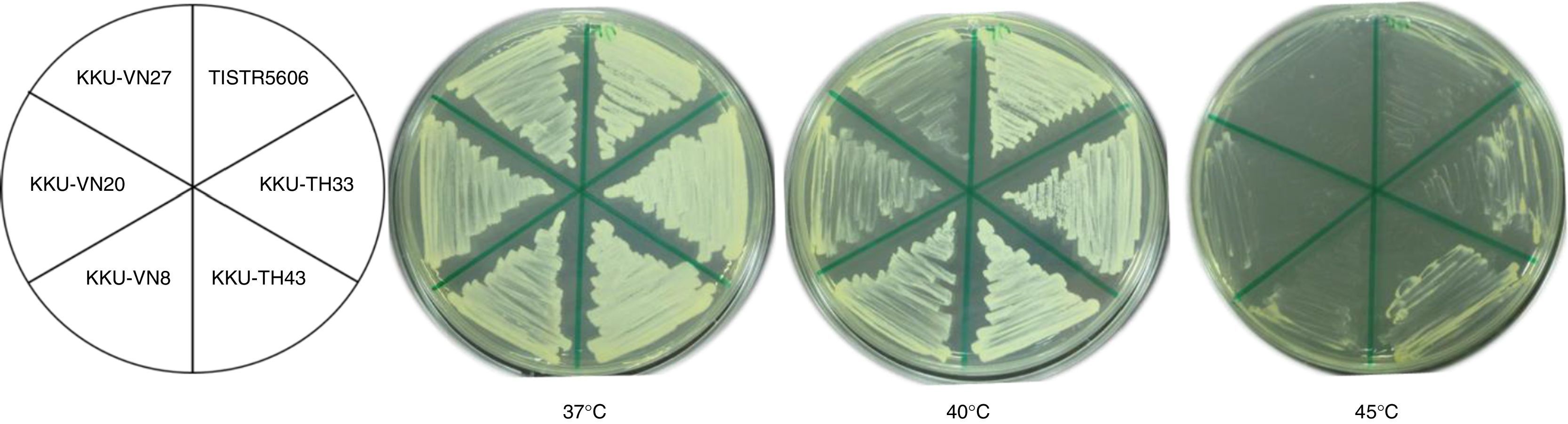
ResultsScreening and isolation of thermotolerant and ethanol-tolerant yeastsTwo hundred and thirty-four yeast isolates were screened and isolated from Thailand, Lao PDR and Vietnam. Twenty-six promising isolates, specifically KKU-TH1, KKU-TH33, KKU-TH43, KKU-TH54, KKU-TH57, KKU-TH105, KKU-TH199, KKU-TH200 and KKU-TH211 isolated from the northeastern region of Thailand, KKU-LA1, KKU-LA4, KKU-LA9, KKU-L14, KKU-L22, KKU-L26 and KKU-L28 isolated from Vientiane city in Lao PDR and KKU-VN7, KKU-VN8, KKU-VN9, KKU-VN20, KKU-VN25, KKU-VN27, KKU-VN28, KKU-VN30, KKU-VN35 and KKU-VN36 isolated from Can Tho city in Vietnam, were selected for preliminary thermotolerance and ethanol tolerance studies. Fig. 1 shows the growth of selected thermotolerant yeast isolates at 37°C, 40°C and 45°C. All isolates grew well at 37°C. Seven isolates from Thailand, six isolates from Lao PDR and two isolates from Vietnam demonstrated good growth at 40°C (data not shown). Only five isolates, specifically KKU-TH33, KKU-TH43, KKU-TH199, KKU-LA1 and KKU-LA4, demonstrated moderate growth at 45°C, while others exhibited weak or no growth (Fig. 1).
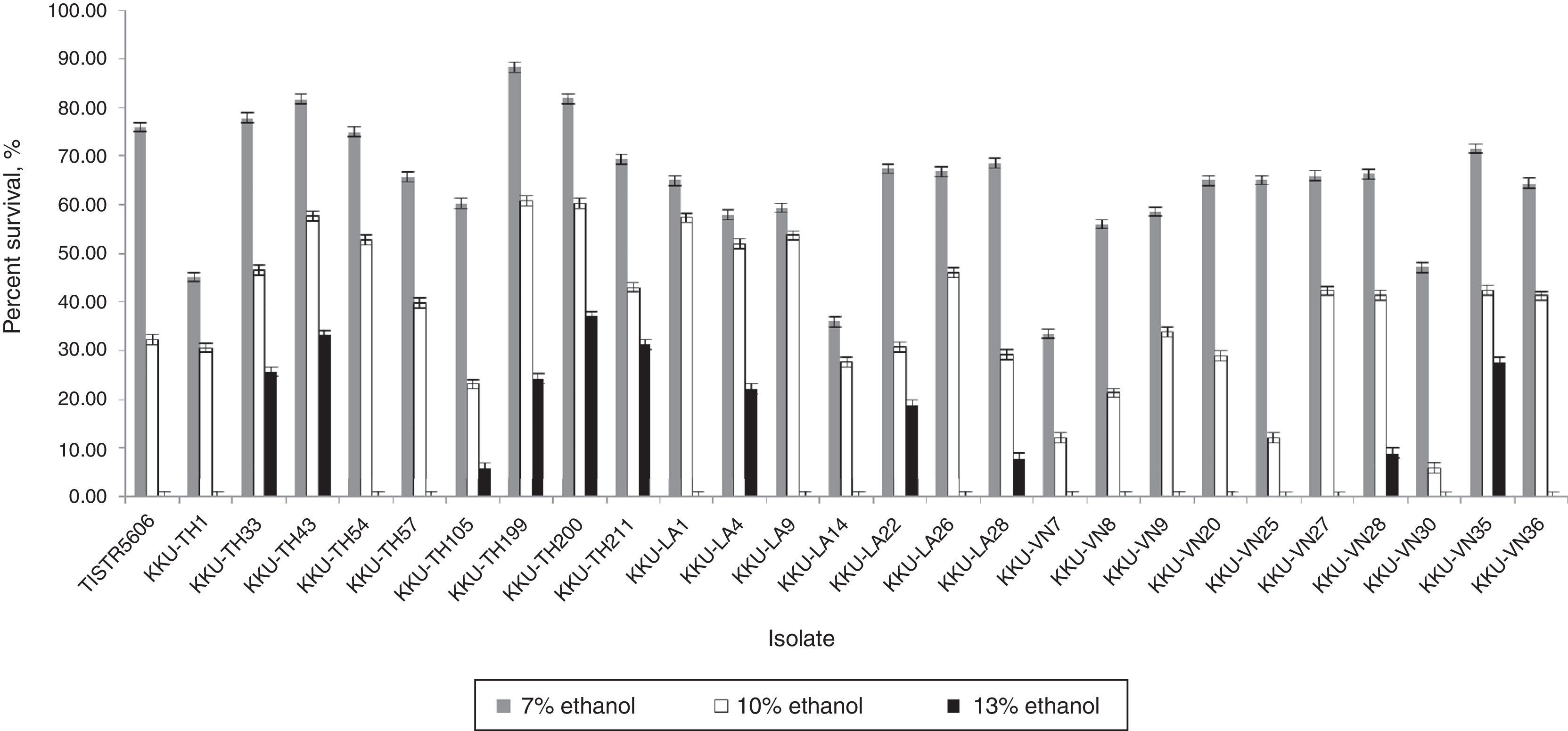
To test for ethanol tolerance, the selected thermotolerant yeast isolates were inoculated into YM medium containing 7, 10 and 13% (v/v) ethanol.39 The majority of the isolates tolerated ethanol concentrations up to 10% (v/v) (Fig. 2). Interestingly, the isolates KKU-TH33, KKU-TH43, KKU-TH200, KKU-TH211 and KKU-VN35 were highly tolerant of high ethanol concentrations up to 13% (v/v).
