Onychomycosis is a fungal infection of the nail caused by high densities of filamentous fungi and yeasts. Treatment for this illness is long-term, and recurrences are frequently detected. This study evaluated in vitro antifungal activities of 12 organic compounds derived from amino alcohols against standard fungal strains, such as Trichophyton rubrum CCT 5507 URM 1666, Trichophyton mentagrophytes ATCC 11481, and Candida albicans ATCC 10231. The antifungal compounds were synthesized from p-hydroxybenzaldehyde (4a–4f) and p-hydroxybenzoic acid (9a–9f). Minimum inhibitory concentrations and minimum fungicidal concentrations were determined according to Clinical and Laboratory Standards Institute protocols M38-A2, M27-A3, and M27-S4. The amine series 4b–4e, mainly 4c and 4e compounds, were effective against filamentous fungi and yeast (MIC from 7.8 to 312μg/mL). On the other hand, the amide series (9a–9f) did not present inhibitory effect against fungi, except amide 9c, which demonstrated activity only against C. albicans. This allowed us to infer that the presence of amine group and intermediate carbon number (8C–11C) in its aliphatic side chain seems to be important for antifungal activity. Although these compounds present cytotoxic activity on macrophages J774, our results suggest that these aromatic compounds might constitute potential as leader molecules in the development of more effective and less toxic analogs that could have considerable implications for future therapies of onychomycosis.
Onychomycosis is a fungal infection of the nails caused by dermatophytic fungi and yeasts.1–5 The main etiological agents are Trichophyton, Epidermophyton, and Candida fungi.6,7 Onychomycosis is an emerging global health problem, as it represents 50% of nail disorders1,8,9 and affects 2–9% of the world population.2,10 This pathological condition affects quality of life, with loss of the patient self-esteem and may result in physical, occupational, and social limitations.2,11,12
Notable among the main factors related to onychomycosis are humidity, wearing closed shoes, repetitive nail trauma, genetic predisposition, and chronic diseases, such as diabetes, HIV, and immunosenescence.2,10,13–16 Moreover, the main etiological agents of onychomycosis in infected areas are Trichophyton rubrum (60%), Trichophyton mentagrophytes (20%), Epidermophyton floccosum (10%), and Candida albicans (5–10%).1–3,9 The T. mentagrophytes complex is particularly difficult to identify because of the morphological features and the inter-specific relationships within this group are unclear, requiring molecular tools to identify species in this complex.17
Despite recent advances in medicine, treating onychomycosis remains challenging due to the anatomical characteristics of nails and the poor efficacy of currently available treatments.1,18–20 Existing therapies for onychomycosis are only partially effective, and recurrence and consequent fungal resistance are common when combined with poor medication compliance.21–25 Furthermore, oral antifungal drugs are associated with undesirable side effects, such as hepatotoxicity.8,10,19 Therefore, new alternatives to prevent, diagnose, and treat onychomycosis are of utmost importance.26 Thus, the discovery of novel antifungal compounds is urgently necessary to develop more effective, economical therapies with fewer side effects. Amine and amide groups are significant functional groups in medications due to their reactive and chemical properties,27 and these groups comprise peptides found in various proteins.28 Our previous data show that amino alcohols have antimicrobial and immunological activities against Trypanosoma cruzi.29–32 Thus, the present study evaluated the antifungal activities of amphiphilic aromatic amino alcohols and amides against different strains of fungi associated with onychomycosis.
