Fresh produce is a generalized term for a group of farm-produced crops, including fruits and vegetables. Organic agriculture has been on the rise and attracting the attention of the food production sector, since it uses eco-agricultural principles that are ostensibly environmentally-friendly and provides products potentially free from the residues of agrochemicals. Organic farming practices such as the use of animal manure can however increase the risk of contamination by enteric pathogenic microorganisms and may consequently pose health risks. A number of scientific studies conducted in different countries have compared the microbiological quality of produce samples from organic and conventional production and results are contradictory. While some have reported greater microbial counts in fresh produce from organic production, other studies do not. This manuscript provides a brief review of the current knowledge and summarizes data on the occurrence of pathogenic microorganisms in vegetables from organic production.
Fresh produce is a generalized term for a group of farm-produced crops, including fruits and vegetables. These foods are an important component of a healthy diet. Consumption of fresh produce is widely promoted by governmental health agencies since it supplies essential nutrients such as vitamins, minerals, dietary fiber and phytochemical compounds at a relatively low calorie density. Furthermore, the consumption of fruits and vegetables has been strongly associated with reduced chronic diseases, risk of heart disease and cancer.1–3 Alternative cropping systems have been developed because of society's increasing concerns about the sustainability of conventional agriculture, intensive use of chemical products and their potential risk to human health and the environment.4,5 Organic agriculture has been on the rise and is attracting the attention of the food production sector in many parts of the world, since it revives eco-agricultural principles that are potentially more environmentally friendly and may provide products with few agrochemical residues.6,7 Organic farming practices which use animal manure as fertilizer can increase the risk of contamination by enteric pathogenic microorganisms and, consequently, pose health risks becoming a major concern for consumers and governments. Furthermore, these foods are often consumed raw, increasing risk of infection if pathogens are present.8
Despite the growing demand for organic fresh produce and its health benefits, a number of foodborne disease outbreaks have been associated with the consumption of these foods.8–14 However, the number of studies focusing on microbial safety of organically produced foods is low. This manuscript provides a brief review of the current knowledge and summarizes data on the risk of pathogenic microorganisms in vegetables from organic production.
Organic farmingThe organic sector has expanded recently worldwide, due to policy support and a growing market demand for these products. Organic farming can be defined as an ecological production system that promotes and enhances biodiversity and biological cycle in soil, crop and livestock. It is based on minimal use of off-farm inputs and on management practices that restore, maintain and enhance ecological harmony.15 The process of certification is also important in organic farming. Certification is intended to assure the consumers that a product marketed as organic was in fact produced according to organic production standards, which vary from country to country, based on their certifying bodies.16
Organic farming is regulated internationally by Codex Alimentarius Guidelines [established by the Food and Agricultural Organization of the United Nations (FAO) and the World Health Organization (WHO)] and by the International Federation of Organic Agriculture Movements (IFOAM) Basic Standards.17 According to the IFOAM,18 the principles of organic agriculture are: (i) health: organic agriculture should sustain and enhance the health of soil, plant, animal, human and planet as one and indivisible; (ii) ecology: organic agriculture should be based on living ecological systems and cycles, work with them, emulate them and help sustain them; (iii) fairness: organic agriculture should be built on relationships that ensure fairness with regard to the common environment and life opportunities and (iv) care: organic agriculture should be managed in a precautionary and responsible manner to protect the health and well-being of current and future generations and the environment.
