Fusarium oxysporum f. sp. lycopersici is a phytopathogenic fungus that causes vascular wilt in tomato plants. In this work we analyze the influence of metal salts such as iron and copper sulphate, as well as that of bathophenanthrolinedisulfonic acid (iron chelator) and bathocuproinedisulfonic acid (copper chelator) on the activity of laccases in the intra (IF) and extracellular fractions (EF) of the wild-type and the non-pathogenic mutant strain (rho1::hyg) of F. oxysporum. The results show that laccase activity in the IF fraction of the wild and mutant strain increased with the addition of iron chelator (53.4 and 114.32%; respectively). With copper, it is observed that there is an inhibition of the activity with the addition of CuSO4 for the EF of the wild and mutant strain (reduction of 82 and 62.6%; respectively) and for the IF of the mutant strain (54.8%). With the copper chelator a less laccase activity in the IF of the mutant strain was observed (reduction of 53.9%). The results obtained suggest a different regulation of intracellular laccases in the mutant strain compared with the wild type in presence of CuSO4 and copper chelator which may be due to the mutation in the rho gene.
Fusarium oxysporum is a highly relevant phytopathogenic fungus due to its economic importance.15 The special form of lycopersici is the causal agent of vascular fusariosis disease in tomato culture. This fungus reaches the vascular system of the plant and subsequently achieves the colonization of the host plant by spreading through the xylem vessels.28
Laccases (bencenodiol: oxygen oxidoreductase, EC 1.10.3.2) are a type of polyphenoloxidase enzymes that are part of the ligninolytic system and with ascorbate oxidases, ceruplasmin and other enzymes belong among blue multicopper oxidases.23,1 The participation of laccases is associated with many biological processes, such as spore resistance, pigmentation, virulence factors,2,37 lignification of plant cell walls,20 lignin biodegradation5 and the protection of the fungus against phenolic compounds released by plants, such as phytoalexins,24 among others. It is known that the manifestation of laccases in fungi is influenced by several factors, including the presence of metals.25 Copper has been demonstrated to regulate the induction transcriptional of the laccases in Trametes versicolor and Trametes pubescens,11,14Pleurotus,32,21 among others.
On the other hand, FeSO4 at a concentration of 1mM did not induce laccase activity with respect to control in Pleurotus pulmonarius at 7 days of growth but there was an inhibition of activity at 10 days of growth. A similar response was observed in Pleurotus ostreatus, since there was an inhibition of activity at 10 days, but in this case there a stimulatory effect of activity was observed at 7 days of growth.33 It was obtained a complete inhibition of activity of a purified laccase of the fungus Trematosphaeria mangrovei with FeSO4 at 1mM.17
In F. oxysporum f. sp. lycopersici the presence of six genes encoding laccase proteins (lcc1, lcc2, lcc3, lcc4, lcc5 and lcc9) has been reported. The mutants lcc1, lcc3 and lcc5 were implemented, in which a significant decrease in the extracellular activity of laccase was observed; however, these mutants showed no loss of pathogenicity on tomato seedlings.8
To know the effect that some metals and chelants may have on the activity of laccases in F. oxysporum, we analyzed the effect of FeSO4, the iron chelator bathophenanthrolinedisulfonic acid (BPDS), CuSO4 and the copper chelator bathocuproinedisulfonic acid (BCDS) on the enzymatic activity of laccase in a wild strain and the mutant rho1::hyg (non-pathogenic) of F. oxysporum f. sp. lycopersici.
Materials and methodsIsolated fungi and growing conditionsStrain 4287 (race 2; wild-type) and the mutant strain rho1::hyg of F. oxysporum f. sp. lycopersici were obtained through Dr. Gonzalez Roncero (University of Cordoba, Spain). The mutant rho1::hyg has interrupted rho1 gene (rho1::hyg) and presents loss of the pathogenicity in tomato seedlings.18
To preserve the strains, the wild-type strain was inoculated in the PDB medium and the mutant strain in the PDB medium with hygromycin (20μg/mL; SIGMA, Saint Louis, Missouri, USA) for 4–5 days at 27°C with shaking. The generated mycelia were removed through filtration under sterile conditions. The filtrate obtained was centrifuged at 6500g for 10min, and the sediment containing the spores was washed three times with sterile water. The strains were stored as microconidial suspensions in 30% glycerol at 70°C until its usage.