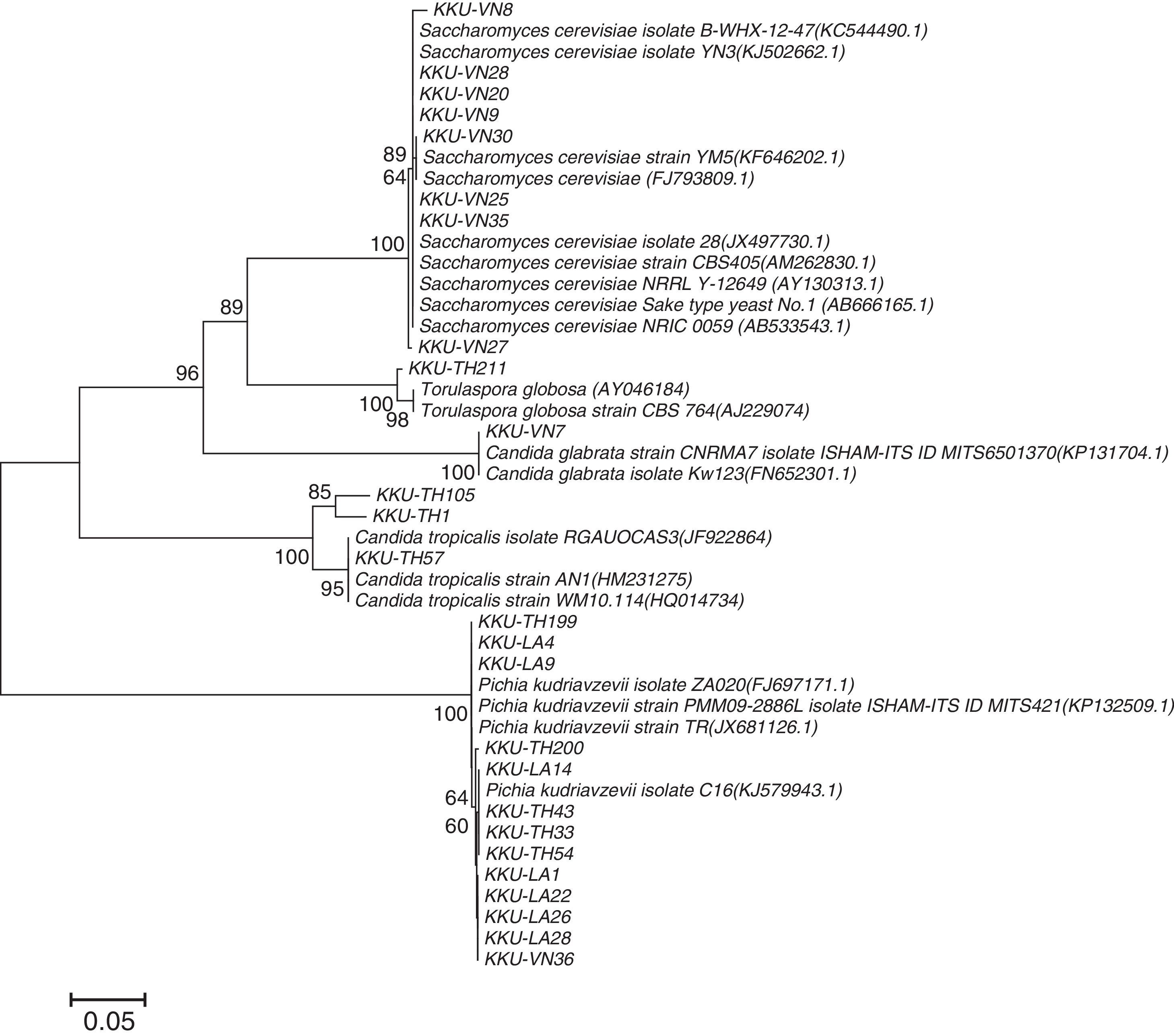
Identification of selected thermotolerant yeastsThe selected thermotolerant yeast colonies exhibited similar physical appearances on YM agar. Colonies were cream-white in color and circular in shape. They possessed a convex profile and were smooth and opaque with a glossy surface. Microscopic characteristics of the selected yeasts included spherical and egg shapes with budding, either isolated or grouped (data not shown). Molecular analysis by PCR showed that the PCR products obtained using the NL1 and NL4 primers consisted of bands of approximately 600bp. However, the PCR products obtained using the ITS1 and ITS4 primers were of different sizes, ranging from 500 to 900bp (data not shown). Phylogenetic relationships were determined by comparing the sequences of the ITS1 and ITS2 regions available in the GenBank sequence database to those of the isolated thermotolerant yeasts (Fig. 3). Twenty-six promising isolates demonstrated sequence identities of 99%. The isolates KKU-TH1, KKU-TH57 and KKU-TH105 were identified as Candida tropicalis. The isolates KKU-TH33, KKU-TH43, KKU-TH54, KKU-TH199, KKU-TH200, KKU-LA1, KKU-LA4, KKU-LA9, KKU-LA14, KKU-LA22, KKU-LA26, KKU-LA28 and KKU-VN36 were identified as P. kudriavzevii. The isolates KKU-VN8, KKU-VN9, KKU-VN20, KKU-VN25 and KKU-VN27 were identified as S. cerevisiae. The isolates KKU-TH211 and KKU-VN7 were identified as Torulaspora globosa and C. glabrata, respectively. All twenty-six thermotolerant yeast strains were employed for subsequent ethanol production experiments.
Phylogenetic relationships of yeast strains and the related taxa based on a combined sequence analysis of the internal transcribed spacer (ITS) 1 and 2 regions. Clustering was performed by the neighbor-joining using maximum likelihood with the software package, Molecular Evolutionary Genetics Analysis (MEGA) version 5.2. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. A scale bar of 0.05 is scale of branch lengths of the evolutionary distances used to infer the phylogenetic tree.
