Leptospirosis is an infectious and acute disease caused by Leptospira spp. that have high epidemic potential. This study verified the main Leptospira spp. serovars detected by MAT from serum of patients with suspicion of leptospirosis from 2008 to 2012 in Minas Gerais State.
MethodsThe laboratory received sera from 4654 patients. All serum were screened by IgM-ELISA according to the manufacturer's instructions. Each sample reactive or indeterminate were tested against twenty-four serovars of Leptospira by MAT.
ResultsIn this study, 597 patients were classified as reactive on MAT. Only 301 patients were confirmed by laboratory test. It was not possible confirmation by laboratory diagnosis of 296 patients. Among the samples classified as reactive on MAT, 273 patients exhibited titers bigger than 800 for one or more serovars; seroconversion was detected in 28 cases. Percentage of 85.1% of the samples reactive on MAT corresponded to males, 39.4% corresponded to patients aged between 20 and 39 years old. The most common serovars found were Icterohaemorrhagiae, Andamana, Patoc, Tarassovi, Copenhageni, Hardjo and Australis. Concerning the samples that exhibited titers bigger than 800, serovar Icterohaemorrhagiae was also the most common, followed by Copenhageni, Andamana, Patoc, Tarassovi, Grippotyphosa and Canicola. In this study, 40% of the cases occurred to the metropolitan area, state capital and 34 neighboring towns.
ConclusionOur results show the possibly spreading serovars in Minas Gerais State and contribute to knowledge of human leptospirosis, aiming at improving the prevention, control of the disease, as well as the treatment of infected patients.
Leptospirosis is an infectious and acute disease that stands out in the world scenario because of its high epidemic potential, especially after long periods of rain.1 It is caused by bacteria from Leptospira genus, further divided into serovars, which were clustered into serogroups, according to their antigenic relations. The advanced molecular techniques allowed the identification of 14 pathogenic and intermediately pathogenic species, and 7 non-pathogenic species, distinguishing three clades in Leptospira genus.2 These clades comprise more than 260 serovars. In the recent years, the classification system based on DNA homology has been used in combination with the classical antigenic classification.2,3
Human leptospiral infections result primarily from direct or indirect exposure to the urine of infected animals. Leptospira invades the human body through cuts and abrasions in the skin, through intact mucous membranes and through waterlogged skin. Other modes of transmission of this infection are also possible.4
Leptospirosis clinical manifestations range from benign aspects to severe forms. Generally, it manifests as an unspecific acute febrile illness, characterized by fever, myalgia and headaches and can be misinterpreted as other diseases, such as influenza, malaria and dengue.4,5 Indeed, there are many other diseases with similar symptoms, hampering the clinical diagnosis of leptospirosis. Therefore, laboratory and epidemiological approaches are crucial for the conclusion of positive cases.4 The clinical manifestations and severity of the disease depend on the inoculum size, Leptospira strain or serovar involved, as well as the age, health and immune status of the infected individual.6
Pathogenic leptospires are widespread in nature, and various animal species, labor activities and territorial occupations have significant importance in maintaining leptospirosis.1,4 In Brazil, environmental and sanitary factors, such as high temperatures, high rainfall rate and floods, favor rapid spread of the disease, being a serious risk to public health.1,7
Bacteriological, microscopic, serological and molecular methods have been used for laboratory diagnosis of leptospirosis.4,8 The choice of which test to use depends on the evolution phase of the disease, its prevalence and the availability of a qualified laboratory.9
Polymerase chain reaction (PCR) has been widely used to diagnose acute leptospirosis, but this method is not always available in reference laboratories of developing countries, where the disease is highly endemic.9,10