For different growth conditions, the germinates were obtained from the PDB culture, which was inoculated with 5×106microconidiamL−1 for 19h at 27°C under shaking. The germs were transferred to a basal medium (BL) (0.4g KH2PO4 L−1, 0.2g MgSO4·7H2O L−1, 1g NH4NO3 L−1, 0.01g FeSO4 L−1, 0.01g ZnSO4 L−1 and 0.01g MnSO4 L−1, 0.2g KCl L−1, 0.25% sucrose) for 6 days; BL without copper (incubation for 3 days) and BL without iron (incubation for 3 days) at 27°C, 220rpm. On the third day of growth in the BL medium without copper, the copper chelator bathocuproinedisulfonic acid (BCDS; SIGMA, Saint Louis, Missouri, USA) at 1mM and CuSO4 at 25μM was independently added in flasks. The same procedure is applied for the BL medium without iron, in which the iron chelator, bathophenanthrolinedisulfonic acid (BPDS; SIGMA, Saint Louis, Missouri, USA) was added at 1mM; and for the duplicate of the BL medium without iron, FeSO4 was added at 25μM. After the addition of BCDS, CuSO4, BPDS and FeSO4, the incubation was continued for 3 days at 220rpm at 27°C. The mycelium and the extracellular fraction were obtained by centrifugation at 6500g for 10min at 4°C.
Obtainment of the homogenateThe mycelia were resuspended in a Buffer Tris–HCl 50mM solution with protease inhibitors (antipain, leupeptin, pepstatin and E64 at 5μg/mL; SIGMA, Saint Louis, Missouri, USA). The cells were fragmented through sonication for 8–10min for 1-min intervals. Subsequently an initial centrifugation was performed at 2500×g for 5min at a temperature of 4°C. The pellet was discarded and the supernatant transferred to a new tube, where a second centrifugation was performed at 6500×g for 45min at 4°C, the pellet discarded and the supernatant (intracellular fraction) obtained. The aforementioned protease inhibitors were added to the extracellular fraction. Both fractions were stored at −20°C until its usage.
Quantification of enzyme activityThe laccase activity was determined using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) [ABTS; SIGMA, Saint Louis, Missouri, USA] as a substrate.7 The laccase activity was quantified through the oxidation of the ABTS substrate (1mM) in 20mM sodium acetate pH 3.5 buffer, by increasing the absorbance at 436nm.14 Final reaction mixture contained 100μL of the sample and 2mL of the ABTS substrate,16 measuring the reaction at 0 and 10min. A unit of laccase activity is defined as the amount of enzyme needed to oxidize a μmol of ABTS min−1 at 25°C.14 All assays were performed in triplicate and three independent batches were performed.
Statistic analysisThe data on enzyme activity were compared between the two strains using ANOVA and Tukey's tests, with Minitab ver. 15 software (Minitab Inc., State College, PA, USA), considering a level of significance at 0.05.