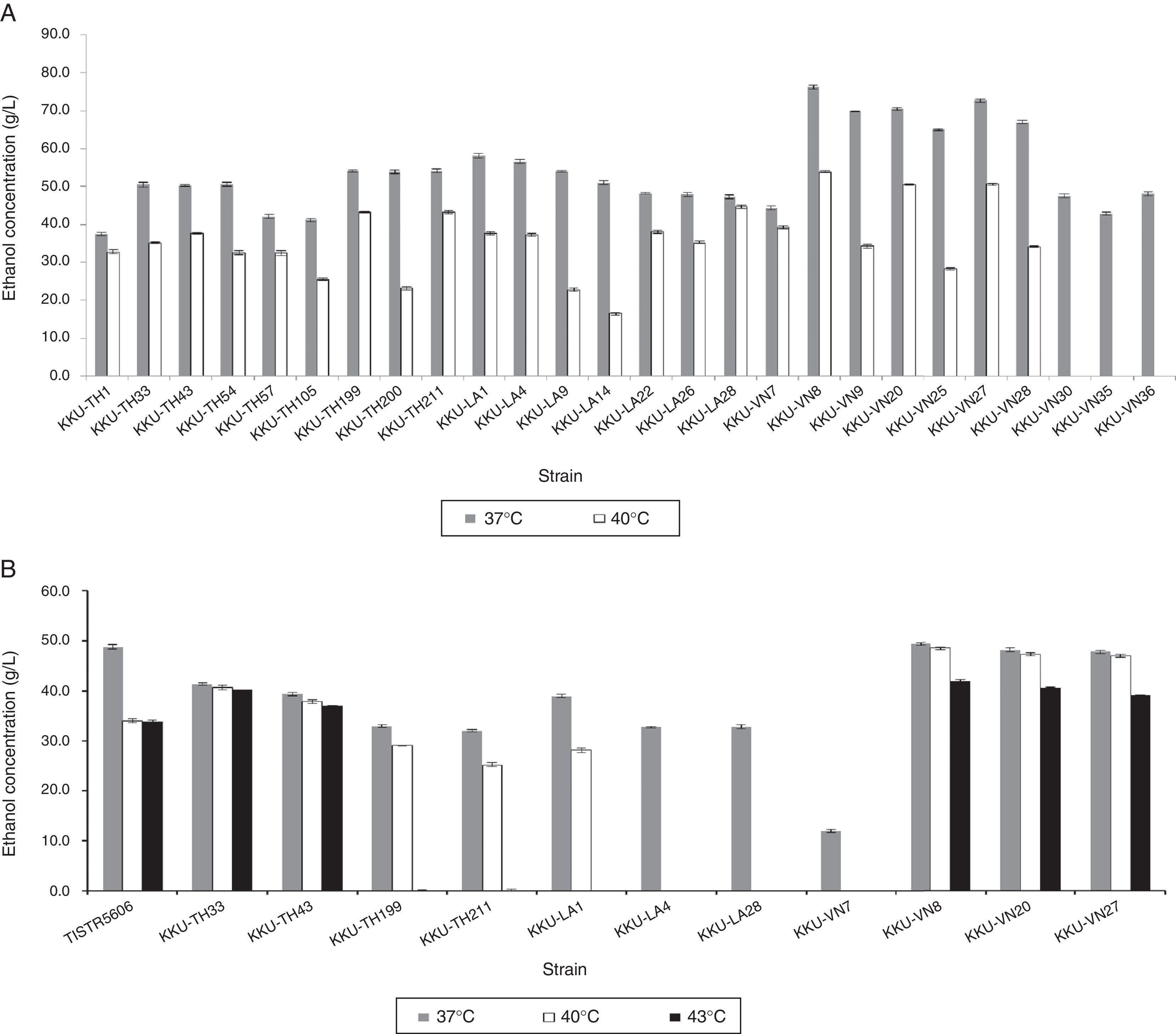
The ethanol production efficiency of the selected thermotolerant yeast strains in YM medium containing 200g/L of glucose was examined. After incubation at 37°C for 48h, all twenty-six strains produced ethanol at concentrations in the range of 37.47–72.69g/L, productivities of 0.78–1.59g/L/h and ethanol yields of 0.27–0.44g/g (Fig. 4A). S. cerevisiae KKU-VN8 exhibited the best performance for ethanol production at 37°C, with an ethanol concentration of 72.69g/L, productivity of 1.59g/L/h and an ethanol yield of 0.44g/g. All strains with the exception of KKU-VN30, KKU-VN35 and KKU-VN36 were chosen for subsequent ethanol production experiments at 40°C, owing to their low yields and ethanol concentrations.
Ethanol production at 40°C identified twenty-three strains demonstrating differential ethanol production, with ethanol concentrations ranging from 16.52 to 53.89g/L, productivities of 0.34–1.12g/L/h and ethanol yields of 0.22–0.44g/g (Fig. 4A). S. cerevisiae KKU-VN8, KKU-VN20, KKU-VN27, P. kudriavzevii KKU-LA28, KKU-TH199 and T. globosa KKU-TH211 produced high ethanol concentrations of 53.89, 50.62, 50.53, 44.68, 43.27 and 43.21g/L, respectively. Based on the criteria described by Sree et al.,8 yeast strains capable of producing ethanol concentrations and yields greater than 35g/L and 0.33g/g, respectively, were selected for further experiments. Therefore, T. globosa KKU-TH211, P. kudriavzevii KKU-TH33, KKU-TH43, KKU-TH199, KKU-LA1, KKU-LA4, KKU-LA28, S. cerevisiae KKU-VN7, KKU-VN8, KKU-VN20 and KKU-VN27 were employed to investigate ethanol production using SSJ as a substrate.
Ethanol production by selected thermotolerant yeasts using SSJ as a substrateEleven thermotolerant yeasts and S. cerevisiae TISTR 5606 as a reference strain were inoculated into SSJ containing 200g/L total sugar and incubated at 37°C with an agitation rate of 150rpm for 48h. The selected thermotolerant yeast strains produced ethanol concentrations in the range of 11.94–47.39g/L with productivities of 0.25–0.99g/L/h and ethanol yields of 0.13–0.45g/g (Fig. 4B). Although the values were lower than those of reference strain, eight potential strains, specifically T. globosa KKU-TH211, P. kudriavzevii KKU-TH33, KKU-TH43, KKU-TH199, KKU-LA1, S. cerevisiae KKU-VN8, KKU-VN20 and S. cerevisiae KKU-VN27, were further investigated for ethanol production at 40°C based on their ethanol yields, which were similar to that of the reference strain.
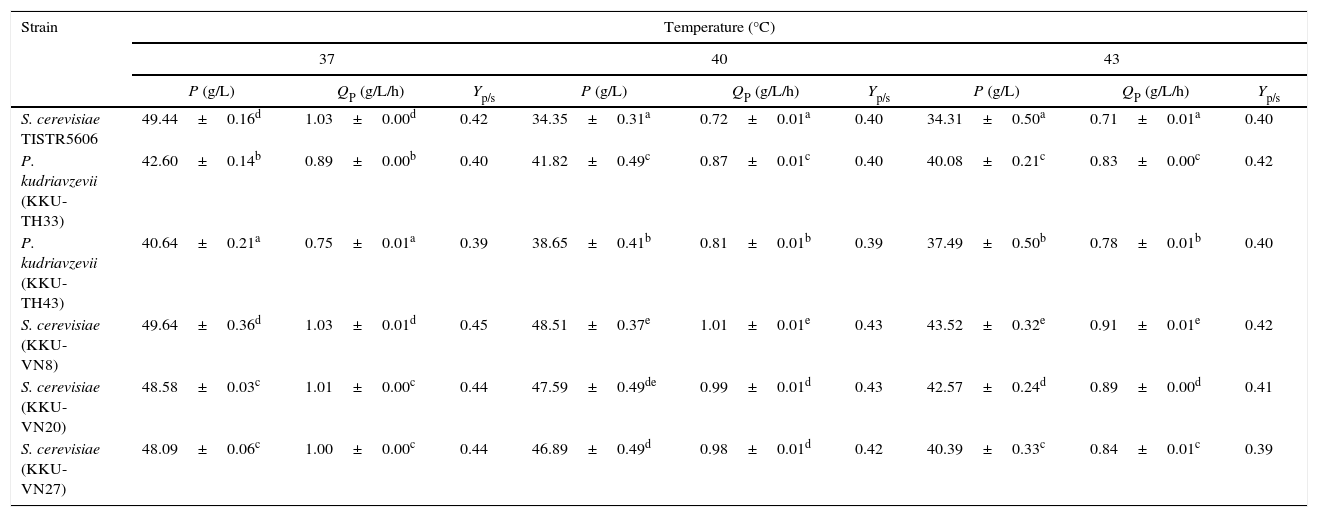
Ethanol fermentation at 40°C by these eight strains resulted in ethanol concentrations that varied from 25.20 to 48.46g/L, with productivities and yields of 0.52–1.01g/L/h and 0.31–0.43g/g, respectively. In terms of ethanol concentrations, productivities and yields of the five strains, specifically P. kudriavzevii KKU-TH33 and KKU-TH43, which were isolated from Thailand, and S. cerevisiae KKU-VN8, KKU-VN20, and KKU-VN27, which were isolated from Vietnam, performed better than the reference strain at 40°C (Fig. 4B). Thus, they were selected to investigate ethanol production at 43°C. These five promising strains, particularly S. cerevisiae KKU-VN8, KKU-VN20 and KKU-VN27, produced much higher ethanol concentrations than that of the reference strain at 40°C and 43°C, although a minute decrease in ethanol concentration was observed at elevated temperatures. When ethanol production performance of the reference strain was evaluated at 40°C and 43°C, ethanol concentrations substantially decreased, suggesting a lower degree of thermal tolerance than the five promising strains.