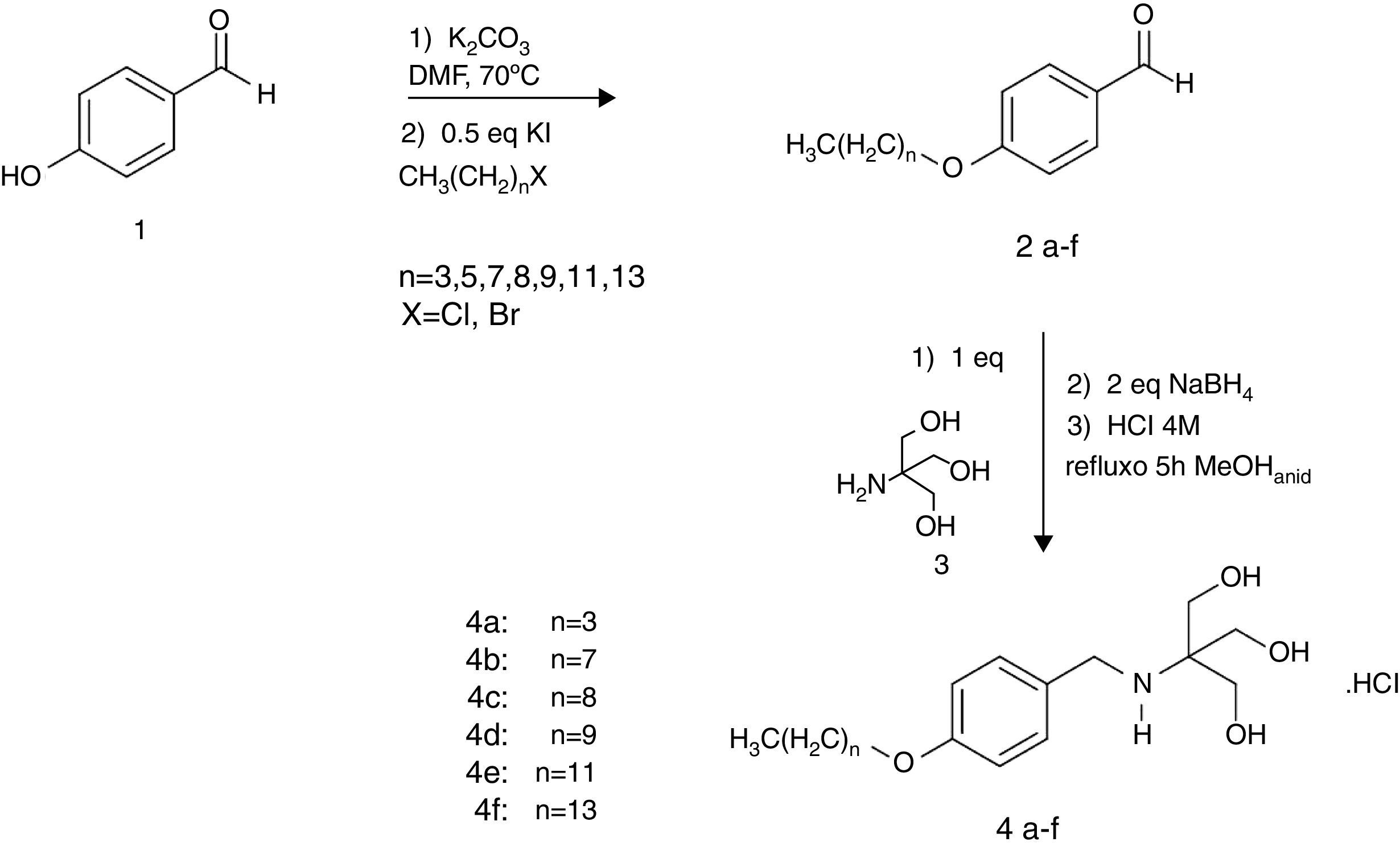
Materials and methodsChemistryAmino alcohols 4a–4f were synthesized from p-hydroxybenzaldehyde 1, as described previously by Almeida et al. (2013) and amides 9a–9f were synthesized from p-hydroxybenzoic acid 5.29 Briefly, compound 1 was previously O-alkylated and then submitted to a direct reductive amination reaction in the presence of 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris), resulting in targeted amino alcohols 4a–4f with 32–87% yield (Fig. 1). Amphiphilic aromatic amides 9a–9f was obtained from O-alkylated esters 7a–7f prepared from carboxylic acid 5. After hydrolysis of the ethyl ester, the carboxyl group was converted to acyl chloride and treated with Tris, resulting in the desired amides (Fig. 2).
Synthesis of the amine series adapted from Almeida et al. (2013).29
Synthesis of the amide series adapted from Almeida et al. (2013).29
Experiments were conducted using T. rubrum CCT-5507 URM 1666 obtained from the Collection of Tropical Crops (CCT) provided by the André Tosello Foundation (Campinas, São Paulo, SP, Brazil), T. mentagrophytes ATCC 11481 and C. albicans ATCC 10231 from the American Type Culture Collection (ATCC) were provided by the National Institute of Quality Control in Health-Oswaldo Cruz Foundation (Rio de Janeiro, RJ, Brazil).