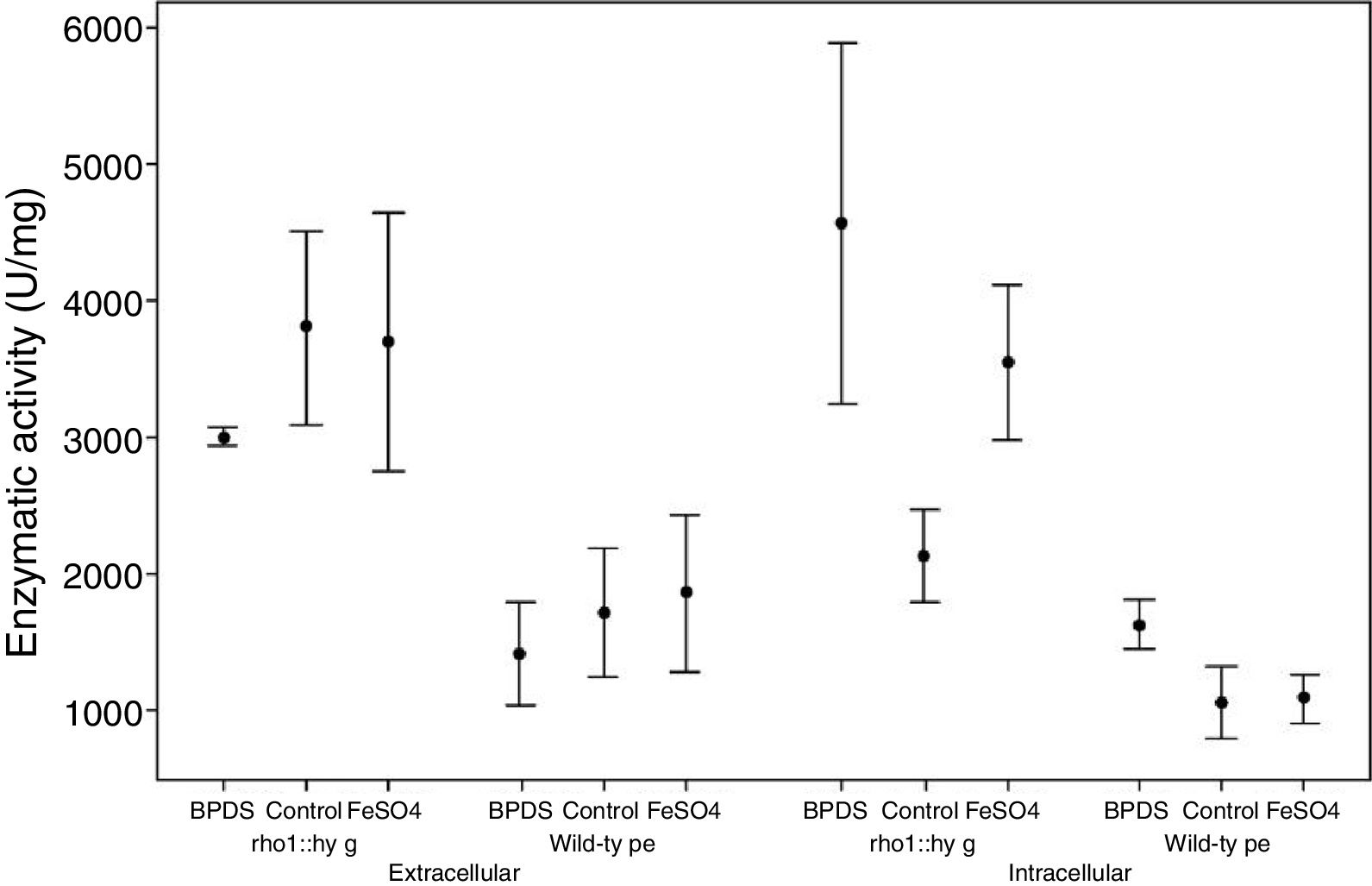
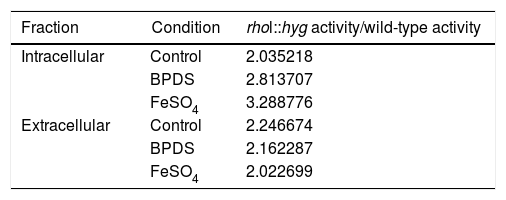
ResultsInfluence of iron on the enzymatic activity of laccasesFrom three independent batches, the enzymatic activity of laccases was measured in the intracellular and extracellular fraction of the wild and mutant strain F. oxysporum f. sp. lycopersici, in the presence of iron and the BPDS iron chelator. Through the ANOVA test, the mutant strain rho1::hyg demonstrated higher extracellular and intracellular enzyme activity (in the control condition, in the presence of BPDS and FeSO4) compared to the wild-type strain. With a normal distribution of the data (p-value>0.1) it is considered that the activity between both strains will always be greater for the mutant strain under the same conditions of the experiment (Table 1).