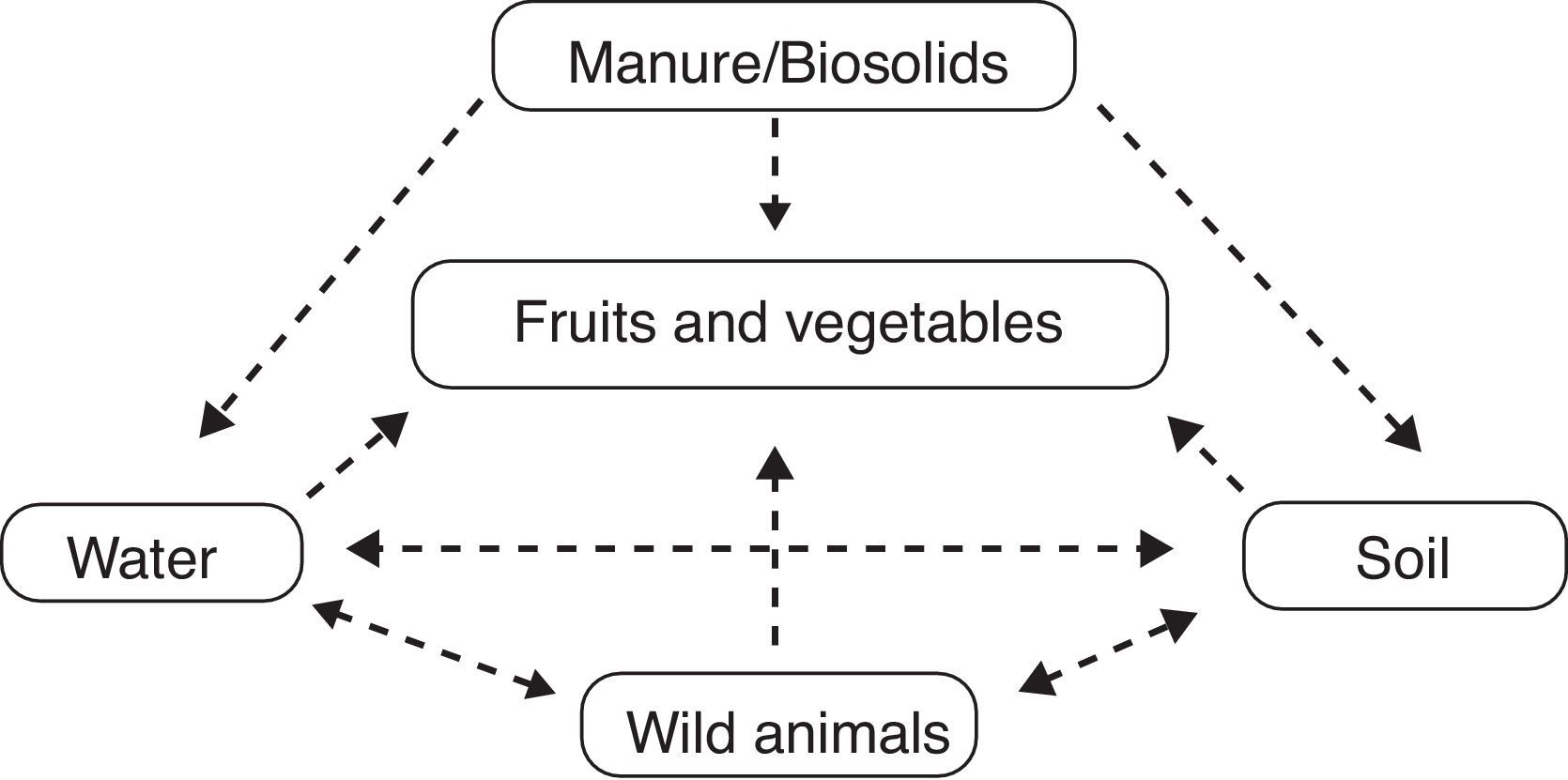
Main sources of contamination of fresh produce by foodborne pathogensFresh produce can become contaminated with pathogenic microorganisms during pre-harvest (in the field) and post-harvest stages and this contamination can arise from environmental, animal or human sources. Pre-harvest sources include soil, irrigation water, inadequately composted or raw animal manure, dust, insects, presence of wild and domestic animals and human handling. Post-harvest sources include human handling, harvesting equipment, transport containers/vehicles, rinse water, improper storage and packaging.8,11,19,20Fig. 1 illustrates the main routes of fresh produce contamination in the field.