Culture is rarely used in clinical setting because it demands prolonged incubation and shows low sensitivity.4,11 Furthermore, this method is not performed in most public and private laboratories in Brazil because of the high costs, besides not being useful for early diagnosis, requiring considerable expertise. However, this technique has an important role in the study of outbreaks and global epidemiology, providing crucial information for the recognition of new patterns of disease presentation and assessing the effectiveness of intervention measures.12
The Microscopic Agglutination Test (MAT) is the serological reference test, and mostly reference laboratories are able to perform it. Even with the development of molecular methods, MAT remains the gold standard method, recommended by the Brazilian Health Ministry and worldwide recognized for laboratory confirmation of leptospirosis.9,12,13 MAT is an indirect diagnosis that detects specific antibodies against Leptospira, although serum samples can react with more than one serovar.4 Information obtained through MAT has been used in epidemiological studies to infer the possible serovars infectious.10,14,15 The occurrence of serovars detected through MAT must be considered as a general idea of the serogroups/serovars in a population and cannot be used to definitely determine the infecting serovars.4,15
The epidemiology of leptospirosis in Minas Gerais, Brazil, is little known. MAT is the only tool available to infer the possible serogroups/serovars that cause the disease, considering the low sensitivity of the isolation method and the limitations of the Central Laboratory of Public Health of Minas Gerais, Brazil, in identifying new strains of Leptospira spp. through traditional (cross-aglutinin absorption test) or molecular methods.16,17
In different epidemiological settings, various animal species can be considered a source of transmission.18 In Minas Gerais, Brazil, there are insufficient data of the association between different host species and human leptospirosis. Inferring potential infective serovars is very important to evaluate the virulence of these microorganisms. This knowledge contributes to a better understanding of the clinical manifestation of leptospirosis.1,19 Thus, the objective of the present study was to verify the main Leptospira spp. serovars detected by MAT from serum samples of patients with suspicion of leptospirosis in the state of Minas Gerais, Brazil, from 2008 to 2012.
MethodsA descriptive study was conducted from the analysis of data obtained from Ezequiel Dias Foundation, Reference Laboratory for Leptospirosis Diagnosis in Minas Gerais, Brazil.
The Ethics Committee in Research at the Federal University of Minas Gerais, Department of Post Graduate Studies in Infectious Diseases and Tropical Medicine, approved this study, since all data regarding to the patients remained anonymous and unlinked (CAAE:05120113.0.00005149). Internal laboratory records were analyzed without exposing the identities of the patients.
From 2008 to 2012, the laboratory received 5370 serum samples from 4654 patients with leptospirosis suspicion. The samples were received with epidemiological records from the Notifiable Diseases Information System (SINAN). All serum samples were screened through IgM ELISA (PanBio Pty Ltd., Brisbane, Australia) according to the manufacturer's instructions. The cut-off value was assessed using the average absorbance of the calibrator multiplied by the specific calibration factor of each lot. The results were expressed in Pan-Bio units, using an indexed value obtained by dividing the sample absorbance by the cut-off value multiplied by 10. The results were interpreted according to the reference values: reactive sample, Pan Bio unit 11; indeterminate, Pan Bio unit 9–11; and nonreactive, Pan Bio unit <9.
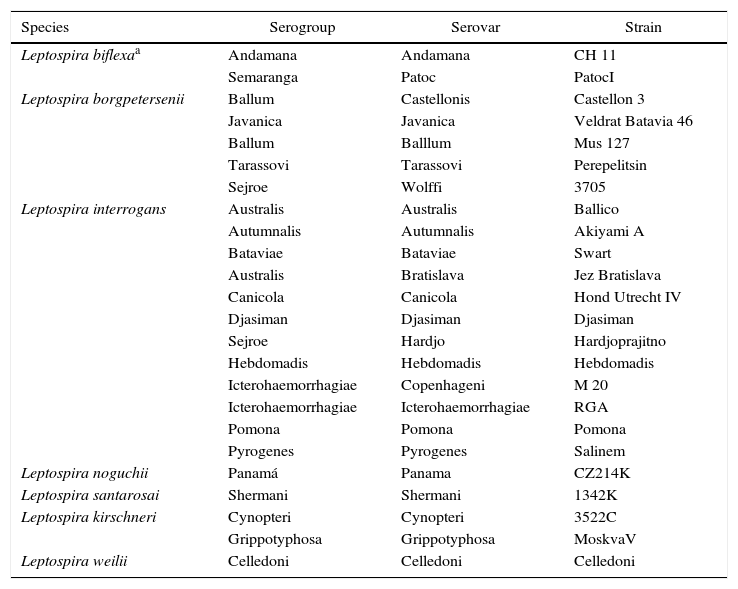
All reactive or indeterminate samples in IgM ELISA were examined by MAT. Each sample was tested against twenty-four serovars (antigens) representing six pathogenic and one non-pathogenic Leptospira species (Table 1). MAT reactive samples for one or more serovars were titrated in 1/100 to 1/6400 dilutions. The considered title was equivalent to the highest serum dilution that showed 50% of agglutinated Leptospira for each tested serovar.