Relation between the activity in the medium of the rho1::hyg mutant and in that of the wild-type strain in the control and in the presence of BPDS and FeSO4.
| Fraction | Condition | rhol::hyg activity/wild-type activity |
|---|---|---|
| Intracellular | Control | 2.035218 |
| BPDS | 2.813707 | |
| FeSO4 | 3.288776 | |
| Extracellular | Control | 2.246674 |
| BPDS | 2.162287 | |
| FeSO4 | 2.022699 |
According to the statistical analysis through the Tukey method, which allows us to analyze the difference in enzymatic activity between the conditions studied for each strain (control group, in the presence of BPDS chelator and in the presence of FeSO4), it was observed that in the extracellular fraction of the wild strain and the mutant strain there exists no difference between the enzymatic activity of the control group with respect to the group in the presence of BPDS or the group in the presence of FeSO4. With respect to the enzymatic activity of the intracellular fraction, in both strains (wild and mutant) a bigger activity in the group with the BPDS chelator was observed with respect to control group (53.4 and 114.32%, respectively), whereas no difference of intracellular enzymatic activity was observed in both strains between the control group and the group with FeSO4 (Fig. 1).
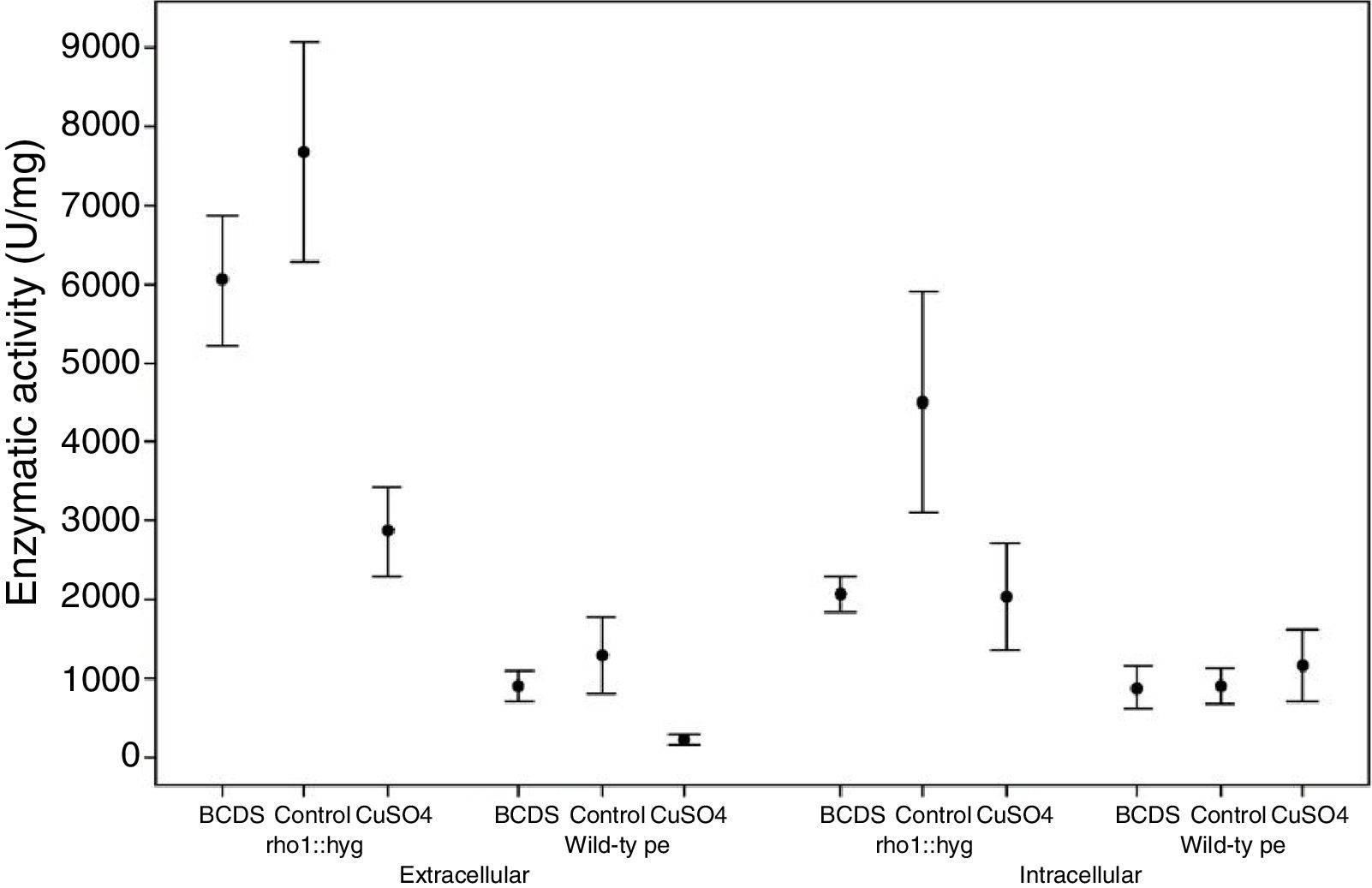
Influence of copper on the enzymatic activity of laccaseFrom three independent batches, the enzymatic activity of laccases was measured in the intracellular and extracellular fraction of the wild and mutant strain of F. oxysporum f. sp. lycopersici, in the presence of copper and the BCDS copper chelator. In both fractions, the ANOVA test performed on all the lots allowed us to know that the mutant strain rho1::hyg had a higher enzymatic activity for the different growth conditions compared to the wild strain. The data maintained a normal distribution (p-value>0.1) (Table 2).