Molecular identificationGenomic DNA was extracted from T. mentagrophytes ATCC 11481 to identify the fungal strain complex. Partial sequencing of the internal transcribed spacer (ITS) region was evaluated using ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC).33 Briefly, the conditions were 100ng DNA, 10pmol of each primer, and an annealing temperature of 58°C. Automated sequencing was evaluated using the Sequencing Platform at Fundação Oswaldo Cruz-PDTIS/FIOCRUZ, Brazil. The sequences were edited using Sequencher 4.9 software and compared with BLAST. The ITS sequence isolated from T. mentagrophytes ATCC 11481 was deposited in Genbank under accession number NCBI/GenBank KX132909. This procedure was not necessary for other strains used herein because these standard strains have been investigated recently by different authors,34,35 confirming the characterization of the strains.
Antifungal activityMinimum inhibitory concentration (MIC) assayThe experiment was conducted using broth dilution. The MIC assay was performed according to Clinical and Laboratory Standards Institute (CLSI) protocols M38-A2,36 M27-A3,37 and M27-S4,38 as described previously. All analyses were performed in triplicate. A filamentous fungal suspension was prepared using 7-day cultures maintained in tubes containing Sabouraud dextrose agar (SDA) at 28±2°C, for yeast the microdilution test is incubated at 35±2°C. Then, three washes were performed by adding 2mL of 0.85% sterile saline and 20μL Tween-80. The suspensions were analyzed using a spectrophotometer (Libra S12; Biochrom, Cambourne, UK) and 89–90% transmittance at a wavelength of 530nm.39 The suspensions were diluted 1:50 (v/v) in RPMI-1640 culture medium (Sigma, St. Louis, MO, USA) buffered with 3 (N-morpholino) propanesulfonic acid (MOPS; JT Baker, Griesheim, Germany), resulting in a suspension containing 0.4–5.0×104CFU/mL. The C. albicans ATCC 10231 cultures were used after 48h of growth in tubes with SDA at 37±2°C. The cultures were diluted and analyzed with a spectrophotometer as described above. Finally, the suspension was diluted in RPMI-1640 culture medium and buffered with MOPS to provide a suspension of 5–25×102CFU/mL.37,40
The amphiphilic aromatic amino alcohol and amide compounds were solubilized in RPMI-1640 culture medium, buffered with MOPS, and tested for filamentous fungi at final concentrations of 7.8–1000μg/mL and 39.06–5000μg/mL for yeasts. Fungal growth was assessed by adding 100μL RPMI-1640 culture medium buffered with MOPS containing the fungal inoculum. The fungi were homogenized for 2min and incubated in a sterile 96-well plate at 28±2°C for seven days (dermatophytes) or 37±2°C for 48h (yeasts). The fungi were analyzed visually using a SMZ800 microscope (Nikon, Melville, NY). Terbinafine, ketoconazole, and amphotericin B were used as reference drugs and assessed in accordance with the M38-A236 and M27-A337 protocols.
Minimum fungicidal concentration (MFC) analysisThe MFC of filamentous fungi was determined by plating 10μL from wells without fungal growth to a new sterile 96-well microplate containing 200μL of Sabouraud dextrose broth (SDB), as described previously.41 The MFC was determined as the lowest concentration of the aromatic compound that reduced the initial fungal count >99.9%. The MFC for C. albicans was evaluated using 5μL from wells without fungal growth, which was transferred to cryotubes containing 1000μL SDB. The analysis was conducted as described above. All experiments were performed in triplicate, and the results are presented as geometric means of the replicates.
Cell viability assayCytotoxic effects in J774 cells were assessed by culturing macrophages (5×105) with different concentrations of the amine compounds (4b–4e) (0.1–100μg/mL) in 96-well tissue culture plates at 37°C in 5% CO2 for 48h. Cell viability was determined by the colorimetric MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide].42 The cytotoxicity results were displayed as percentage of cell viability comparing with untreated cells (100% of viability). Absorbance of the solubilized MTT formazan product was spectrophotometrically measured at 540nm using a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA). All samples were run in triplicate and analyzed on the same day to minimize day-to-day variation.
ResultsThe sequence obtained by partial sequencing of the ITS region (NCBI/GenBank KX132909) was isolated from ATCC 11481 and was 100% concordant with that of T. mentagrophytes JX122271 deposited in Genbank. The MIC and MFC values of the compounds, as well as those of terbinafine, ketoconazole, and amphotericin B, were assessed in the fungal strains T. rubrum, T. mentagrophytes, and C. albicans (Table 1).
