This work described a novel halotolerant phage, JMT-1, with a spherical morphology. JMT-1, which was isolated from a hypersaline lake, could produce clear plaques on Chromohalobacter sp. LY7-3. The purified virions are spherical, have no visible tail, and are about 30–50nm in diameter. JMT-1 has a wide host range, and this study showed that the phage can infect at least five halophilic bacteria. The proteins of JMT-1 were analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis, and six proteins were detected. Results show that JMT-1 is a bacteriophage with a linear double-stranded DNA. Meanwhile, the genome is approximately 23kb in length and is sensitive to the restriction endonucleases Bam I, EcoR I, Hind III and Kpa I. JMT-1 has a high titer, approaching 1.5×109pfu/mL after dilution to 10−6pfu/mL. The phage is also sensitive to chloroform but not to temperature, pH, and lowered salt concentration. JMT-1 is a spherical lytic halotolerant phage with a wide host range and has the tolerance to specific extreme environments. These data could provide references for studying phage resources in extreme environments and would also provide the useful methods for isolation and identification of other valuable phage in the salt lake environment.
Viruses from the aquatic systems are the most abundant biological group in hypersaline environments.1 Although some viruses in hypersaline environments have been characterized through culture-dependent methods, the knowledge on halophilic bacterial viruses and viruses that could infect bacteria is limited. In nearly 6300 known prokaryote viruses,2 only 90 are extremely halophilic, and very few of these are reported to infect bacteria.3
Even though some bacteria are found in some extreme habitats, such as hypersaline lakes, a number of studies indicated that halophilic bacteria typically live in extreme salinities.4 Meanwhile, viruses, such as halophilic bacterial phage, also exhibit halophilic characteristics in hypersaline environment.5 However, reports about such bacteriophage are very few. Nearly 50 prokaryotic haloviruses were isolated from globally distant locations, but only four can infect bacteria.6 Considering that bacteriophage could be both nonextremophiles and extremophiles, Ackermann and Prangishvili7 studied the infection ability of bacteriophage.
A group of round haloviruses, halosphaerovirus, has been recently expanded by the addition of SH1 and other three haloviruses, such as PH1, HHIV-2, and SNJ1.8,9 The members of this group are related by their capsid proteins. However, the genome lengths and replication strategies of halosphaerovirus are different. In this group, the SH110 and HHIV-211 genomes are linear and double-stranded DNA (dsDNA) (∼30.5kb) with terminal protein, indicating that their replication starts with protein priming.12 Meanwhile, the SNJ1 genome has a 16.3kb circular dsDNA.13 Among these viruses, SH1 has been the most comprehensively studied, with focus on its host range, thermal stability, genome sequence, transcription mapping, and virion structure.8 However, information about other haloviruses is scarce.
In this research, JMT-1, a strain of lytic bacteriophage was isolated from Yuncheng Saline Lake. The biological characteristics of this bacteriophage were reported. Results of this study will provide references for the research of extreme environment phage resources and guidance for saline lake bacteriophage separation and identification.
Materials and methodsBacterial strainThe halophilic strain Chromohalobacter sp. LY7-3 used as the host bacteria in this study was isolated from Yuncheng Saline Lake, China.
Culture mediaThe media used in this study are prepared based on the description provided in the online website http://www.haloarchaea.com/resources/halohandbook/index.html. Artificial salt water (SW) contained a total of 30% (w/v) salt, which comprised 4M NaCl, 150mM MgCl2, 150mM MgSO4, 90mM KCl, and 3.5mM CaCl2, and was adjusted to pH 7.5 per liter. This research utilized 15% SW as the virus dilution solution (VDS). Overlay plates, which contained 15mM Tris–HCl (pH 7.5), 1% yeast extract, 0.5% peptone, and 2.5%, 5%, 10%, 15%, or 25% total SW, of modified growth medium (MGM) were prepared. Agar was added for the solid (15g/L) or top-layer (5g/L) media.14
Isolation of lytic bacteriophage JMT-1A total of 100mL saline lake water and 50mL Luria-Bertani medium were mixed and cultured at 37°C on the shaker for 24h. Then, the medium was centrifuged at 8000rpm for 30min. Subsequently, the precipitate was resuspended with 0.5mL VDS and filtered using a 0.22μm microporous membrane. The filtrate was then stored at 4°C.
The 150μL filtrate and 50μL Chromohalobacter sp. LY7-3 suspension were incubated at room temperature (RT) for 15min. Then, the mixture was blended with 3mL of top-layer MGM (the concentration of SW ranged from 2.5% to 25%) and spread on the solid MGM. The mixture was cultured at 37°C for 12h. After culturing, a single plaque on a Chromohalobacter sp. LY7-3 lawn plate was obtained and resuspended with 100μL VDS as the concentrated stock. To test the infection ability of the novel virus, 10μL concentrated stock was added on the 4mL top-layer MGM with 100μL Chromohalobacter sp. LY7-3 and cultured for 24h at 37°C. The novel virus was named JMT-1.