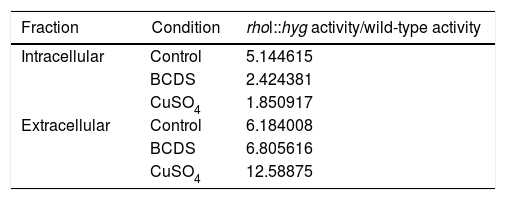
Relation between the activity in the medium of the rho1::hyg mutant and in that of the wild-type strain in the control and in the presence of BCDS and CuSO4.
| Fraction | Condition | rhol::hyg activity/wild-type activity |
|---|---|---|
| Intracellular | Control | 5.144615 |
| BCDS | 2.424381 | |
| CuSO4 | 1.850917 | |
| Extracellular | Control | 6.184008 |
| BCDS | 6.805616 | |
| CuSO4 | 12.58875 |
According to the statistical analysis performed through the Tukey method, it was observed that in the extracellular fraction, the enzymatic activity in the wild and mutant strain presented less activity in the group with CuSO4 compared to their respective control groups (a reduction of the activity of 82 and 62.6% with respect the control group, respectively). There was no effect between the control group and the group with addition of the BCDS chelator of the extracellular fraction (in both strains). On the other hand, in the intracellular fraction of the wild-type strain there was no difference in laccase activity between the control group, the group with CuSO4 and the group with addition of the BCDS chelator; however, in the mutant strain the CuSO4 group demonstrated less activity with respect control group (a reduction of the activity of 54.8%). Also in the same fraction, the mutant strain showed less activity of laccase in the BCDS chelator group compared with control group (a reduction of 53.9%) (Fig. 2).