The sources and routes of contamination of fruits and vegetables.
The soil is a habitat for many organisms, including human pathogens, which can contaminate plants through the seeds, roots or surface. Both conventional and organic produce can be fertilized with natural sources of nutrients such as animal manure and plant debris. Since animal manure is the main fertilizer type in organic farming, where no chemical treatment against bacteria is allowed, it gives rise to concern about the possible contamination of produce with microbial pathogens such as Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes.21,22 A key strategy used to reduce the concentration of enteric pathogens in manure is composting, the biological decomposition of organic matter by microorganisms under controlled conditions.23 Compost can provide certain benefits to plants when applied to the soil. If composting is done incorrectly (i.e. for too short a time or at too low a temperature), the result can increase microbial proliferation and risk of pathogen contamination.24,25
The irrigation water can also be a source of contamination. The most common sources of water for irrigation include wells, rivers, reservoirs and lakes, all of which are susceptible to contamination by human pathogens. The presence of pathogenic microorganisms in irrigation water and their transfer to vegetables has been reported.26–28 Moreover, vegetable cultivation in open areas allows the access of animals (birds, insects, rodents, domestic and wild animals), which can defecate in the fields and, therefore, be a source of contamination. Studies conducted by Islam et al.29,30 showed that pathogens such as S. Typhimurium and E. coli O157:H7 can survive for a long period in soil (>150 days) and vegetables (>60 days) grown after experimental contamination using contaminated fertilizer (manure) or irrigation water.
Contaminated equipment, poor hygiene and improper post-harvest handling can compromise the microbial safety of a product. Proper storage conditions, including temperature, air circulation and relative humidity may be needed for pathogen control. The storage of fresh produce under refrigeration (≤4°C) is an important strategy to reduce the metabolic rate of the plant and prevent or limit the growth of pathogens.31
Pathogens isolated from or associated with organic produceAn increased number of foodborne disease outbreaks have been associated with the consumption of fresh produce recently.8–14,32 According to a report by the Center for Science in the Public Interest (CSPI), fresh produce was the cause of most foodborne illnesses occurred in the U.S. between 2004 and 2013, including 193,754 illnesses from 9,625 outbreaks.33 Studies have isolated pathogens including Salmonella spp., L. monocytogenes and pathogenic E. coli from fresh and fresh-cut vegetable samples in many countries.34–49 Other studies have compared the microbiological quality of vegetables from organic and conventional production.21,22,24,50–59 Ceuppens et al.51 assessed the microbiological quality of lettuce production in Brazil and detected generic E. coli more frequently and at higher average concentrations in lettuce samples from organic farms (23.1% and 3.22logCFU/g) vs. conventional farms (16.7% and 2.27logCFU/g). Gomes Neto et al.52 evaluated the microbiological quality of 180 iceberg lettuce samples from conventional (n=60), organic (n=60) and hydroponic (n=60) cropping systems in Brazil and observed that samples from organic systems were the most contaminated both by bacteria and intestinal parasites, while the lowest contamination level was observed in hydroponically grown lettuce. Maffei et al.24 analyzed 130 samples of different organic (n=65) and conventional (n=65) vegetable varieties (also in Brazil), and observed that some organic varieties had greater microbial counts with the highest incidence of generic E. coli in organic loose-leaf lettuce (90% of samples positive).
Mukherjee et al.55 analyzed 476 organic and 129 conventional produce samples from farms in Minnesota, USA, and found greater prevalence of E. coli in organic vs. conventional samples. The largest prevalence of E. coli was in organic lettuce (22.4% of samples). Oliveira et al.21 analyzed 72 lettuce samples of organic and conventional agriculture in Spain and found similar results, with a greater prevalence of E. coli in lettuce samples from organic (22.2%) than conventional (12.5%) agriculture. Wießner et al.22 investigated the effect of different organic manures in comparison with mineral fertilizer on the risk of pathogen transfer in lettuce plants in Germany and observed that microbial counts tended to be slightly higher after organic fertilization, although the differences were not statistically significant (p<0.05). Although these studies show that organic produce generally seems to be more contaminated than conventional produce, this effect was not seen in every study.