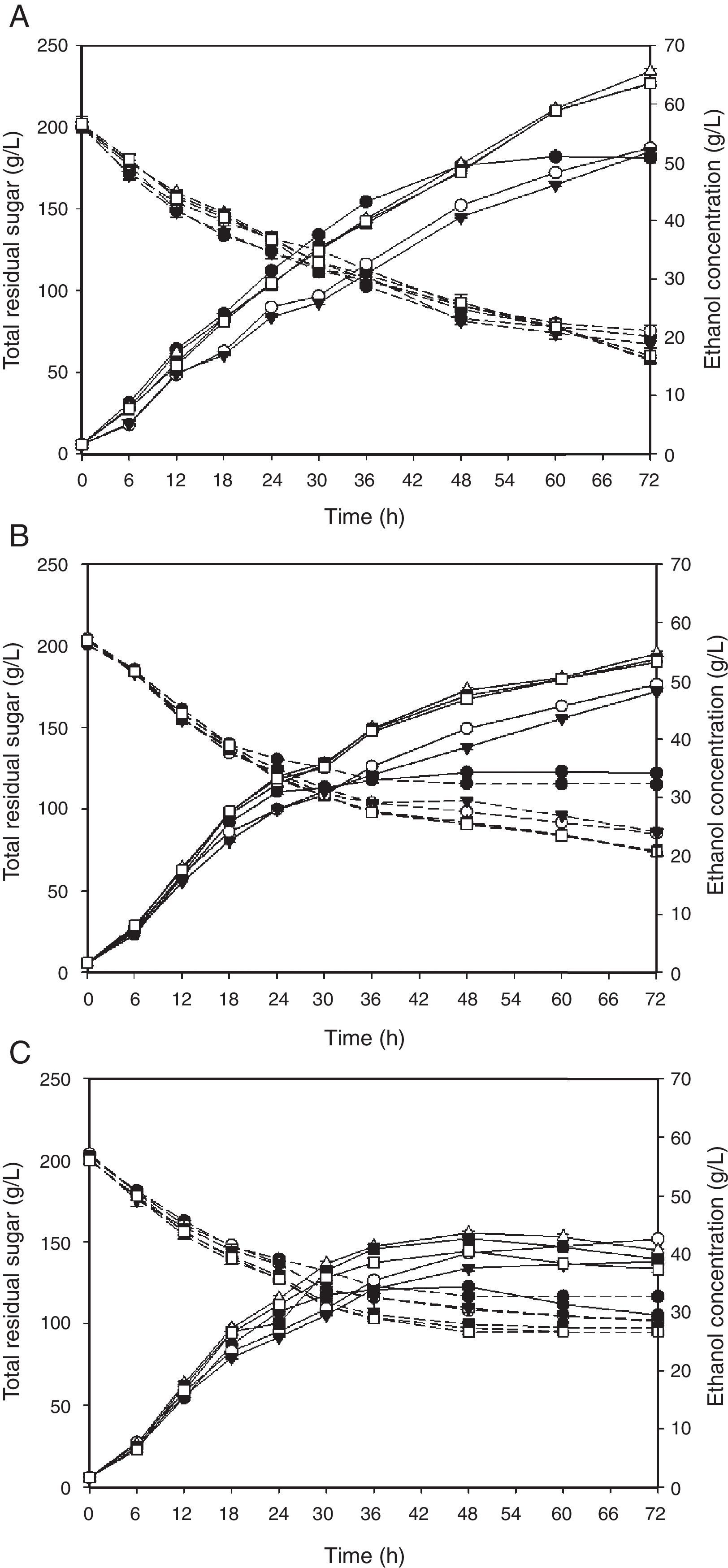
Batch culture profiles of the selected thermotolerant yeasts, specifically P. kudriavzevii KKU-TH33, and KKU-TH43, S. cerevisiae KKU-VN8, KKU-VN20, and KKU-VN27 and the reference strain S. cerevisiae TISTR 5606, using SSJ at 37°C, 40°C and 43°C for 72h were investigated. Ethanol concentration continued to increase during fermentation over 72h at 37°C. Although the ethanol concentrations and yields of S. cerevisiae KKU-VN8, KKU-VN20 and KKU-VN27 and the reference strain were approximately the same at 48h of fermentation, S. cerevisiae KKU-VN8, KKU-VN20 and KKU-VN27 demonstrated much greater values when the fermentation time reached 72h (Fig. 5). Because the ethanol production of the reference strain was stable at 48h, the fermentation parameters of all strains were compared during this time period (Table 2). At 40°C and 43°C, ethanol concentrations, productivities and yields of all five strains were much higher than those of the reference strain (Table 2, Fig. 5).
Batch culture profiles of ethanol production from SSJ at the initial sugar concentration of 200g/L with pH 4.5 at 37°C (A), 40°C (B) or 43°C (C) by S. cerevisiae TISTR 5606 (●), P. kudriavzevii KKU-TH33 (○), P. kudriavzevii KKU-TH43 (▾), S. cerevisiae KKU-VN8 (▵), S. cerevisiae KKU-VN20 (■) and S. cerevisiae KKU-VN27 (□): total residual sugars (dash line), ethanol concentration (solid line). The results were expressed as mean±SD.
Ethanol production efficiency of thermotolerant yeast strains at 37, 40 and 43°C for 48h using SSJ.
| Strain | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 37 | 40 | 43 | |||||||
| P (g/L) | QP (g/L/h) | Yp/s | P (g/L) | QP (g/L/h) | Yp/s | P (g/L) | QP (g/L/h) | Yp/s | |
| S. cerevisiae TISTR5606 | 49.44±0.16d | 1.03±0.00d | 0.42 | 34.35±0.31a | 0.72±0.01a | 0.40 | 34.31±0.50a | 0.71±0.01a | 0.40 |
| P. kudriavzevii (KKU-TH33) | 42.60±0.14b | 0.89±0.00b | 0.40 | 41.82±0.49c | 0.87±0.01c | 0.40 | 40.08±0.21c | 0.83±0.00c | 0.42 |
| P. kudriavzevii (KKU-TH43) | 40.64±0.21a | 0.75±0.01a | 0.39 | 38.65±0.41b | 0.81±0.01b | 0.39 | 37.49±0.50b | 0.78±0.01b | 0.40 |
| S. cerevisiae (KKU-VN8) | 49.64±0.36d | 1.03±0.01d | 0.45 | 48.51±0.37e | 1.01±0.01e | 0.43 | 43.52±0.32e | 0.91±0.01e | 0.42 |
| S. cerevisiae (KKU-VN20) | 48.58±0.03c | 1.01±0.00c | 0.44 | 47.59±0.49de | 0.99±0.01d | 0.43 | 42.57±0.24d | 0.89±0.00d | 0.41 |
| S. cerevisiae (KKU-VN27) | 48.09±0.06c | 1.00±0.00c | 0.44 | 46.89±0.49d | 0.98±0.01d | 0.42 | 40.39±0.33c | 0.84±0.01c | 0.39 |
P, ethanol concentration (g/L); QP, volumetric ethanol productivity (g/L/h); Yp/s, ethanol yield (g/g).
abcdef values bearing different superscripts within the same column are significantly different using Duncan's multiple range test at the level of 0.05. The results were expressed as mean±SD.