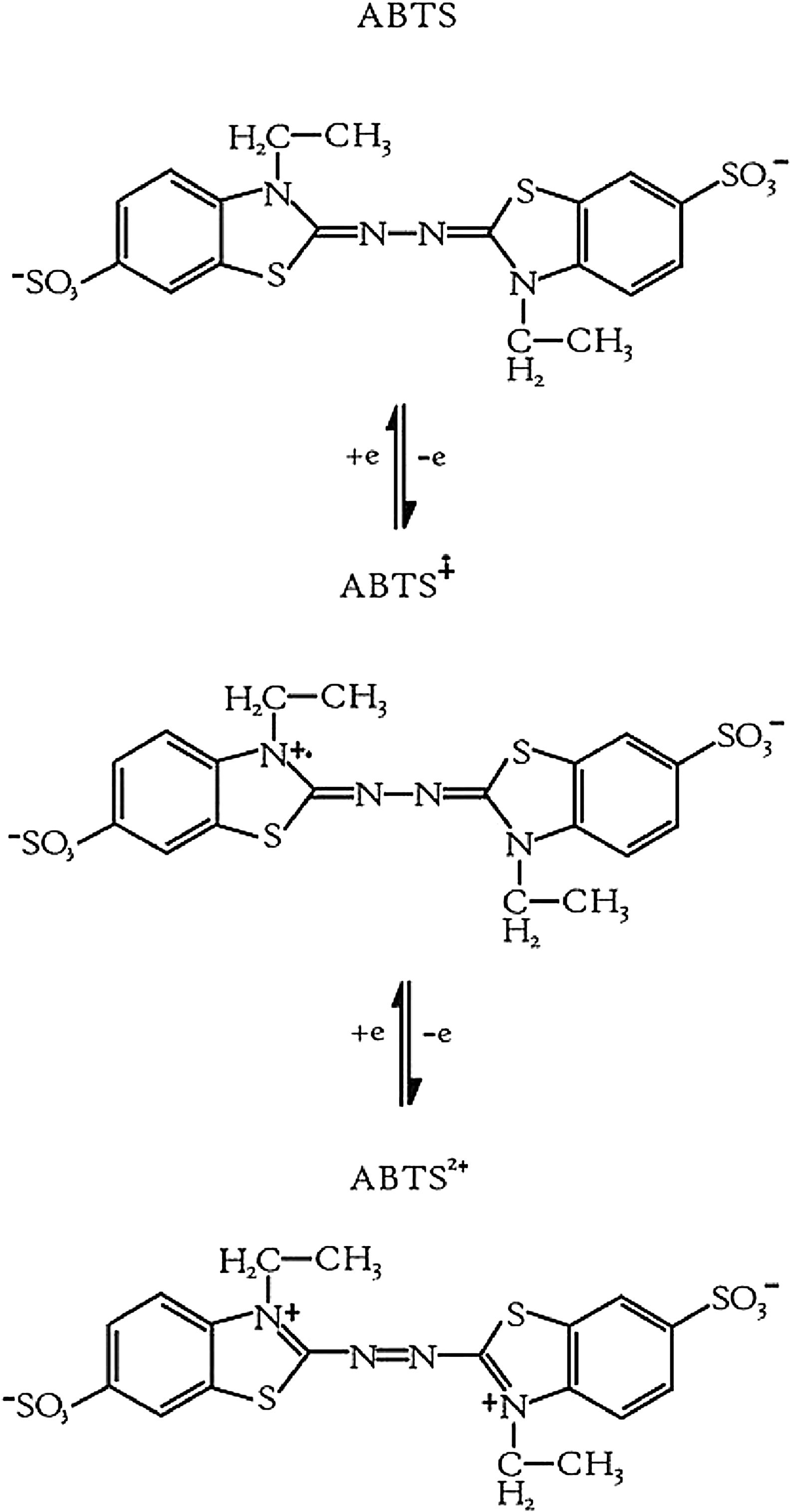
DiscussionLaccases are a type of phenoloxidase enzymes widely distributed in nature, as they have been found in plants, bacteria and fungi.19 Among the functions attributed to laccases are lignification,20 the protection of the fungus against phenolic compounds,24 among others. ABTS is an organic redox mediator used in the laccase reaction. ABTS undergoes two step oxidation reaction during the enzymatic reaction by laccase (first for the obtention of ABTS cation radical and then the ABTS dication)22 (Fig. 3).