In vitro inhibitory activity of amphiphilic aromatic amino alcohols and amides.
| Compounds | Fungal strains | |||||
|---|---|---|---|---|---|---|
| Trichophyton rubrum CCT 5507 URM 1666 | Trichophyton mentagrophytes ATCC 11481 | Candida albicans ATCC 10231 | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | |
| 4a | 62.5 | 500 | 125 | 125 | >5000 | ND |
| 4b | 62.5 | 125 | 15.62 | 15.62 | 78 | 312.5 |
| 4c | 7.8 | 15.62 | 15.62 | 15.62 | 31.25 | 31.25 |
| 4d | 31.25 | 31.25 | 31.25 | 125 | 62.5 | 125 |
| 4e | 15.62 | 62.5 | 7.8 | 62.5 | 15.65 | 15.65 |
| 4f | >1000 | ND | 62.5 | 125 | 500 | >5000 |
| 9a | >1000 | ND | >1000 | ND | >5000 | ND |
| 9b | >1000 | ND | >1000 | ND | >5000 | ND |
| 9c | >1000 | ND | >1000 | ND | 625 | >5000 |
| 9d | >1000 | ND | >1000 | ND | >5000 | ND |
| 9e | >1000 | ND | >1000 | ND | >5000 | ND |
| 9f | >1000 | ND | >1000 | ND | >5000 | ND |
| Terbinafine | 0.19 | 0.19 | 0.03 | 0.03 | NE | NE |
| Ketoconazole | 1 | 4 | 0.25 | 0.25 | NE | NE |
| Amphotericin B | NE | NE | NE | NE | 0.125 | 0.5 |
ND, not determined (MIC >1000μg/mL or MIC >5000μg/mL); NE, not evaluated; MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration. Results are expressed as μg/mL.
As described in Table 1, the amine series (4b–4e) was effective against T. rubrum CCT 5507 URM 1666 (MIC≤62.5μg/mL and MFC≤500μg/mL), T. mentagrophytes ATCC 11481 (MIC≤31.25μg/mL and MFC≤125μg/mL), and C. albicans ATCC 10231 (MIC≤62.5μg/mL and MFC≤312.5μg/mL). Compound 4c almost abolished growth of T. rubrum CCT 5507 URM 1666 (MIC=7.8μg/mL and MFC=15.62μg/mL) and T. mentagrophytes ATCC 11481 (MIC=15.62μg/mL and MFC=15.62μg/mL). Notably, this effect was quite similar to that caused by terbinafine (0.03–1μg/mL) and ketoconazole (0.25–16μg/mL) (Table 1), which were the clinical antifungal reference drugs (Table 1). Moreover, compounds 4a and 4f moderately inhibited growth of T. rubrum and T. mentagrophytes, suggesting that antifungal activity may be related to the number of carbon atoms (8C–12C) in the aliphatic chain. Nevertheless, amides 9a–9f failed to inhibit any of the filamentous fungal or yeast strains (Table 1), except amide 9c, which demonstrated activity against C. albicans ATCC 10231 (fungistatic at 625μg/mL). Amphotericin B, which is used clinically, inhibited growth of C. albicans (MIC=0.125μg/mL and MFC=0.5μg/mL) (Table 1).