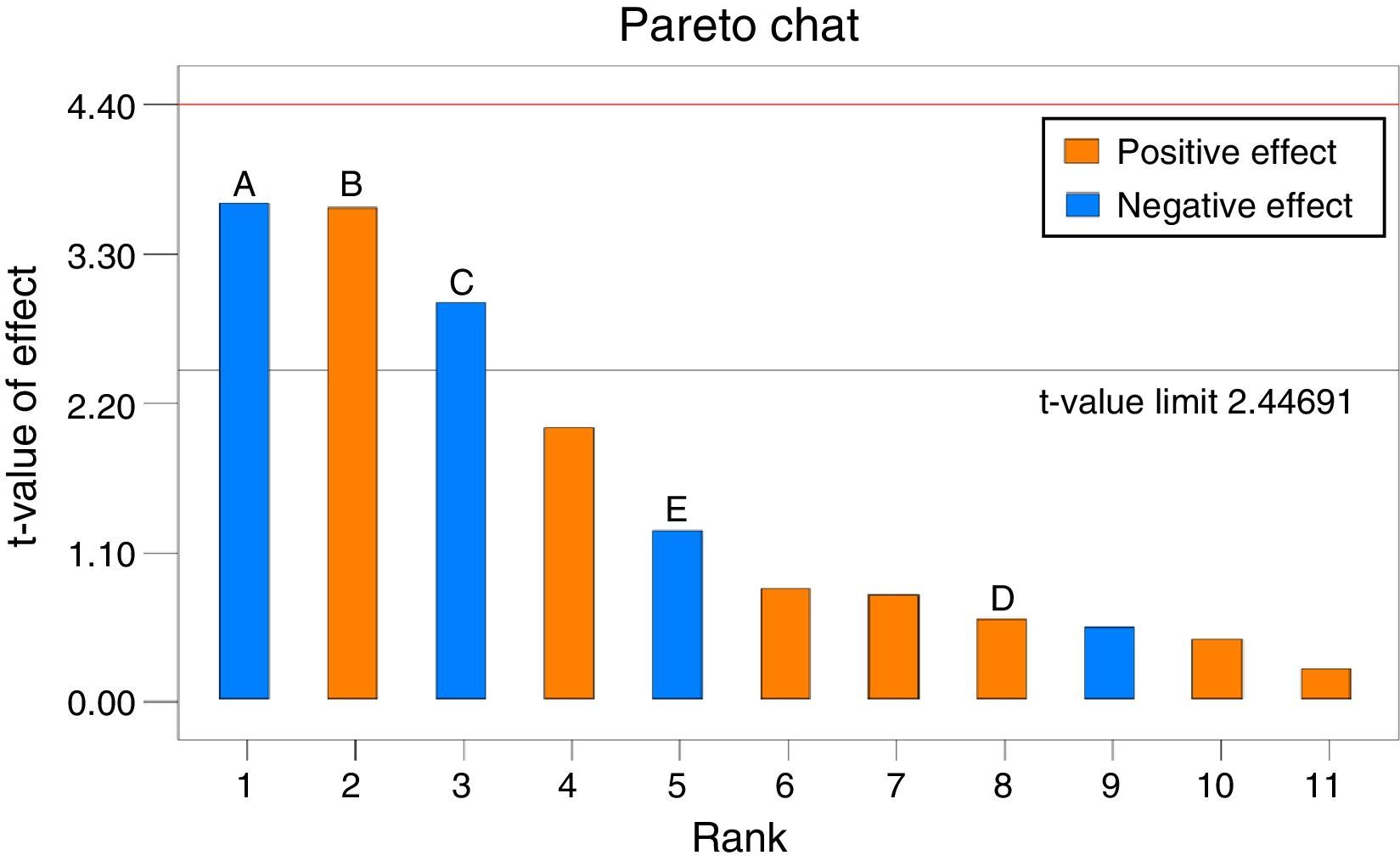
The significance of each variable for ethanol production by S. cerevisiae KKU-VN8 was determined using the Plackett–Burman design implemented with Design Expert software. The experimental design matrices as well as the response variable were evaluated. The ethanol concentrations measured in 12 experiments ranged from 41.87 to 57.76g/L (Additional information section, Table 1). To identify significant variables affecting ethanol production, a Pareto chart of standardized effects was constructed (Fig. 6). Based on the t-value limit, three variables, specifically initial sugar concentration (A), initial pH (B) and (NH4)2SO4 concentration (C), were considered significant variables; variables A and C exerted negative effects on ethanol production, while variable B exerted a positive effect.
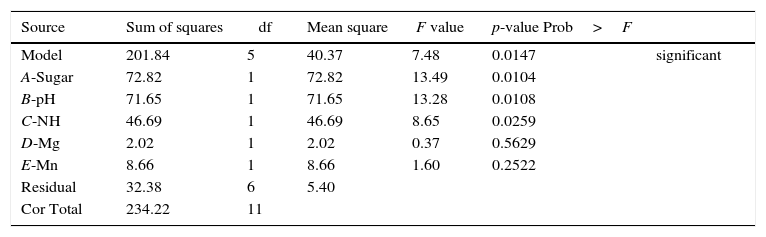
The statistical significances of the variables and the established model were confirmed by ANOVA (Table 3). In this study, the established model was highly significant because the p-value of the experiment was less than 0.05; thus, the model is reliable. As shown in Table 3, variables A, B and C exerted significant effects on ethanol production from SSJ, as demonstrated in the Pareto chart analysis. Therefore, these three variables were chosen for optimization experiments using the Box–Behnken design to identify the best combination of variables resulting in maximum ethanol production efficiency.
Analysis of variance for selected factorial model for ethanol concentration.
| Source | Sum of squares | df | Mean square | F value | p-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 201.84 | 5 | 40.37 | 7.48 | 0.0147 | significant |
| A-Sugar | 72.82 | 1 | 72.82 | 13.49 | 0.0104 | |
| B-pH | 71.65 | 1 | 71.65 | 13.28 | 0.0108 | |
| C-NH | 46.69 | 1 | 46.69 | 8.65 | 0.0259 | |
| D-Mg | 2.02 | 1 | 2.02 | 0.37 | 0.5629 | |
| E-Mn | 8.66 | 1 | 8.66 | 1.60 | 0.2522 | |
| Residual | 32.38 | 6 | 5.40 | |||
| Cor Total | 234.22 | 11 |
The experimental design matrices and the ethanol concentration (g/L), as the response variable, were evaluated. Ethanol concentrations measured in 16 experiments ranged from 31.18 to 59.10g/L (Additional information section, Table 2). To develop a quadratic polynomial regression model and a second-order polynomial equation (1) to predict the final ethanol concentration (P, g/L) as a function of the fermentation variables, including the initial sugar concentration (A), the initial pH (B) and the (NH4)2SO4 concentration (C), the ethanol concentrations were used, and the prediction equation was as follows:
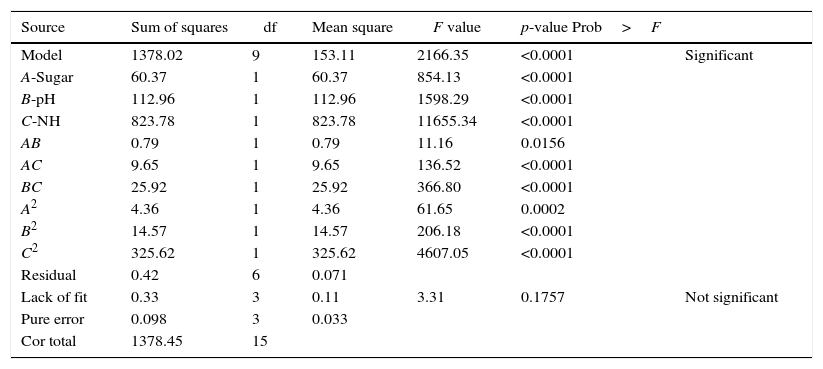
The statistical significance of the model was determined by ANOVA (Table 4). The established model was highly significant (p-value less than 0.05). In addition, the linear terms of the initial sugar concentration (A), initial pH (B), and (NH4)2SO4 concentration (C), the interactions between each variable (AB, AC and BC) and the quadratic terms of each variable (A2, B2 and C2) were also significant. The R2 value of the regression (0.9997) suggested that 99.97% of the variability in the response was explained by this model. The p-value obtained from the “lack-of-fit tests” (0.1757) was not significant (p-value greater than 0.05). Based on these results, the model is clearly reliable.