Oxidation of ABTS by laccase. ABTS cation radical (a) and ABTS dication (b).22
In studies by Cañero and Roncero in 20088 demonstrated the expression of various laccase genes during the infection process of the tomato plant with F. oxysporum f. sp. lycopersici (lcc1, lcc3 and lcc9). In addition, the location of each of the laccase proteins through the amino acid sequence was identified, demonstrating that Lcc1 and Lcc2 have an intracellular localization, Lcc3 is transmembrane and Lcc4, Lcc5 and Lcc9 have an extracellular localization. An analysis of the promoter regions of the lcc1, lcc2, lcc3, lcc4, lcc5 and lcc9 genes demonstrate metal-responsive elements (MRE), stress-responsive elements (STRE), xenobiotic-responsive elements (XRE), an ACE1 copper-responsive transcription factor and a PacC ambient pH response factor. Laccase activity has been reported to increase with low concentrations of sucrose, glycerol and polygalacturonic acid.8 Therefore, in this work a basal medium with a low concentration of sucrose as a carbon source was used.
Some trace elements are essential for fungal metabolism; however, at high concentrations they could be toxic.6,4 Some trace elements such as Fe, Zn and others act through metal-responsive promoter interaction, for post-transcriptional regulation.34,13,10 In fungi, it is known that regulation in the manifestation of laccases can be mediated by the presence of various metals.25
In the results obtained in this work, a higher enzymatic activity of laccase was observed in the rho1::hyg mutant strain compared to the wild-type strain under all tested conditions (control group, presence of iron, copper, and BPDS and BCDS chelators). These results correspond with to the reported by Reyes-Medina and Macías-Sánchez,26 since under the conditions utilized in that work they also detected greater activity in the mutant strain with respect to the wild-type, suggesting that this effect could be influenced by the absence of Rho protein. The results obtained at the extracellular and intracellular levels suggest that the presence of FeSO4 has no apparent effect on the enzymatic activity of laccase in the wild strain of F. oxysporum f. sp. lycopersici in comparison to its basal condition, which could be due to FeSO4 not acting as an inductor of the extracellular laccase (Lcc4, Lcc5 and Lcc9) or intracellular laccases (Lcc1 and Lcc2), or that the concentration used (25μM) is not the concentration necessary to generate the induction of laccases. A similar behavior is observed for the mutant strain, where the addition of FeSO4 does not appear to affect laccase activity. The effect of Fe on laccase activity differs depending on the microorganism and the concentration used; for example, the effect of this metal at a concentration of 2mM on the activity of the purified laccase on Aspergillus nidulans demonstrated a relative activity of 139% with respect to that obtained in the control condition.36 However, in Streptomyces cyaneus a reduction of the activity of laccase purified under the presence of FeSO4 (concentrations since 0–50mM) was observed.3 On the other hand,38 observed in Trametes velutina an increase in the extracellular enzymatic activity when adding iron from a concentration of 0.04mM.27 tested the effect of FeSO4 at a concentration of 5mM on laccase activity in Chalara (syn. Thielaviopsis) paradoxa, observing an inhibition of the activity with respect to the control.
In P. ostreatus an increase in laccase activity was observed in the presence of FeSO4 at 7 days of incubation; however, at 10 days of incubation there was less activity with respect to its control. On the other hand, the activity of laccase in Pleurotus pulmonarius was similar in presence of FeSO4 and the control group at 7 days of incubation, and there was a decrease of the activity in the presence of Fe compared to the control group at 10 days of incubation.33
The BPDS is known as a potent iron chelating agent.31 In the present study, no differences were observed between the control group and the group with addition of BPDS in the extracellular fraction of the wild and mutant strain. In contrast, in the intracellular fraction, there was observed that in both strains the BPDS group presented greater activity compared to the control group, which would indicate that a lower iron concentration is required than the used in the control condition to induce the activity of the laccases.