Purification of the bacteriophage JMT-1Chromohalobacter sp. LY7-3 was infected with JMT-1 on the double-plate MGM to produce high-titer phage stocks. When more than 90% lysis of Chromohalobacter sp. LY7-3 was observed, the top-layer MGM was collected using 4mL VDS. The cultures were blended on a vortex for 2min and then centrifuged at 10,000rpm for 3min. The supernatant was filtered via a 0.22μm microporous membrane to purify JMT-1. After the collection, JMT-1 was precipitated with 10% (w/v) polyethylene glycol for 12h at 4°C and then centrifuged at 12,000rpm for 15min. The precipitation was dissolved in 1mL VDS.
Negative-stain electron microscopyA 10μL drop of the purified JMT-1 was placed on a clean surface, and the virus particles were allowed to adsorb onto a Formvar film 300-mesh copper grid for 1min. The excess liquid on the film was absorbed with filter paper and washed by water. Then, a 20μL drop of water was placed on a clean surface, the grid was buckled on the water, and the film was quickly removed by absorption with filter paper. The JMT-1 on the film was negatively stained with a 20μL drop of 0.2% uranyl acetate for 50s and absorbed with filter paper before air-drying. The sample was examined using Philips CM 120 BioTwin transmission electron microscope (Royal Philips Electronics), which was operated at 120kV acceleration voltage.
Host range test of JMT-1Ten strains of the halophilic archaea, with the 16S rDNA genbank access numbers, including Halobacillus sp. LY7 (HQ683726.1), Thalassobacillus sp. LY18 (HQ683736.1), Virgibacillus sp. GQ15 (KM055003.1), Chromohalobacter sp. LY7-4 (JF796143.1), Idiomarina sp. W33 (JN112009.1), Gracilibacillus sp. SK1 (JQ045293.1), Virgibacillus sp. SK31 (JN998439.1), Virgibacillus sp. GQ8 (KM974807.1), Bacillus sp. GQ39 (JN998395.1), and Halomonas sp. GQ44 (JQ421337.1) isolated from Yuncheng Saline Lake (China), were screened for JMT-1 susceptibility. The lysates from JMT-1-infected halophilic archaea cultures were spotted onto the lawns of each strain (using media with 18% MGM) and incubated for 2–5 days at 37°C.
Nucleic acids analysis of JMT-1The isolation of protease-treated nucleic acids from purified JMT-1 preparations was performed based on the method of Porter et al.10 To identify the nucleic acids that were separated from JMT-1, DNase I (20U/μg) and RNase A (5U/μg) were added to the nucleic acids for 1h at 37°C. For the JMT-1 restriction fragment length polymorphism (RFLP) analysis, restriction enzymes (Bam I, EcoR I, Hind III, Kpa I, Dde, Sal I, Sac I, and Xho I) were purchased from TransGen Biotech and used per the manufacturer's instructions. The total nucleic acids, the Dnase I- or RNase A-treated nucleic acids, and restriction fragments were separated using 1% (w/v) agarose gels in Tris–acetate–EDTA electrophoresis buffer.
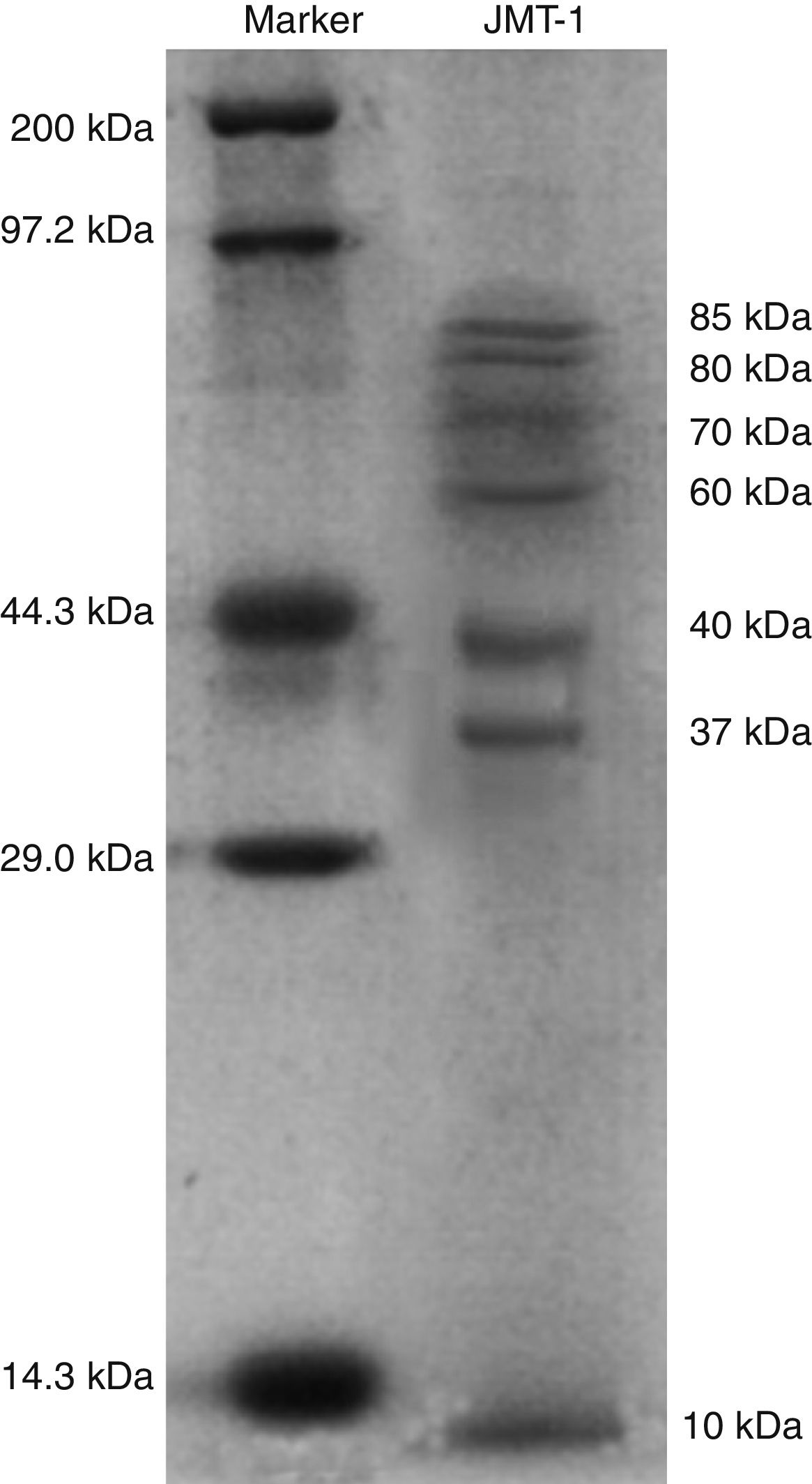