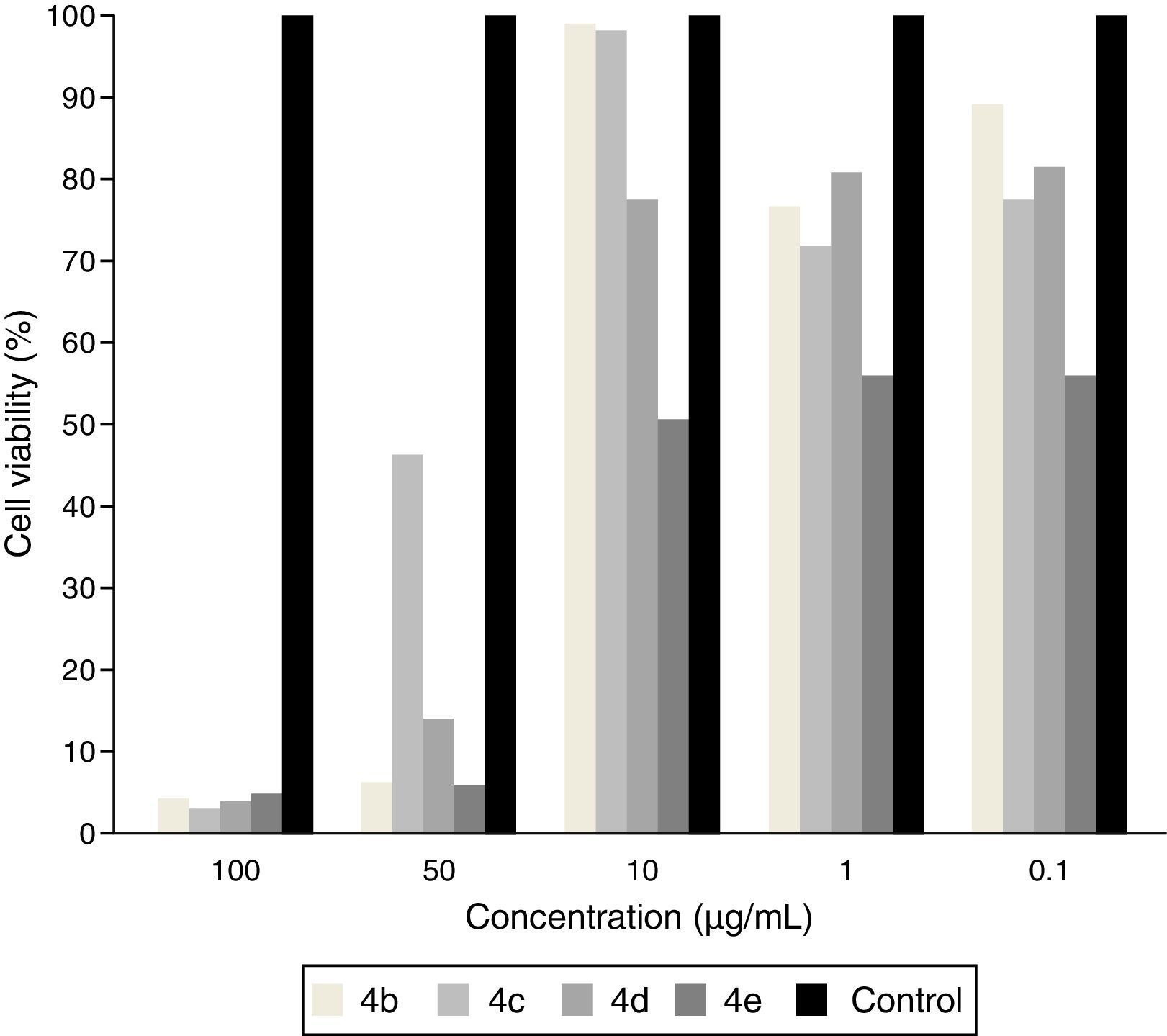
Aiming to rule out the possibility of cytotoxic activity of compounds 4b–4e, we accessed in another set of experiments the amine molecules on macrophages J774. Results depicted in Fig. 3 show that compounds 4b–4d at 0.1–10μg/mL displayed lower cytotoxic effect on macrophage (cell viability more than 80%). The compound 4e decreased the viability of macrophages by 44.1%, 44.4% and 49.9% at the concentration 0.1, 1 and 10μg/mL, respectively (Fig. 3). All compounds 4b–4e reduced significantly the cell viability only at the concentration of 50 and 100μg/mL.
DiscussionWe identified a new partial sequence in the ITS region from the T. mentagrophytes ATCC 11481-NCBI/GenBank KX132909 complex due to the difficulties of identifying different species using only phenotypic characteristics, as reported previously by Packeu et al. (2013).43 Moreover, this procedure was not necessary for other strains used herein because these standard strains have been investigated recently by different authors,18,34,44,45 confirming the characterization of the strains.