Copper plays an important role in the regulation of laccases since it is known that the addition of low concentrations of copper to the culture medium can stimulate laccase production21; in addition, laccases have four copper atoms in their catalytic center, which have different properties.23 With the data obtained in this work, in the group with the addition of CuSO4, indicate a decrease in the activity of laccase in the extracellular fraction of the wild and mutant strain, as well as in the intracellular fraction of the mutant strain, indicating that CuSO4 at a concentration of 25μM inhibits the activity of laccases in F. oxysporum. f. sp. lycopersici.11 reported that an increase in the concentration of copper causes an increment in the laccases activity in T. versicolor. However, the use of different concentrations of the metal on the induction of laccases in other microorganisms has demonstrated that at a certain concentration a maximum is reached in the activity and after this the activity considerably decreases and in some cases it is canceled completely. In the Grammothele subargentea fungus, it was observed that in the concentration range of 0.6–1.2mM of CuSO4 major enzymatic activity of laccase occurred, whereas at higher concentrations of the metal (1.5 and 1.8mM), the activity decreases30; a similar situation is observed in T. versicolor, where the laccase activity is induced in the presence of CuSO4 up to 17%; however, it decreases significantly with high levels of Cu2+ (80mM).16 A particular case is that of Trametes hirsuta, in which there was observed the irregular behavior of enzymatic activity under induction conditions with CuSO4 with respect to the culture time and the used concentration of the metal; in that experiment being possible to distinguish several peaks of activity, followed by periods of inhibition.29 In this work, there is no difference in the activity of the laccase of the control group and the group with copper in the intracellular fraction of the wild strain, where possibly a higher concentration of copper is required to observe an effect on the enzymatic activity of the laccases. This also indicates that the laccases present in the intracellular fraction of the wild strain do not present as much sensitivity to copper, unlike the laccases present in the intracellular fraction of the mutant strain, and in the extracellular fraction (wild and mutant strain).
BCDS is a chelating agent for copper ions.35 Endo et al.12 used a chelator concentration similar to this work. They observed a reduction of the enzymatic activity between the condition and its control. With the results obtained in our work, in the presence of the BCDS chelator in the extracellular fraction, no differences were observed with respect to the control for the two strains utilized, as well as in the intracellular fraction of the wild strain, not being the same case for the mutant strain, where there is a diminishing in the activity in the group with the addition of BCDS, being the only case where the inhibition of the enzymatic activity is observed with the presence of this chelator.9 analyzed the influence of copper in the expression of laccase genes in F. oxysporum utilizing CuSO4 and BCDS; in both cases there were seen no differences in the expression of the lcc1, lcc2, lcc3, lcc4, lcc5 and lcc9 genes; however, if there was a higher extracellular enzymatic activity in the wild-type strain in the presence of CuSO4 compared to the medium without CuSO4 (growth for 5 days in a minimal medium containing sucrose with and without 250μM of CuSO4), these results differ from those obtained in this work, since we observed less activity in the presence of copper, even though a smaller amount of CuSO4 (25μM) was utilized; however, the incubation was performed for a shorter period of time (3 days), where it is possible to observe a different behavior over time, which may be due to regulation mechanisms of the metal.
In this work, it was of interest to learn about the in vitro behavior of the laccase enzymes’ activity in the intra and extracellular fraction of the wild strain of F. oxysporum f. sp. lycopersici (pathogen strain), and of the mutant strain rho1::hyg (non-pathogen strain), and its response to inductors like Fe and Cu, and with the iron chelator (bathophenanthrolinedisulfonic acid), and copper chelator (bathocuproinedisulfonic acid). Nevertheless, these do not constitute a field treatment for the control of fusariosis. However, knowledge of the behavior of laccase activity in the wild and mutant strains under these conditions may be used as a guide in the analysis and modulation of these metallic ions in the soil, thus creating soil conditions that can be less appropriate for the establishment of fusariosis.
With the results obtained in this work it can be suggested that the intracellular laccases of the wild and mutant strain demonstrate a differential response to the presence of copper ions. The difference observed could be regulated through of rho1 gene. Because the role of copper in the function of laccase proteins, this response could be attributed to its insertion in the inactive apoprotein. As perspectives of this work, the conditions that presented a bigger effect on the laccase activity can be used to observe the effect that present in the sporulation, pigmentation, and expression of transcription factors, among other processes.
FundingThis study was funded by the Instituto Politécnico Nacional, Secretaría de Investigación y Posgrado (20131056).
Conflicts of interestThe authors declare that they have no conflicts of interest.
This research was supported by the Secretaría de Investigación de Posgrado of the Instituto Politécnico Nacional (20131056).