Capsid protein analysis of JMT-1JMT-1 was precipitated and resuspended with double distilled H2O (ddH2O). Then, 35μL JMT-1 was dissolved in 15μL Laemmli sample buffer with 48mM β-mercaptoethanol15 and heated in boiling water for 5min. The mixture was separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the gels were stained for 30min with Coomassie Brilliant Blue buffer (CBB) (0.25% Coomassie Brilliant Blue, 5% methanol, and 7.5% acetic acid). The gels were destained with 40% methanol and 10% acetic acid several times until the protein bands have been clearly revealed.
Stability of JMT-1After centrifugation, the bacteriophages were collected and diluted in VDS, and virus titers were determined via plaque assay on Chromohalobacter sp. LY7-3. Each experiment was performed in triplicate, and representative data are shown. Chloroform sensitivity was examined through exposure of Chromohalobacter sp. LY7-3 titers in MGM to chloroform in a volume ratio of 1:5 (chloroform to phage). The incubation was conducted with severe agitation at RT for 1min then at 30°C for 30min. Subsequently, 10μL supernatant and 50μL Chromohalobacter sp. LY7-3 were blended at RT for 15min, and the mixture was cultured on MGM for 12h to determine the titer. The effect of a low-ionic environment was examined through the dilution of JMT-1 (in MGM) with ddH2O at a volume ratio of 1:1000 (phage to ddH2O). The incubation was performed every 5min (with a total of eight times, from 0min to 35min), and the mixture was quickly cultured in MGM for 12h to detect the titer. The pH stability of JMT-1 was determined via the dilution of JMT-1 in MGM into the appropriate pH buffer (the VDS was buffered with appropriate Tris–HCl, pH from 4 to 10). After 30min, 20μL JMT-1 and 50μL Chromohalobacter sp. LY7-3 were cultured in MGM for 12h to detect the titer. The thermal stability of JMT-1 was examined via 30min incubation of the phages in MGM at different temperatures (30, 40, 50, 60, 70, 80, 90, and 100°C). Afterward, the phages were quickly incubated at 4°C, diluted in VDS, and cultured with Chromohalobacter sp. LY7-3 in MGM for 12h to determine the titer.
ResultsBacteriophage isolation and host range testThe water sample from Yuncheng Saline Lake, a hypersaline lake in Shanxi Province, China, was screened for haloviruses via direct plating on lawns of Chromohalobacter sp. LY7-3 using overlay plates of MGM with 18% salt. A high titer of similar plaque morphology was observed on the plates, and the novel halovirus, JMT-1, was isolated from one of these plaques.
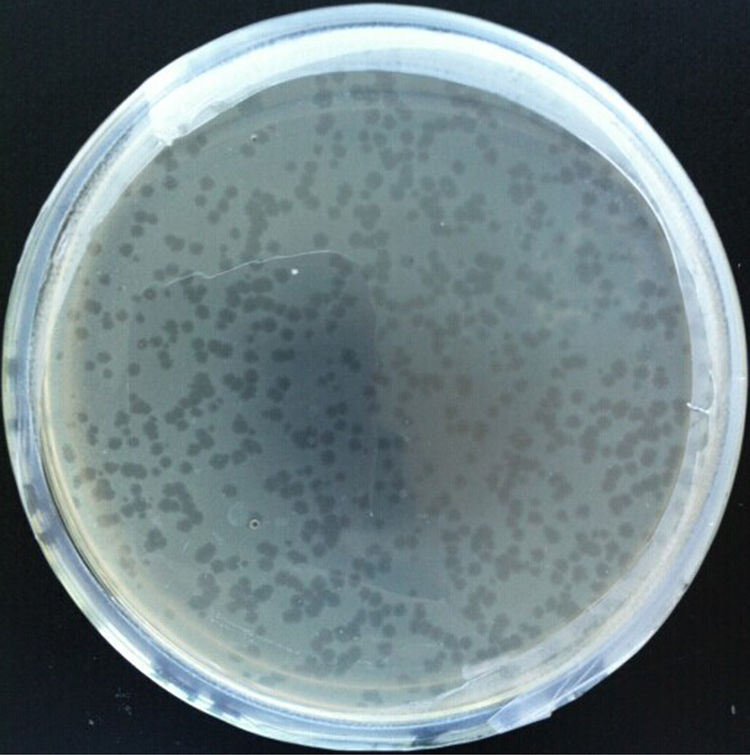
The top layer of MGM was harvested, and the selected haloviruses were grown with Chromohalobacter sp. LY7-3 on the overlay plates of MGM. After two-day culturing at 37°C, the plaques fully developed on the overlay plates of MGM. The plaques were 2–3mm in diameter, clear, and have homogeneous smooth edges on the MGM (Fig. 1).