Importantly, the amphiphilic character of the organic compounds reflects their ability to interact and penetrate biological membranes inducing different effects,29,32,46 such as increased antimicrobial activity.47 Lipophilicity is an important characteristic of antifungal agents, such as terbinafine and ketoconazole, and is also evident in drugs like amphotericin B, which have proven effectiveness to treat systemic fungal infections.46 The relevance of the lipophilic chain in amino alcohols was reported by Almeida et al. (2013),29 who assessed antibacterial activity on some bacterial strains, such as Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, and methicillin-resistant S. aureus, demonstrating that lipophilic compounds show potent antimicrobial activities. In this study, we extended and added to these findings by demonstrating that amino alcohols 4b–4e showed significant antifungal activity, although lipophilic compound 4a and the most lipophilic amino alcohol 4f only displayed antifungal activity against two of the fungal strains tested.
The evaluation of the structure–activity relationships of amines showed that increasing the number of carbons in the aliphatic chain to eight favored antifungal action, corroborating and extending previously published data.48
This reduction in antifungal potential can be associated with size of the carbonic side chains 3C and 13C for 4a and 4f compounds, respectively. The results obtained with compound 4e contradict that higher lipophilicity of the compound permits penetration into the target cell and consequently its pharmacological action, as reported by Gushchina et al. (2015).49 However, this hypothesis was confirmed by Almeida et al. (2013),29 who demonstrated the same profile with amino alcohol compounds, which show less antibacterial action as the carbon chain is elongated. Dolezal et al. (2002) reported marked activity against Clorella vulgaris after associating lipophilicity with its alkoxy substituent.50 Smolarz et al. (2005) discovered that the antifungal potential of 2-carboxy-3,5-dimethoxy-E-stilbene against Trichophyton spp. strains is related to the same physicochemical characteristics reported above.51 Although amides are found in different pharmaceutical compounds,52,53 compounds 9a–9f did not significantly inhibit growth of filamentous fungi or yeast, except compound 9c, which has an alkyl chain bearing eight carbon atoms.
The discrepancy between the antifungal activities of amino alcohols 4a–4f and those of amides 9a–9f suggests that amine groups could contribute to antifungal action, as only amine compounds are in a hydrochloride form, which helps with their water solubility and absorption. Shah et al. (2015) observed similar results when they assessed amine and amide compounds toxic to C. albicans.54 Thus, antifungal potential is related to the size of the lateral aliphatic chain and can directly improve efficacy.55 Surprisingly, our data suggest that intermediate size aliphatic amine chains showed a notable effect, justifying additional experiments.
Previous studies have reported a direct correlation between in vitro cytotoxicity and in vivo acute toxicity in animals and humans56; therefore, investigating the toxicological effects of new antifungal compounds in normal cells are important. Herein, the cytotoxicity of compounds 4b–4e was evaluated using an MTT assay with macrophages. Compound 4e decreased viability of macrophages by 44.1%, 44.4%, and 49.9% at concentrations of 0.1, 1, and 10μg/mL, respectively. Our data also show that compounds 4b–4e reduced cell viability only at 50 and 100μg/mL, indicating that higher concentrations reduced macrophage viability and fungal growth. These results suggest that less toxicity was associated with the presence of an intermediate number of carbons in the side chain, as compounds with eight carbons in the aliphatic chain were more cytotoxic than those with 7, 9, or 11 carbons. Thus, additional in vitro cytotoxic assays should be conducted to determine the safety profile of any potential drug candidate for therapeutic applications in animals and humans.
According to the World Health Organization (WHO), resistant microorganisms (including bacteria, fungi, viruses, and parasites) can withstand attack by antimicrobial drugs, so standard treatments can become ineffective and infections persist, increasing the risk of spread to other microbes, resulting in resistance.57 The 2014 WHO report on global surveillance of antimicrobial resistance revealed that antimicrobial resistance, including antifungal resistance, is no longer a prediction for the future, as it is occurring worldwide now and is increasing the risk of being unable to properly treat common infections in the community and hospitals. For this reason, fostering innovative research and developing new vaccines, diagnostics, infection treatment options, and other tools is urgently needed.22,58–60
In summary, the data presented here demonstrate that amphiphilic aromatic amino alcohols (4b–4e) markedly inhibited standard strains of fungi, such as T. rubrum, T. mentagrophytes and C. albicans. Although these compounds have presented cytotoxic effect on macrophages; they might constitute potential as leader molecules in the development of innovative antifungal (aiming effectiveness and safety) for the treatment of fungal infection, including onychomycosis.
Conflicts of interestNo conflict of interest was declared.
This research was supported by CAPES, CNPq, FAPEMIG, and PROPESQ/UFJF.