Serovars used in microscopic agglutination test.
| Species | Serogroup | Serovar | Strain |
|---|---|---|---|
| Leptospira biflexaa | Andamana | Andamana | CH 11 |
| Semaranga | Patoc | PatocI | |
| Leptospira borgpetersenii | Ballum | Castellonis | Castellon 3 |
| Javanica | Javanica | Veldrat Batavia 46 | |
| Ballum | Balllum | Mus 127 | |
| Tarassovi | Tarassovi | Perepelitsin | |
| Sejroe | Wolffi | 3705 | |
| Leptospira interrogans | Australis | Australis | Ballico |
| Autumnalis | Autumnalis | Akiyami A | |
| Bataviae | Bataviae | Swart | |
| Australis | Bratislava | Jez Bratislava | |
| Canicola | Canicola | Hond Utrecht IV | |
| Djasiman | Djasiman | Djasiman | |
| Sejroe | Hardjo | Hardjoprajitno | |
| Hebdomadis | Hebdomadis | Hebdomadis | |
| Icterohaemorrhagiae | Copenhageni | M 20 | |
| Icterohaemorrhagiae | Icterohaemorrhagiae | RGA | |
| Pomona | Pomona | Pomona | |
| Pyrogenes | Pyrogenes | Salinem | |
| Leptospira noguchii | Panamá | Panama | CZ214K |
| Leptospira santarosai | Shermani | Shermani | 1342K |
| Leptospira kirschneri | Cynopteri | Cynopteri | 3522C |
| Grippotyphosa | Grippotyphosa | MoskvaV | |
| Leptospira weilii | Celledoni | Celledoni | Celledoni |
The results were interpreted, for laboratory confirmation, according to the criteria established by the Brazilian Ministry of Health. A laboratory-confirmed case of leptospirosis was defined through MAT. A positive MAT was defined for any of the following criteria: (1) seroconversion observation of two samples from the same patient: one sample of the acute phase, 1–14 days after the symptoms started, as non-reactive; and a second sample of the convalescence phase, 14–21 days up to 60 days after the symptoms started, with a title ≥200; (2) demonstration of four-fold microagglutination title rise between paired sera collected within 21 days; or (3) a title equal or higher than 800 with a single sample of serum.13