The host range of JMT-1 was detected after the phages had been isolated. JMT-1 was unable to form plaques on the lawns of five different species (Halobacillus sp. LY7, Salimicrobium sp. GQ15, Chromohalobacter sp. LY7-4, Gracilibacillus sp. SK1, and Halomonas sp. GQ44) but could form the structures on Clostridium sp. GQ8, Bacillus sp. GQ39, Virgibacillus sp. SK31, Thalassobacillus sp. LY18, and Halomonas sp. W33. This finding indicates that the novel bacteriophage has a wide host range.
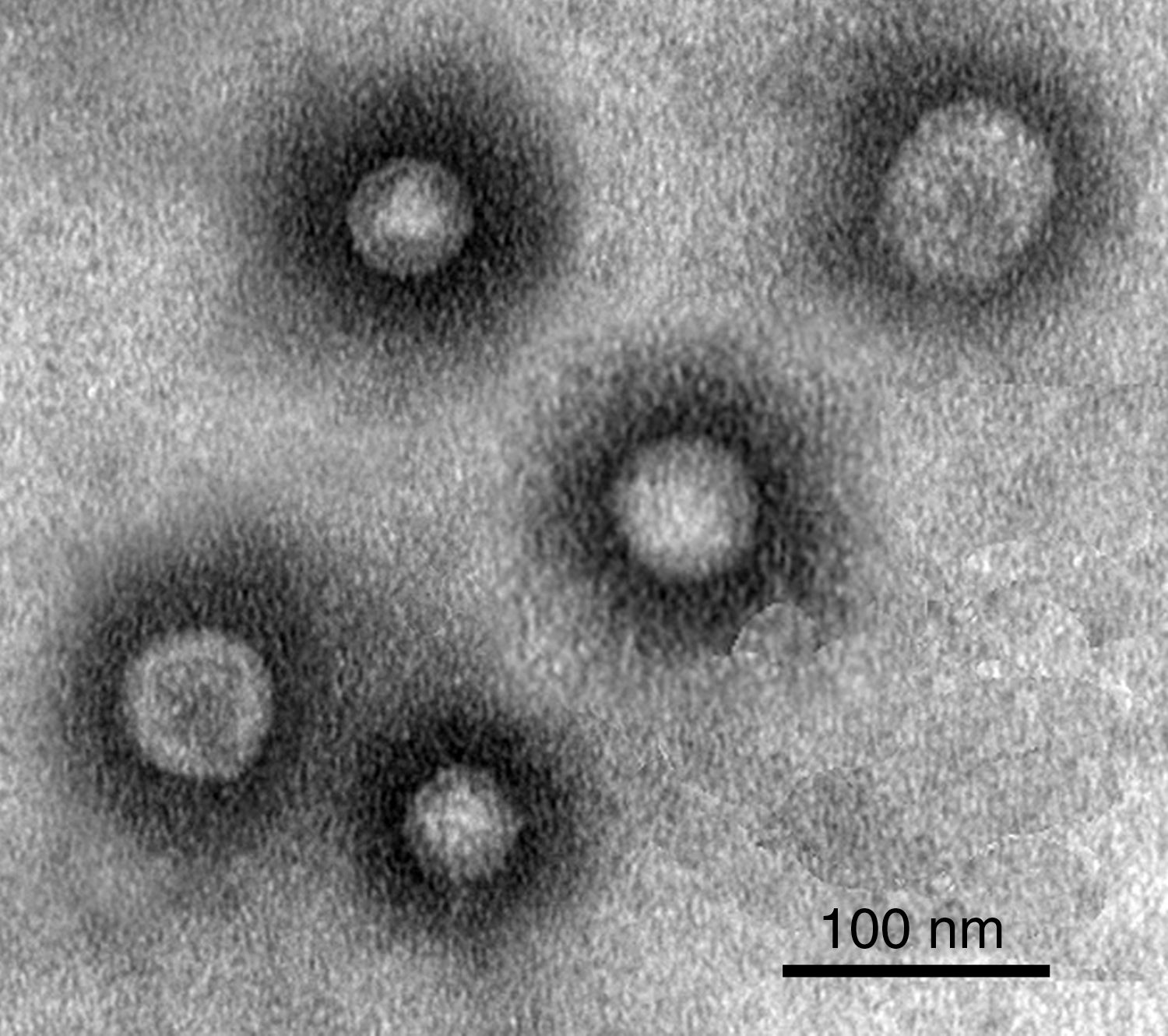
Morphology of JMT-1Negative-stain electron microscopy of JMT-1 revealed spherical particles with an average diameter of about 30–50nm, most of the particles had a stable diameter, nearly 40nm. The figure also suggested that, JMT-1 was a spherical particle that had a distinct outer layer, but no discernable tail (Fig. 2).