Bohaychuk et al.50 analyzed 673 fresh produce samples collected from Alberta public and farmer's markets in Canada (including organic produce) and observed that the levels of E. coli in organically and conventionally grown produce was not significantly different (p<0.05). Khalil and Gomaa53 analyzed 380 samples of unpackaged whole conventional and 84 packaged whole organic leafy greens collected from retail markets in Alexandria, Egypt, and observed the mean total bacterial count for organic samples were statistically significantly less (p<0.05) than those of the corresponding conventional samples and E. coli was detected in 100% of all leafy greens. Marine et al.54 evaluated leafy greens from organic (n=178) and conventional (n=191) farms in the Mid-Atlantic Region of the United States. They observed that the farming system was not a significant factor for E. coli, aerobic mesophiles or Salmonella, but with (non-statistically significant) higher total coliform counts from organic farm samples.
Mukherjee et al.56 conducted a 2-year study to evaluated 2029 preharvest produce samples (473 organic, 911 semiorganic, and 645 conventional) in Minnesota and Wisconsin, USA, and concluded that the microbiological quality of preharvest produce from the three types of farms was very similar. Phillips and Harrison57 evaluated the microflora of organic (n=108) and conventional (n=108) spring mix samples obtained from a commercial California (USA) fresh-cut produce where manure is not used in the cultivation practices. The authors observed that the mean microbial population for conventional samples was not statistically different (p>0.05) from the corresponding mean populations for organic samples. The mean population of each microbial group was significantly higher in unwashed vs. washed product. Ryu et al.58 analyzed the microbiological quality of 11 types of environmentally friendly and conventionally grown vegetables sold at retail markets in Korea and did not observe significant difference (p>0.05) in the overall microbiological quality among samples. Tango et al.59 analyzed the microbiological quality of 354 Korean leafy vegetable samples (165 conventional and 189 organic) and their results did not support the hypothesis that organic produce poses a greater risk of pathogen contamination vs. conventional produce.
Only Ceuppens et al.,51 Marine et al.,54 Mukherjee et al.55 and Tango et al.59 detected pathogenic bacteria in the fresh produce samples analyzed above. Ceuppens et al.51 isolated Salmonella from one organic lettuce sample. Marine et al.54 isolated Salmonella from eight (2.16%) of 369 leafy greens [four organic (2.24%) and four conventional (2.09%) samples]. Mukherjee et al.55 isolated Salmonella from one organic lettuce and one organic green pepper sample. Tango et al.59 reported positive results for Bacillus cereus (n=17), Staphylococcus aureus (n=13) and L. monocytogenes (n=10) in 63 organic samples, vs. 3, 8 and 6, respectively for conventional samples. These authors also detected E. coli O157:H7 in 1 out of 55 conventional samples.
Some scientific studies have been conducted to determine the presence of pathogenic bacteria exclusively in organic produce.60–67 Among these, only Chang et al.,61 Loncarevic et al.,62 McMahon and Wilson,64 Nguz et al.65 and Rodrigues et al.66 detected the presence of pathogenic bacteria. Chang et al.61 reported the presence of E. coli O157:H7 in four out of 210 organic vegetables collected in supermarket and groceries in Selangor, Malaysia. Loncarevic et al.62 isolated L. monocytogenes serogroups 1 and 4 from two of 179 organically grown leaf lettuce samples in Norway. McMahon and Wilson64 investigated the occurrence of selected enteric pathogens and Aeromonas species in organic vegetables in Northern Ireland. They found Aeromonas spp only (34% of the samples) including A. schubertii (21%), A. hydrophila (5.8%), A. trota (5.8%), A. caviae (3.5%) and A. veronii biovar veronii (2.3%). These Aeromonas species were previously reported to be potentially pathogenic and responsible for gastrointestinal infections in humans.68,69 Nguz et al.65 assessed the microbiological quality of fresh-cut organic vegetables (washed with chlorine solution at 150μgmL−1) produced in Zambia and detected the presence of L. monocytogenes, Salmonella and S. aureus in 20%, 23.1% and 80.0% of samples, respectively. Rodrigues et al.66 isolated Salmonella from only one of 36 Brazilian organic lettuce samples.