Data were analyzed from Excel tables. The map was built using Tab for Windows – TabWin, 3.6b version from 02/07/2010, available at http://www2.datasus.gov.br/DATASUS/index.php?are=060805.
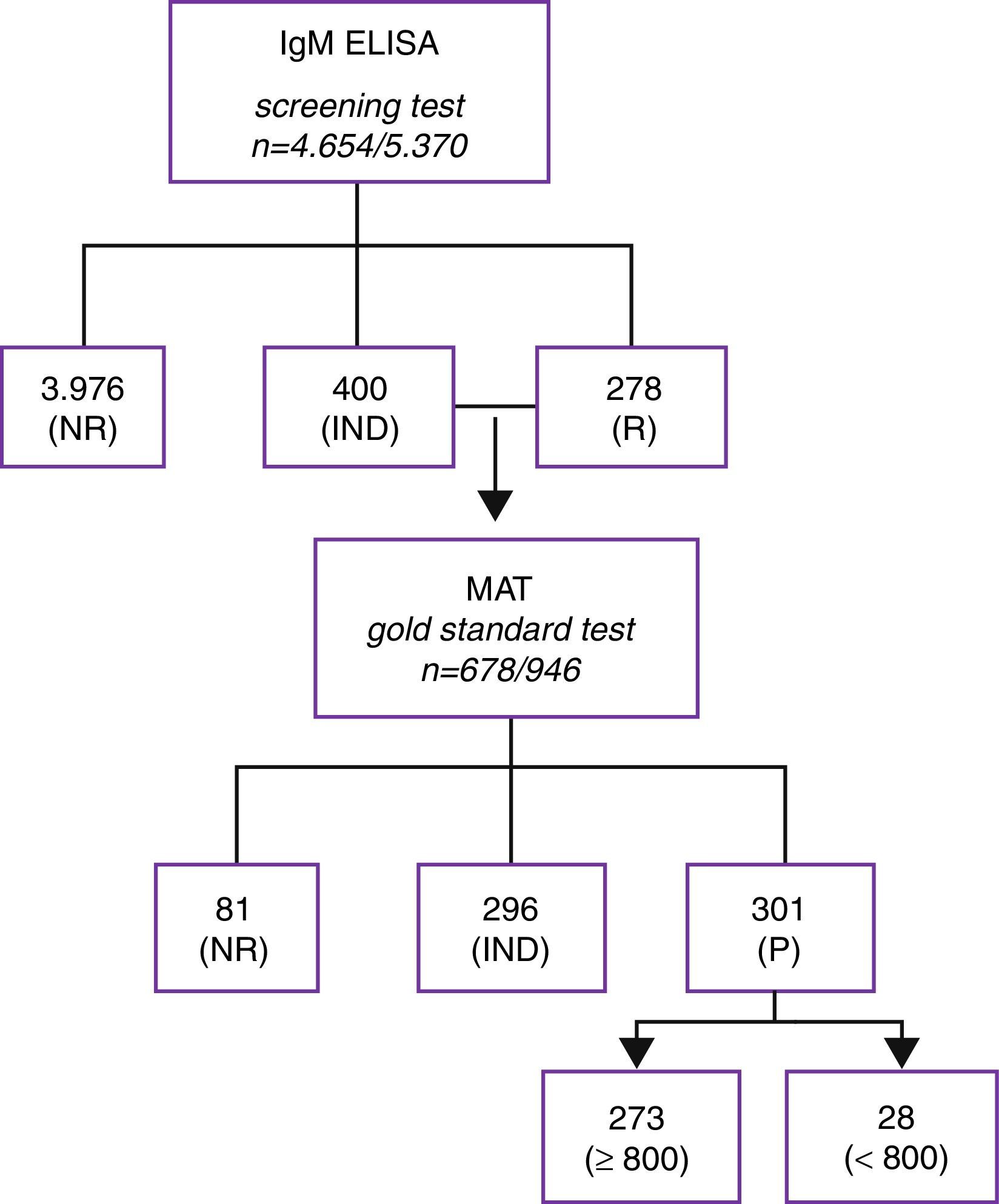
ResultsThe samples classified as indeterminate or reactive on IgM ELISA were either confirmed or rejected through MAT. As recommended by the World Health Organization and by the Brazilian Ministry of Health, the cases of leptospirosis suspicion analyzed herein were laboratory-confirmed through MAT.12,13 A number of 946 serum samples from 678 patients that were indeterminate or reactive on IgM ELISA were tested through MAT, and 81 of them (122 samples) did not show any reaction on MAT, while 597 patients (824 serum samples) presented reactivity on MAT. Among these, only 301 patients (50.4%) were laboratory-confirmed through MAT, according to the criteria established by the Brazilian Ministry of Health. Moreover, among the 597 MAT-reactive patients, it was not possible to define a laboratory diagnostic conclusion for samples from 296 patients (49.6%). Among the samples classified as positive on MAT, 273 patients (45.7%) exhibited titers ≥800 for one or more serovars; and seroconversion was detected in 28 cases (4.7%), with titers <800 (Fig. 1). Coagglutination of two or more serovars was observed for 697 serum samples (84.6%).
Flowchart showing serologic diagnosis of patients with clinical suspicion of leptospirosis. IgM ELISA screening test and laboratory confirmation by MAT in the state of Minas Gerais, Brazil from 2008 to 2012 (n=patient/samples; NR, non-reactive; IND, indeterminate; R, reagent; P, positive).