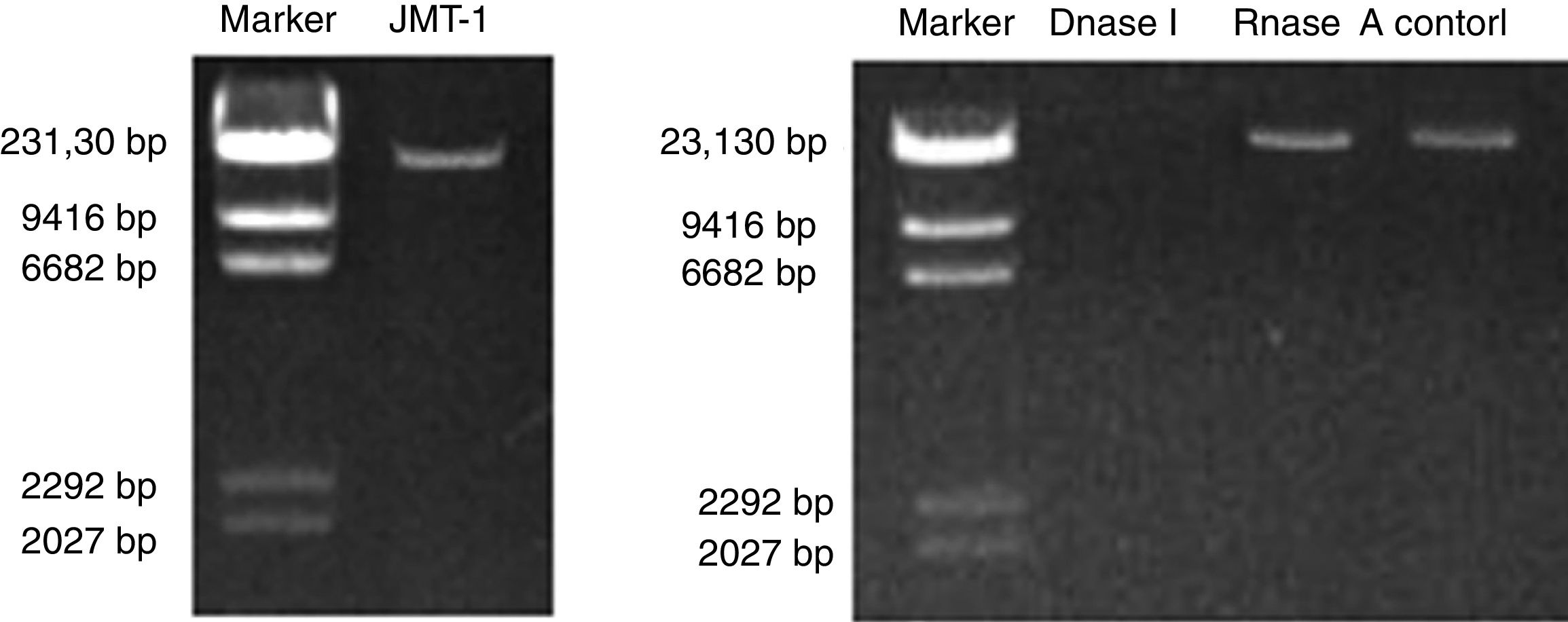
Genome characteristics of JMT-1Nucleic acids were extracted from purified JMT-1, and the measured length of JMT-1 genome is approximately 23kb (Fig. 3A). The nucleic acids extracted from JMT-1 were treated with RNase A or DNase I. Results show that the genome was sensitive to DNase I but cannot be digested by RNase A (Fig. 3B). This finding indicates that the genome of JMT-1 is a DNA.