The incidence of pathogens in organic vegetables varies according to the study (Table 1). Salmonella spp prevalence varies between 0.4 and 23.1%, L. monocytogenes between 1.1 and 20% and S. aureus between 20.6 and 80%. B. cereus prevalence can be as high as 26.9% and as high as 34% for Aeromonas spp. E. coli O157:H7 prevalence can be as high as 1.9%. Since fresh produce is often consumed raw even low prevalence rates may mean increased risk of foodborne disease from these foods.
Incidence of pathogenic bacteria on organic vegetables.
| Country | Pathogen | Number of samples | Reference | |
|---|---|---|---|---|
| Total n | Positive n (%) | |||
| Brazil | Salmonella spp. | 75 | 1 (1.33) | Ceuppens et al.51 |
| Brazil | Salmonella spp. | 36 | 1 (2.77) | Rodrigues et al.66 |
| Korea | B. cereus | 63 | 17 (26.9) | Tango et al.59 |
| L. monocytogenes | 63 | 10 (15.8) | ||
| S. aureus | 63 | 13 (20.6) | ||
| Malaysia | E. coli O157:H7 | 210 | 4 (1.90) | Chang et al.61 |
| Northern Ireland | Aeromonas spp. | 86 | 29 (34.0) | McMahon and Wilson64 |
| Norway | L. monocytogenes | 179 | 2 (1.11) | Loncarevic et al.62 |
| USA | Salmonella spp. | 178 | 4 (2.24) | Marine et al.54 |
| USA | Salmonella spp. | 476 | 2 (0.42) | Mukherjee et al.55 |
| Zambia | L. monocytogenes | 80 | 16 (20.0) | Nguz et al.65 |
| Salmonella spp. | 160 | 37 (23.1) | ||
| S. aureus | 80 | 54 (80.0) | ||
Data based on studies that presented positive results (presence of pathogens).
Information on foodborne outbreaks from organic produce is limited. However, some cases involving Shiga toxin-producing E. coli (STEC) and organic produce have been reported in the past few years. An outbreak involving STEC O157:H7 linked to organic spinach and spring mix blend occurred in the U.S. in 2012 and affected 33 persons from five states: 46% were hospitalized and two developed hemolytic uremic syndrome (HUS).70 STEC O104:H4 was responsible for an outbreak linked to organic raw sprouts in Germany, with thousands of infections.71,72
Control measuresCurrent strategies to control microbial contamination during the production of fresh produce (regardless of cultivation method) are based on the implementation of Good Agricultural Practices (GAP). A GAP framework considers the implementation of best practices regarding worker's health and hygiene, soil and water quality, sewage treatment, wildlife and livestock management, manure and biosolids management, field sanitation and hygiene, and harvest and transportation.20 The implementation of food safety management tools such as Good Hygienic Practices (GHP) and Hazard Analysis and Critical Control Points (HACCP) at postharvest steps also helps to reduce, eliminate or prevent the occurrence of hazards. The washing step before consumption is also important as it may reduce microbial load on vegetables surfaces. Washing with sanitizer may also reduce contamination and prevent cross-contamination by pathogenic microorganisms.73–76
Concluding remarksData summarized above from a variety of studies focusing on different crops and different countries highlight the potential risks of pathogenic microorganisms in fresh produce, including organic products. Although a number of studies have indicated that organic produce may pose a greater risk than conventional grown produce, this trend is not universal across all studies. Further efforts are needed to understand and control the disease risk associated with organic produce from harvest through consumption.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for scholarship (830642/1999-4) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for scholarship (2016/09601-5) and financial support (2013/07914-8).