Analysis of variance for response surface of quadratic model for the Box–Behnken design.
| Source | Sum of squares | df | Mean square | F value | p-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 1378.02 | 9 | 153.11 | 2166.35 | <0.0001 | Significant |
| A-Sugar | 60.37 | 1 | 60.37 | 854.13 | <0.0001 | |
| B-pH | 112.96 | 1 | 112.96 | 1598.29 | <0.0001 | |
| C-NH | 823.78 | 1 | 823.78 | 11655.34 | <0.0001 | |
| AB | 0.79 | 1 | 0.79 | 11.16 | 0.0156 | |
| AC | 9.65 | 1 | 9.65 | 136.52 | <0.0001 | |
| BC | 25.92 | 1 | 25.92 | 366.80 | <0.0001 | |
| A2 | 4.36 | 1 | 4.36 | 61.65 | 0.0002 | |
| B2 | 14.57 | 1 | 14.57 | 206.18 | <0.0001 | |
| C2 | 325.62 | 1 | 325.62 | 4607.05 | <0.0001 | |
| Residual | 0.42 | 6 | 0.071 | |||
| Lack of fit | 0.33 | 3 | 0.11 | 3.31 | 0.1757 | Not significant |
| Pure error | 0.098 | 3 | 0.033 | |||
| Cor total | 1378.45 | 15 |
R-Squared (R2)=0.9997; Adj R-Squared=0.9992.
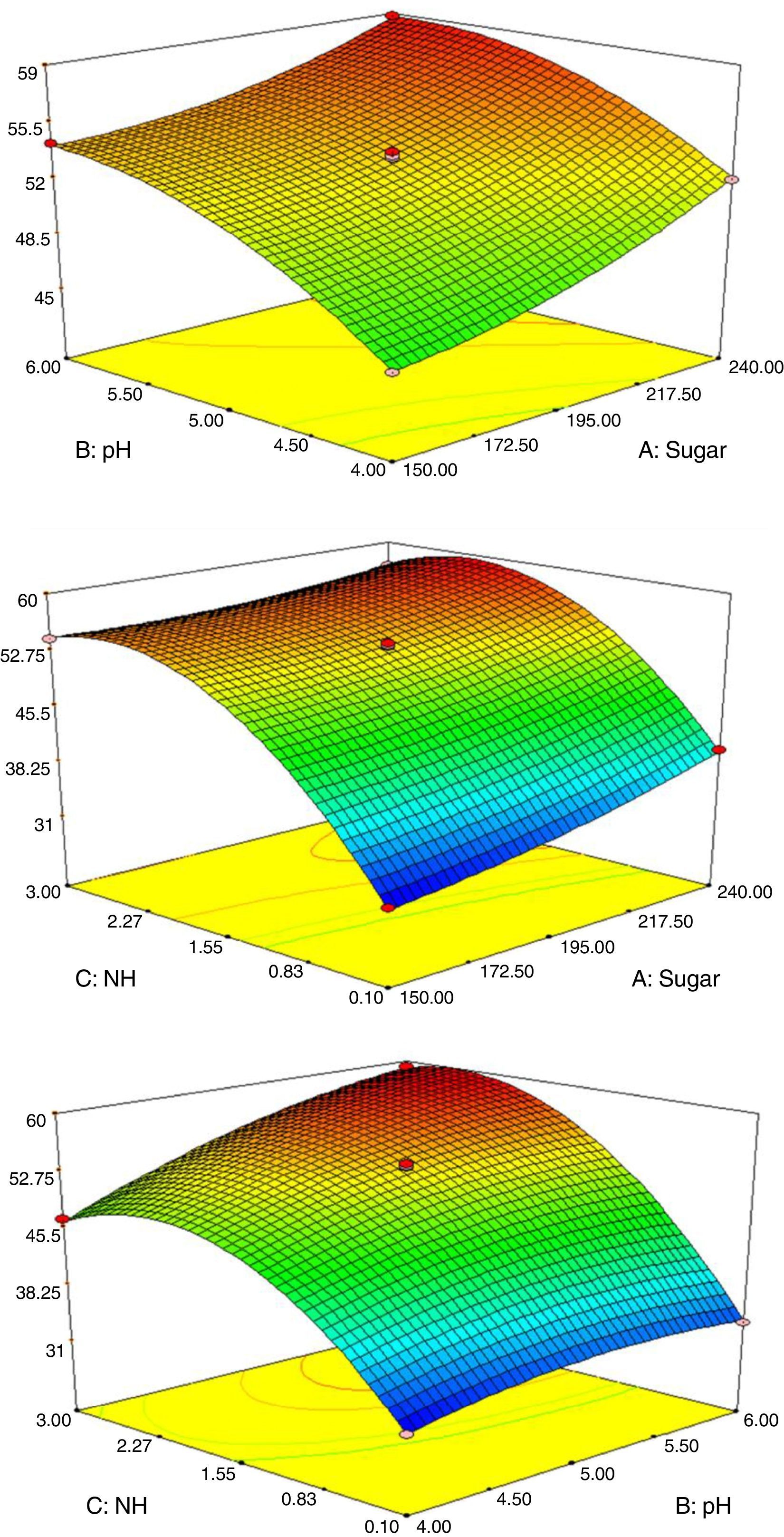
3-D response surface plots illustrating the effects of all selected variables on ethanol production by S. cerevisiae KKU-VN8 at 40°C are shown in Fig. 7. Fig. 7A shows the effects of the initial sugar concentration and the initial pH on ethanol concentration. Ethanol concentration increased when the levels of both variables increased. In addition, ethanol concentration gradually increased when levels of the initial sugar concentration and the (NH4)2SO4 concentration increased (Fig. 7B). Fig. 7C shows the effects of initial pH and (NH4)2SO4 concentration on the ethanol concentration when the initial sugar concentration is fixed at the center point. According to the results, the ethanol concentration increased when the initial pH and the (NH4)2SO4 concentration increased to a certain level.
Response surface plots of effects of the initial sugar concentration and the initial pH (A), the initial sugar concentration and the ammonium sulfate concentration (B) and the initial pH and the ammonium sulfate concentration (C) on efficiency of ethanol production. Increase in the initial sugar concentration, the initial pH and the ammonium sulfate concentration resulted in varying ethanol concentrations from 31.18g/L (blue) to 59.10g/L (red).