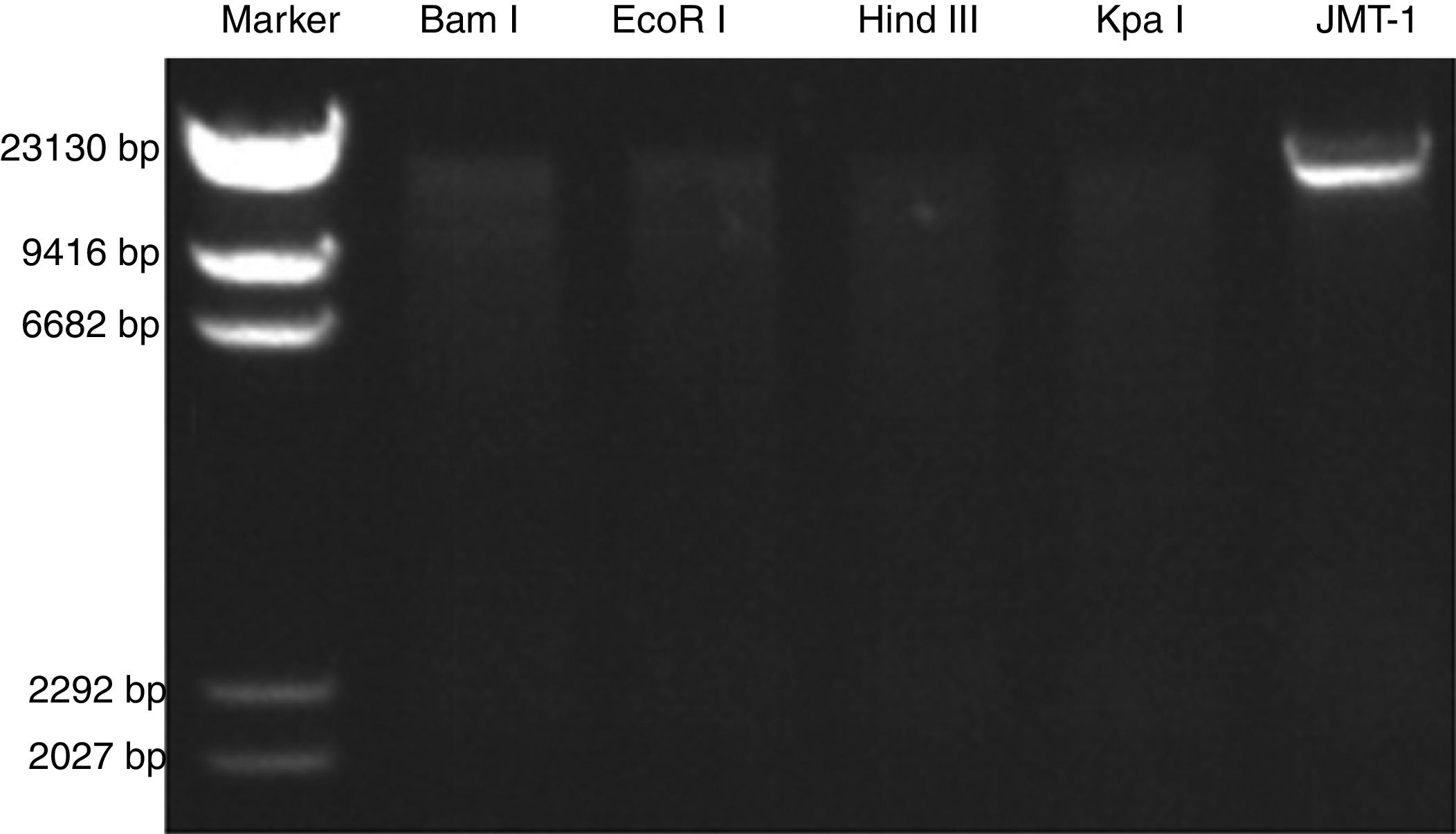
The JMT-1 RFLP analysis was conducted using eight restriction endonucleases. As shown in Fig. 5, the genome of JMT-1 was sensitive to four endonucleases, including Bam I, EcoR I, Hind III, and Kpa I. Given that Bam I and EcoR I are dsDNA endonucleases, result indicates that the genome of JMT-1 is a dsDNA (Fig. 4).
RFLP of JMT-1. The genome of JMT-1 is examined with restriction endonucleases. The nucleic acids are sensitive to Bam I, EcoR I, Hind III and Kpa I, and this means that the genome of JMT-1 is ds RNA as Bam I and EcoR I are dsDNA endonucleases. M, marker; Bam I, EcoR I, Hind III, Kpa I, the endonucleases used in JMT-1 RFLP.
Purified phage particles were subjected to SDS-PAGE analysis after washing them thrice with 0.1M ammonium acetate to remove any residual bacterial protein. Proteomic patterns were obtained following Coomassie Brilliant Blue G250 staining and destaining steps. The protein pattern of QHHSV-1 was determined through SDS-PAGE. Seven JMT-1 protein bands were detected with molecular weights ranging from 10kDa to 80kDa. Four of these seven protein bands were considered as the primary protein bands with molecular weights of 85, 80, 70, and 43kDa, respectively (Fig. 5).