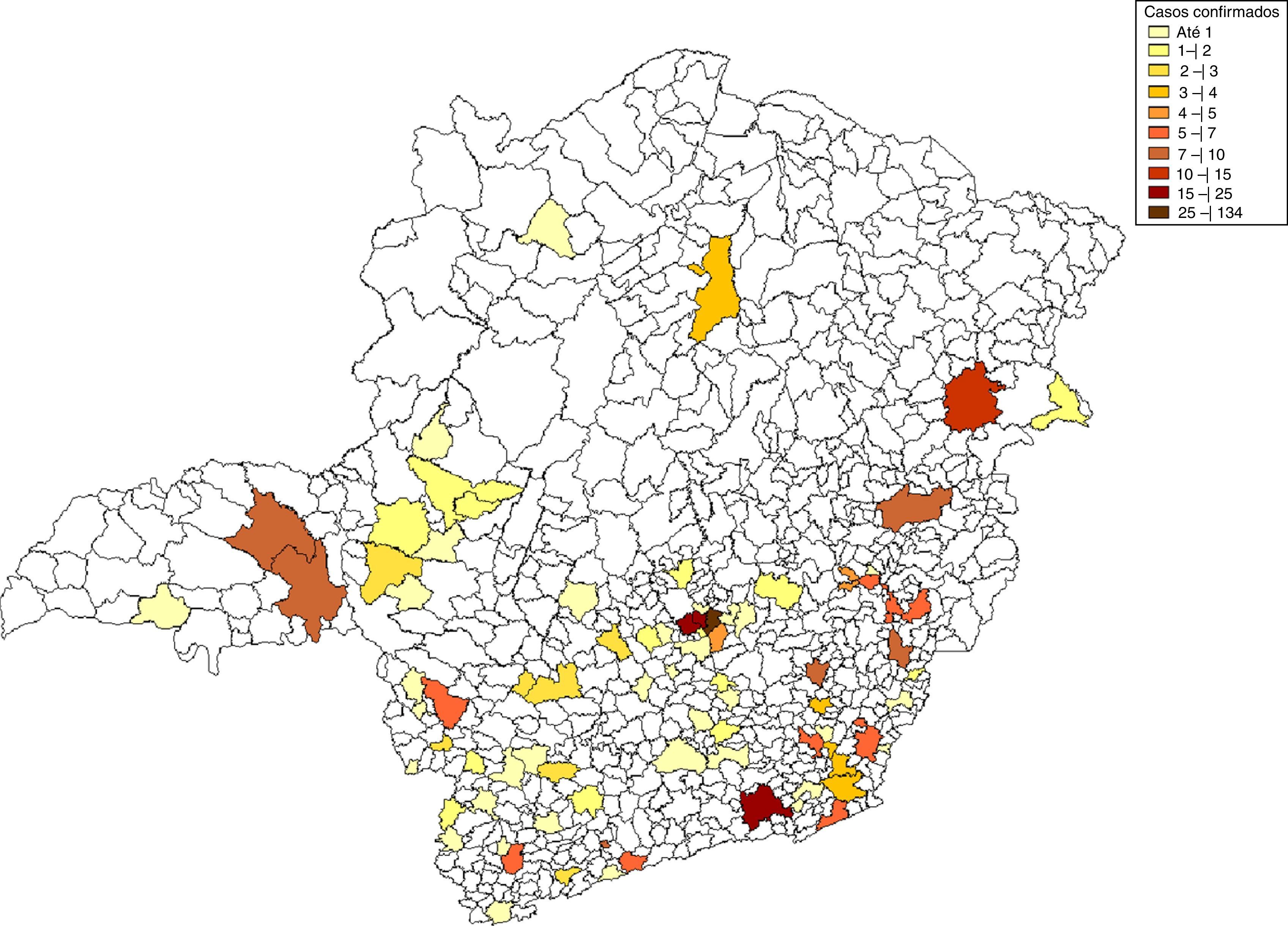
The percentage of 85.1% of the total samples classified as reactive on MAT corresponded to males, and 39.4% corresponded to patients aged between 20 and 39 years old. In this study, 40% of the cases occurred to the metropolitan area, which comprises of the state capital (Belo Horizonte) and 34 neighboring towns (Fig. 2).