To verify the predicted optimal values, repeated ethanol production experiments utilizing SSJ at 40°C with S. cerevisiae KKU-VN8 were performed. The following final optimal values of the selected variables from the repeated experiments were achieved: initial sugar concentration of 238.52g/L, initial pH of 5.82 and (NH4)2SO4 concentration of 2.04g/L. The maximum ethanol concentration (65.67g/L), volumetric ethanol productivity (1.37g/L/h) and theoretical ethanol yield (93.24%) were achieved under the optimal conditions and were reliably similar to the predicted values (data not shown).
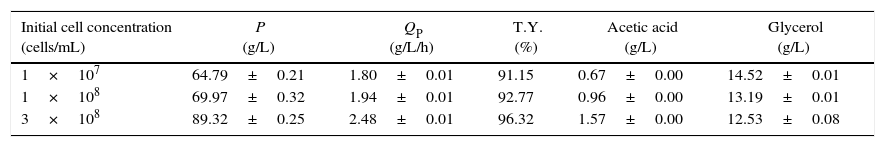
Effects of the initial cell concentration of S. cerevisiae KKU-VN8 on ethanol production efficiency using SSJ as a substrateSince 49.64% of the remaining sugars were observed in the medium after 48h of the optimized ethanol fermentation, it was plausible that increasing the initial cell concentration correlated with increased ethanol production. Thus, the experiments varying the initial cell concentration from 1×107cells/mL to 3×108cells/mL were conducted. Effects of the initial cell concentrations on ethanol production at 40°C using SSJ under optimized conditions are summarized in Table 5. By increasing the initial yeast cell concentrations from 1×107cells/mL to 3×108cells/mL, ethanol production after the fermentation time of 36h greatly increased. It was demonstrated that the maximal ethanol concentration of 89.32g/L with the productivity and the theoretical ethanol yield of 2.48g/L/h and 96.32%, respectively, were achieved when the initial cell concentration of 3×108 cells/mL was used (Table 5). The by-products, i.e., acetic acid and glycerol were quantified after 36h of fermentation. Acetic acid and glycerol concentrations of 1.57 and 12.53g/L, respectively, were observed (Table 5).
Ethanol production from SSJ by S. cerevisiae KKU-VN8 at 40°C for 36h.
| Initial cell concentration (cells/mL) | P (g/L) | QP (g/L/h) | T.Y. (%) | Acetic acid (g/L) | Glycerol (g/L) |
|---|---|---|---|---|---|
| 1×107 | 64.79±0.21 | 1.80±0.01 | 91.15 | 0.67±0.00 | 14.52±0.01 |
| 1×108 | 69.97±0.32 | 1.94±0.01 | 92.77 | 0.96±0.00 | 13.19±0.01 |
| 3×108 | 89.32±0.25 | 2.48±0.01 | 96.32 | 1.57±0.00 | 12.53±0.08 |
P, ethanol concentration (g/L); QP, volumetric ethanol productivity (g/L/h); T.Y., theoretical yield of ethanol (%).
The results were expressed as mean±SD.
Heat and ethanol stresses induce functionally overlapping stress responses in yeast cells. Yeasts respond to heat and ethanol stresses by synthesizing heat-shock proteins following exposure to temperatures above 35°C or ethanol levels greater than 4–6% (v/v).40 The combination of a temperature of 35°C and an ethanol concentration of 4% (v/v) is suitable and chosen to screen for thermotolerant yeasts.3 If a yeast is able to grow at a temperature slightly above the maximum temperature (Tmax) of mesophilic yeast, which ranges from 35 to 40°C, it is considered thermotolerant.41 Thus, the results from this study indicated that putative thermotolerant yeasts were obtained. Many studies have reported thermotolerant yeasts growing at temperature ranges similar to those described in this study; however, differential ethanol production was observed.8,12,15,17,42 The differences in ethanol production might be due to differences in natural sources for the isolation and strains of thermotolerant yeasts, in which this study differs from those reported by Limtong et al.,3 Yuangsaard et al.,15 Kwon et al.,17 Kaewkrajay et al.,43 and Charoensopharat et al.44
A high ethanol concentration above 10% (v/v) negatively affects yeast growth because it inhibits cell division and cell viability, resulting in a decreased specific growth rate or increased cell death.45,46 Therefore, yeasts that tolerate stress conditions, particularly ethanol stress, are required for ethanol production. The results obtained in our study in terms of ethanol tolerance were consistent with those of Negi et al.,32 and Tikka et al.,39 who found that S. cerevisiae strain N and strain YMI, respectively, tolerated ethanol concentrations up to 12% (v/v). However, the mutant S. cerevisiae UVNR56 tolerated higher ethanol concentrations than those observed in this study because S. cerevisiae UVNR56 was treated with UV-C radiation, which potentially modified the genes involved in certain stress-tolerant mechanisms such that ethanol tolerance was improved to 15% (v/v).47
High temperatures and high ethanol concentrations are major stress factors for yeast during the fermentation process, inhibiting cell division and the specific growth rate.48 In addition, these factors affect various transport systems such as the general amino acid permease and glucose uptake processes.5,49 The yeast isolates obtained in our study, which possess high temperature and ethanol tolerance levels, potentially develop both short-term and long-term stress response mechanisms to cope with the deleterious effects of these stresses, including the synthesis of heat shock proteins (HSPs) and trehalose as well as alterations to the lipid composition of the plasma membrane.50–53
The observed physical appearances and microscopic characteristics of the yeasts were in good agreement with the results of other investigators.12,42 For molecular and phylogenetic analyses, both ITS regions and D1/D2 domain were employed to identify thermotolerant yeasts at species levels. According to Chen et al.,54 the ITS1 and ITS2 regions are useful to differentiate closely related species, particularly those with identical D1/D2 domain DNA sequences. In addition, these regions distinguish yeast strains exhibiting differences of 10bp or more in terms of PCR product lengths.55 Thus, species identification accuracy should depend on both the D1/D2 domain of the large subunit of 26S rDNA as well as the ITS1 and ITS2 regions. There are five yeast species identified in this study, i.e., C. tropicalis, P. kudriavzevii, S. cerevisiae, T. globosa and C. glabrata. Although C. tropicalis was previously reported as a leukemia-causing medical pathogen, it is also a candidate for ethanol production from olive prunings56 and rice straw hydrolysate.57P. kudriavzevii was previously reported to produce ethanol from sugarcane juice,4 cornstalk hydrolysate17 and cassava hydrolysate.15S. cerevisiae is frequently employed and demonstrates high potential in industrial ethanol production. Substrates utilized for ethanol production by S. cerevisiae are cassava powder hydrolysate,12 wheat straw hydrolysate18 and SSJ.10T. globosa is reported to be utilized for the biological control of fungal growth,58 oil production for biodiesel59,60 and ethanol production.61 However, C. glabrata is reported to be a medical pathogen.62,63