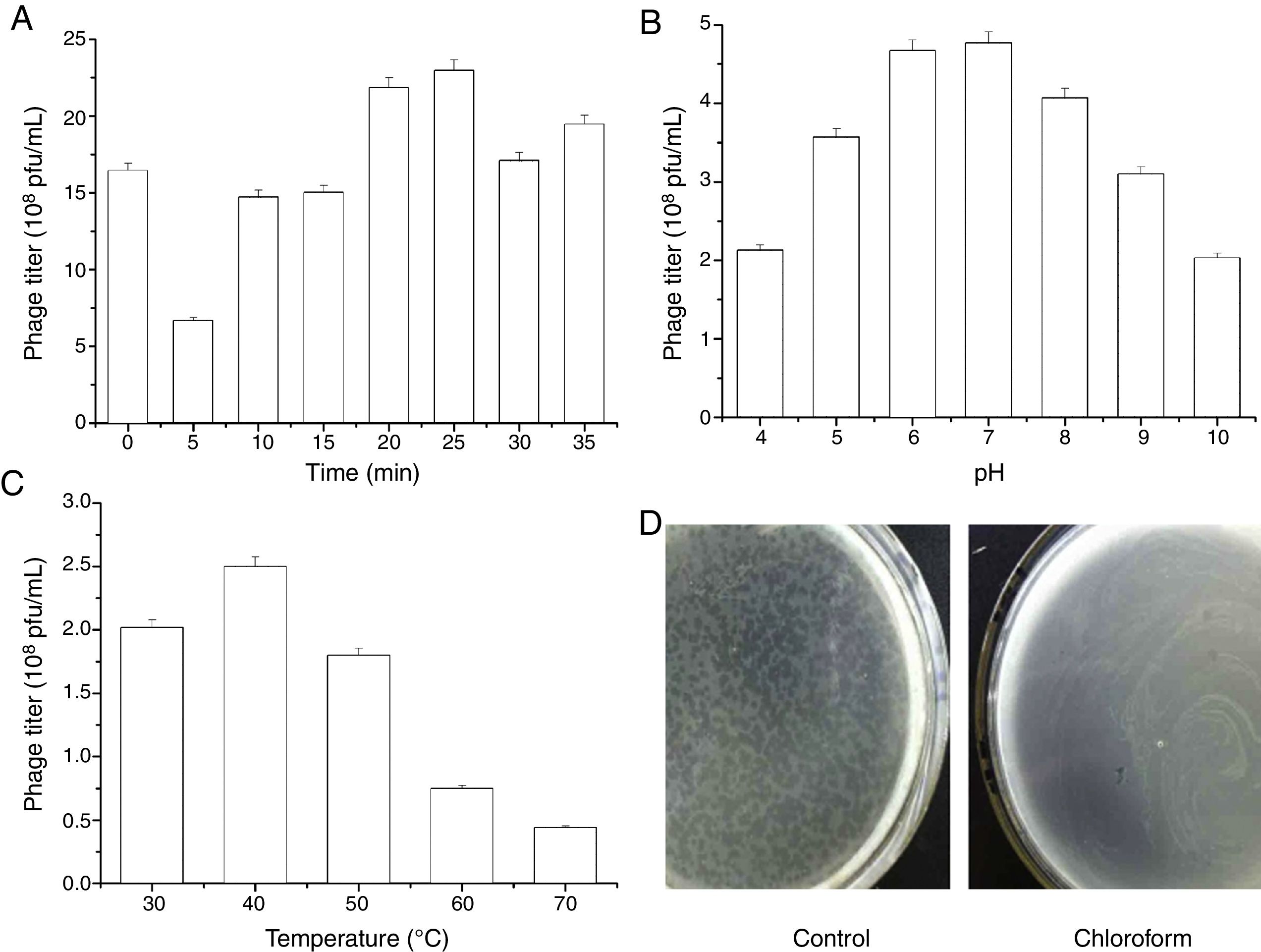
Stability of JMT-1The stability of JMT-1 was tested under several conditions (Fig. 6):
- (1)
As a halovirus living in the saline lake, the stability of JMT-1 in low ionic environment was the first characteristic that we wanted to determine. The test lasted for 35min. For the first 5min, the phage titer decreased sharply (from 16.5×108pfu/mL to 6.4×108pfu/mL) and then increased unstably, the highest titer appeared at 25min, and the titer was up to 22.6×108pfu/mL (Fig. 6A). This observation indicates that the phage is affected by the low ionic environment although JMT-1 has a certain degree of tolerance, and JMT-1 is a halotolerant phage.
- (2)
JMT-1 was stable between pH 4 and 10. The optimal pH was determined by testing the stability of the phage JMT-1 under different pH conditions. JMT-1 was most stable at pH 6.0–8.0, and the phage survival decreased slightly at lower or higher pH values (Fig. 6B). Results suggest that extreme pH values might affect JMT-1 stability, and that the phage is more stable in a neutral environment than in an acidic or alkaline one.
- (3)
Fig. 6C presents the thermal stability curve of JMT-1, indicating that the phage was stable from 30°C to 50°C and showed a rapidly reduced titer (from 2.48×108pfu/mL to 0.69×108pfu/mL) when the temperature increased up to 70°C. At 40°C, JMT-1 showed the highest titer (2.48×108pfu/mL). Compared with the titer at 40°C, the survival rates of JMT-1 at 50, 60, and 70°C were 72.2%, 32.2%, and 21.9%, respectively. This finding suggests that JMT-1 has a high stability in different temperature environments and is extremely heat stable, because 32.2% and 21.9% of the phages remained alive after 30min of incubation at 60 and 70°C, respectively.
- (4)
JMT-1 is sensitive to chloroform (Fig. 6D). As shown in Fig. 6D, JMT-1 cannot live in MGM after treatment with chloroform, indicating the presence of lipids in the viral capsid or a surrounding lipid layer.