The most common serovars found in this period were Icterohaemorrhagiae, followed by Andamana, Patoc, Tarassovi, Copenhageni, Hardjo and Australis. Concerning the samples that exhibited titers ≥800, serovar Icterohaemorrhagiae was also the most common, followed by Copenhageni, Andamana, Patoc, Tarassovi, Grippotyphosa and Canicola.
DiscussionNew cases of leptospirosis are notified among urban and rural populations in Minas Gerais, Brazil, throughout the year. In Brazil most cases concentrate in urban regions, due to disordered growth, high population density and poor sanitary conditions.20
The main advantages of using the IgM ELISA for the screening of serum samples from patients with suspicion of leptospirosis are the fast acquisition of negative results and the low costs. Our work showed 50.4% of MAT laboratory-confirmed cases, while studies performed in other state of Brazil showed different results depending on the specificities of each population.21,22
The cases which were not laboratory-confirmed do not rule out the possibility of leptospirosis infection, since epidemiologic and clinical information must be considered. In the acute-phase of the disease, molecular methodologies may be promising complementary tools for the confirmation of non-conclusive serologic diagnosis.23
Despite being the gold standard method for the diagnosis of leptospirosis, MAT analyses may result in cross-reactions especially in acute-phase disease, due to the presence of lipopolysaccharides that are common to both pathogenic and non-pathogenic serovars. The patients’ serum may recognize antigenically similar serovars or may contain non-specific antibodies. Some authors claim that the high titers detected through MAT indicate infecting serovars.4 Patients from endemic areas can be infected by more than one serovar, and recurrent infections may activate immunologic memory, leading to coagglutination on MAT.14
Although both genders are vulnerable to this disease, independently on the age, men in productive phase are more exposed and, consequently, more affected. Leptospirosis leads to economic losses especially due to hospitalization and medical assistance.24
Leptospirosis is endemic in all regions of Brazil, and the prevalence of serovars may vary according to the geographic region and the contact established among the various hosts. Icterohaemorrhagiae serovar was the most prevalent in Minas Gerais, and previous reports show similar results in other States of Brazil.16,22 In 1995, a study performed in Belo Horizonte, Minas Gerais, showed that Icterohaemorrhagiae serovar presented the highest titers on MAT, being characterized as the infecting serovar.25
In the present study, we highlighted other pathogenic serovars, as Copenhageni, Tarassovi, Cynopteri, Grippotyphosa, Canicola, Hardjo, Australis, which showed expressive occurrence and/or high reactivity among the tested samples. In a study performed in Salvador, Bahia, the highest titers corresponded to Copenhageni serovar. In Uberlândia, Minas Gerais, Canicola, Tarassovi and Wolffi serovars prevailed in humans and were related to the serum prevalence in the canine population.26 Some studies have tried to correlate the type of infecting serovar with the clinical manifestation of the disease. However, a recent study showed that infections caused by the same serovar show different clinical manifestations and variable severity among patients.22,27
The high incidence and/or reactivity of Icterohaemorrhagiae serovar in the samples that presented some reactivity on MAT (laboratory-confirmed or strongly suggestive cases) allows us to infer that the studied human population was in contact with rodents, which are the main hosts of this serovar, being responsible for the maintenance of the disease in urban areas.
The epidemiology of leptospirosis in Minas Gerais is not well established and all literature data were obtained from the results of MAT, the same methodology used in the present study. Therefore, considering the limitations of the methodologies used for detection and identification of Leptospira spp. serovars, MAT is the most suitable one.
ConclusionsOur results showed the possibly spreading serovars in Minas Gerais state and this information contributes to a better knowledge of human leptospirosis, aiming at improving the prevention and control of the disease, as well as the treatment of infected patients.
Authors’ contributionsMAAO has made design of the study, laboratory analysis, acquisition of data and drafted the manuscript. MAC and EAL were involved in laboratory analysis. JCSF was involved in of data analysis. RSD has made substantial contributions to revising of manuscript. JCS revising it critically of intellectual content and final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of interestThe authors declare that there are not conflicts of interest.
The authors would like to thank Michelle Lara Samuel and Gilsiléia Gomes Rebouças from Service of Fungal and Bacterial Disease/Funed, Brazil.