The ethanol concentrations produced by yeast strains obtained in our study were greater than those reported by Sree et al.,8 when using glucose as a substrate under the same fermentation conditions at 40°C. The same trend was clearly observed when SSJ was used as a substrate for ethanol production. The promising strains, particularly S. cerevisiae KKU-VN8, produced much higher ethanol concentrations than that of the reference strain as well as those reported by Sree et al.,8 Tomás-Pejó et al.,64 and Kaewkrajay et al.,43 at 40°C and 43°C. These results may be attributable to the higher levels of stress tolerance exhibited by the potential strains during fermentation, leading to better ethanol production and the unique stress-tolerant mechanisms, allowing the yeasts cope with environmental stresses during ethanol fermentation, including the synthesis of Hsps and trehalose.50–53 Moreover, the batch culture profiles showed that ethanol production and sugar consumption decreased as temperature increased, potentially because some sugars were converted to by-products such as glycerol, succinate, acetic acid and fusel oil.6 Yeast cells are affected by various stress factors, including nutrient deficiency, high temperature, organic acids, osmotic pressure and ethanol stress.9 These stresses inhibit not only yeast growth and viability but also affect other metabolic processes. To cope with these problems, Hsps, trehalose and glycogen metabolism are induced to protect yeast cells from these stresses.50,51,53,65
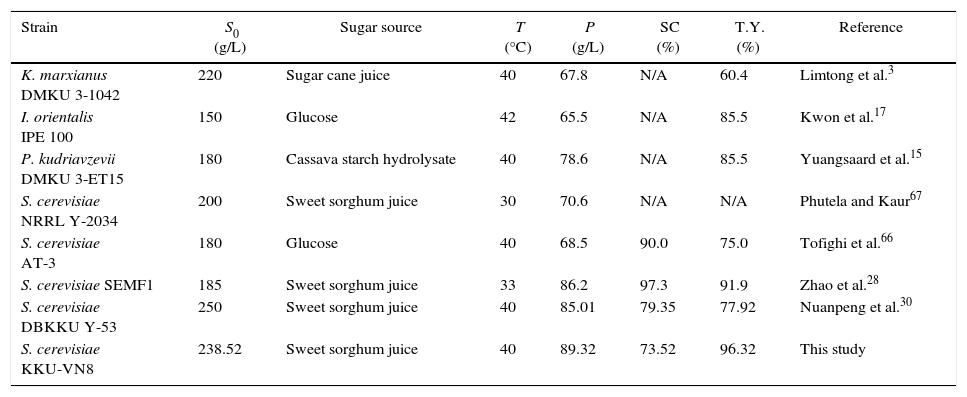
Significant variables and their interactions as well as the response variables were determined with the reliable model and validated. The Box–Behnken design was employed and demonstrated the best combination of variables, resulting in maximal ethanol production efficiency. A comparative analysis of the ethanol production from SSJ by the newly isolated thermotolerant S. cerevisiae KKU-VN8 strain and other raw materials used in combination with different yeast strains reported in the literature is shown in Table 6. The ethanol concentration produced by S. cerevisiae KKU-VN8 was higher than that of K. marxianus DMKU 3-1042 using sugar cane juice as a substrate,3Issatchenkia orientalis IPE10017 and S. cerevisiae AT-366 using glucose, P. kudriavzevii DMKU 3-ET15 using cassava starch hydrolysate,15S. cerevisiae NRRL Y-2034,67S. cerevisiae DBKKU Y-5330 and S. cerevisiae SEMF128 using SSJ. Although the percentage of sugar consumption by S. cerevisiae KKU-VN8 was lower than those of Zhao et al.,28 Nuanpeng et al.,30 it exhibited a substantially greater theoretical ethanol yield than those previously reported (Table 6). The differences in ethanol production efficiency are potentially attributable to differences in raw materials, yeast strains and initial cell concentration used for fermentation. In particular, the determination of the appropriate initial cell concentration is essential for the improvement of substrate utilization and ethanol yield, as typically observed in industrial ethanol fermentation.7 Charoensopharat et al.,44 Nuanpeng et al.,30 and Laopaiboon et al.10 reported that increasing the initial cell concentration improved the ethanol concentration and productivity. Our study also demonstrated that a high initial cell concentration had a positive effect on sugar utilization, which was increased from 50.36% to 73.52%, leading to the maximal ethanol concentration and theoretical ethanol yield. Acetic acid and glycerol were formed during ethanol fermentation as observed in this study; however, they were detected at low amount, which were comparable to other studies.68–70 In this study, SSJ supplemented only with (NH4)2SO4 was directly used by S. cerevisiae KKU-VN8 to produce ethanol, which differs from other conditions reported in the literature.3,15,24–28,30,66
Comparison of ethanol production by S. cerevisiae KKU-VN8 and other yeast strains reported in the literatures.
| Strain | S0 (g/L) | Sugar source | T (°C) | P (g/L) | SC (%) | T.Y. (%) | Reference |
|---|---|---|---|---|---|---|---|
| K. marxianus DMKU 3-1042 | 220 | Sugar cane juice | 40 | 67.8 | N/A | 60.4 | Limtong et al.3 |
| I. orientalis IPE 100 | 150 | Glucose | 42 | 65.5 | N/A | 85.5 | Kwon et al.17 |
| P. kudriavzevii DMKU 3-ET15 | 180 | Cassava starch hydrolysate | 40 | 78.6 | N/A | 85.5 | Yuangsaard et al.15 |
| S. cerevisiae NRRL Y-2034 | 200 | Sweet sorghum juice | 30 | 70.6 | N/A | N/A | Phutela and Kaur67 |
| S. cerevisiae AT-3 | 180 | Glucose | 40 | 68.5 | 90.0 | 75.0 | Tofighi et al.66 |
| S. cerevisiae SEMF1 | 185 | Sweet sorghum juice | 33 | 86.2 | 97.3 | 91.9 | Zhao et al.28 |
| S. cerevisiae DBKKU Y-53 | 250 | Sweet sorghum juice | 40 | 85.01 | 79.35 | 77.92 | Nuanpeng et al.30 |
| S. cerevisiae KKU-VN8 | 238.52 | Sweet sorghum juice | 40 | 89.32 | 73.52 | 96.32 | This study |
S0, initial sugar concentration (g/L); P, ethanol concentration (g/L); SC, Sugar consumption (%); T.Y., theoretical yield of ethanol (%); N/A, not available.
In this study, five thermotolerant yeast strains, specifically P. kudriavzevii KKU-TH33 and KKU-TH43 and S. cerevisiae KKU-VN8, KKU-VN20 and KKU-VN27, were successfully isolated. Enhanced stress tolerance levels were observed at high temperatures up to 45°C and ethanol concentrations up to 13% (v/v). The ethanol production capabilities of these strains were greater than that of the reference strain at elevated temperatures. Maximal ethanol production at 37°C was achieved by S. cerevisiae KKU-VN8, which produced an ethanol concentration of 72.69g/L, a productivity of 1.59g/L/h and an ethanol yield of 0.44g/g using glucose as a substrate. When SSJ only was employed as a raw material, this strain also exhibited the highest ethanol production at 40°C, with the ethanol concentration, the productivity and the yield of 48.51g/L, 1.01g/L/h and 0.43g/g, respectively. The BBD was used to identify the optimal conditions for ethanol production from SSJ at high temperatures by S. cerevisiae KKU-VN8. When the initial yeast cell concentration of 3×108 cells/mL was used, the maximal ethanol concentration of 89.32g/L with the productivity and the theoretical ethanol yield of 2.48g/L/h and 96.32%, respectively, were achieved under the optimized conditions. Ethanol concentration increased by approximately 45.7% when compared to the unoptimized condition. Our findings provide significant information regarding this candidate yeast for use as an ethanol producer and represent a promising starting point to improve ethanol production processes in the near future. To gain further insights into the molecular mechanisms by which yeast cells adapt to adverse environmental conditions and acquire thermotolerance during ethanol fermentation at high temperatures, the expression of genes involved in specific metabolic processes such as carbohydrate and fatty acid metabolism, the heat shock response, oxidative stress defense, cellular signaling and protein folding and degradation will also be determined.
Conflicts of interestThe authors declare no conflicts of interest.
This research was financially supported by the National Research University Project of Thailand through the Biofuel Research Cluster of Khon Kaen University, Thailand (NRU 543042 and NRU-KKU-Ph.D 54310). We are grateful to the Fermentation Research Center for Value Added Agricultural Products (FerVAAP) and the Department of Biotechnology, Faculty of Technology, Khon Kaen University for supporting the equipment and facilities for this research.