Stability of JMT-1 to various treatments and conditions. Virus preparations (infected-cell supernatants in 18% (w/v) MGM) were exposed to various conditions, after which JMT-1 titer was determined (in duplicate) on Chromohalobacter sp. LY7-3 cells. (A) The effect of lowered salt concentration. Virus was diluted 1:1000 in double-distilled H2O and incubated at room temperature, culture on MGM, the titer of sample was detected. (B) The effect of pH. Virus was diluted 1:100 in Tris–HCl buffers at the different pHs, samples were cultured on MGM, the titer of JMT-1 was detected. (C) The effect of temperature. JMT-1 was incubated for 30min at temperatures between 30 and 70°C (30°C, 40°C, 50°C, 60°C, 70°C, respectively), samples were cultured on MGM, the titer of JMT-1 was detected. (D) The effect of chloroform. Chloroform was mixed with virus (1:4 ratio) and incubated at room temperature, cultured on MGM.
The characteristics of JMT-1 indicate that it is a novel virus infecting bacteria from salt field environment. As shown in Fig. 1, the presence of plaque indicated that JMT-1 is a lytic phage. JMT-1 is a spherical particle that has a distinct proteinaceous outer layer, no discernable tail, and a lytic growth pattern. In addition, JMT-1 possesses a DNA genome with a length of ∼23kbp. Meanwhile, the restriction endonucleases Bam I and EcoR I were able to digest JMT-1, indicating that the genome of the phage is composed of dsDNA.
Only a few halotolerant viruses, such as JMT-1, have been reported. SH116 and PH1,17 which are halophilic viruses isolated from a saline lake in Australia, are similar to JMT-1 in terms of their living environment. However, some distinctions exist between JMT-1 and SH1/PH1. A visible difference is that SH1 and PH1 have one and two distinct proteinaceous outer layers, respectively, whereas JMT-1 does not have such structure. SH1 and PH1 share similar genome and proteome, but JMT-1 has smaller DNA and lower number of proteins. SH1 and PH1 are sensitive to low ionic strength conditions, high temperature, and acidic pH. By contrast, JMT-1 is stable in such conditions. This observation indicates that JMT-1 exhibits better adaption to a changing environment compared with SH1 and PH1.
Given the special kind of ecosystem, numerous research on bacteria and viruses living in salt field environment have been carried out extensively for nearly a century or more. However, the progress on halophilic phage research is quite limited. Meanwhile, reports on halophilic phage-infected eukaryotic microorganisms in salt field environments do not exist. Research on the morphological diversity of halophilic, spindle-shaped, spherical-, and star-shaped phages is relatively few. However, studies on halophilic phages with “head” and “tail” are very common.18 In this study, JMT-1 is a spherical phage, which was isolated using a simpler method. A group of round haloviruses, the halosphaerovirus, has been recently expanded with the addition of SH1 and other three haloviruses, including PH1, HHIV-2, and SNJ1.9,17 Compared with these viruses, JMT-1 has a smaller genome and proteome.
In this study, the concentration and purification procedures for JMT-1 yielded about 90% of the original infectivity in the lysate. Compared with PRD119 or PM2,20 the halophilic phage containing dsDNA has a better yield. Our data showed that JMT-1 has a high titer, reaching 1.5×109pfu/mL after dilution to 10−6pfu/mL. The infectivity of JMT-1 stability in the original lysate, which was stored in the growth medium at 4°C, is little decay noted in several months (at least 6 months). In addition, JMT-1 is not sensitive to lowered salt conditions (the titer could up to 22.6×108pfu/mL in lowered salt conditions) and higher temperature, at the temperature 70°C. the phage still has a titer of 0.69×108pfu/mL. Such stability is helpful in studying JMT-1 infection and its lysis mechanism, analyzing its life history, or determining the pathogenic mechanism of pathogenic bacteria through animal experiment.
Viruses have been acknowledged to be the most distinct source of biodiversity. However, characterizing viruses in natural systems remains difficult. The reasons are possibly the lack of universally present and phylogenetically informative genes.21 However, culture-independent methods, such as transmission electron microscopy,1 pulsed-field gel electrophoresis,22–25 and metatranscriptome26 and metagenome analyses,25,27 have shown that viruses from hypersaline environments are among the most abundant biological entities in the aquatic systems. Indeed, the halovirus assemblage was reported to be highly active in such environments.26 This study describes conditions where a halophilic virus-host system exists, and how the bacteriophage JMT-1 was purified. Thus, this work sets the basis for further studies on JMT-1. We also observed the diversity between JMT-1 and other halophilic viruses, such as SH1 and PH1. Reports indicated that the gross morphology of the SH1 virion is similar to that of the membrane-containing phages, PRD128 and PM2,20 whereas its genome structure is similar to that of PRD1. In terms of the diverse stability of viruses in different environments, JMT-1 has higher chances of survival in much more extreme conditions compared with other halophilic viruses. This finding might provide addition motivation regarding the potential application of such halophilic bacteriophage.
Conflicts of interestThe authors declare that they have no conflict of interest.
This work was supported by Program for the Top Young Academic Leaders of Higher Learning Institutions of Shanxi (2016), Key Disciplines Construction Foundation of the “1331 Project” of Shanxi Province (098-091704), Natural Science Foundation of Shanxi Province (201701D121088) and PhD Start-up Foundation of Yuncheng University (Grant No. YQ-2016015).