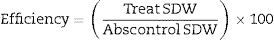The family Leguminosae comprises approximately 20,000 species that mostly form symbioses with arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing bacteria (NFB). This study is aimed at investigating and confirming the dependence on nodulation and biological nitrogen fixation in the specie Piptadenia gonoacantha (Mart.) Macbr., which belongs to the Piptadenia group. Two consecutive experiments were performed in a greenhouse. The experiments were fully randomized with six replicates and a factorial scheme. For the treatments, the two AMF species and three NFB strains were combined to nodulate P. gonoacantha in addition to the control treatments. The results indicate this species’ capacity for nodulation without the AMF; however, the AMF+NFB combinations yielded a considerable gain in P. gonoacantha shoot weight compared with the treatments that only included inoculating with bacteria or AMF. The results also confirm that the treatment effects among the AMF+NFB combinations produced different shoot dry weight/root dry weight ratios. We conclude that AMF is not necessary for nodulation and that this dependence improves species development because plant growth increases upon co-inoculation.
The family Leguminosae comprises approximately 3000 species throughout Brazil and is the third largest angiosperm family, with approximately 20,000 species and 700 genera,1 only surpassed by Orchidaceae and Asteraceae.2 Most species are associated with nitrogen-fixing microorganisms and arbuscular mycorrhizal fungi (AMF),3,4 which are the two main symbiotic microorganisms in terrestrial plants. New microbial species and rhizobia plant infection mechanisms were discovered through studying this bacterial diversity.5,6 Certain native legume species in the subfamily Mimosoideae exhibit atypical characteristics with a high exploitation potential for the two symbioses. Synergy between the symbionts has also been reported; mycorrhizal fungi can aid in increasing biological nitrogen fixation, and nitrogen-fixing bacteria influence mycorrhizal colonization.7,8
The species studied herein, Piptadenia gonoacantha (Mart.) J. F. Macbr., is arboreal and naturally occurs in southern and southeastern Brazil. It is economically and socially useful because it is used in the furniture construction, energy, cellulose, and paper sectors, among other fields.10 This species is also used considerably in degraded site restoration projects because it can biologically fix nitrogen.11
A recent discovery showed that the legumes P. gonoacantha and Piptadenia paniculata, both Atlantic Rainforest natives,12 did not nodulate in pots with soil and sand as substrates when they were not co-inoculated with AMF.11 Asai13 reported this observation and indicated that certain legumes do not nodulate in autoclaved soils without co-inoculation by mycorrhizal fungi. Crush14 provided the first conclusive observations on this synergistic effect.
However, the effects of the substrates on symbiosis formation remains uncertain; most likely, an underabundance of phosphorus limits symbiosis formation even if mycorrhizae are necessary for nodulation because phosphorus is important for forming nodules in the root system. In addition to affecting nodulation, the mycorrhizal fungus aids in better development of the plant species because biological nitrogen fixation demands high levels of energy, which is provided by the plant as ATP. However, the great phosphorus deficiency in tropical soils limits the maximum development of the symbiosis. Thus, increased phosphorus absorption by AMF yields increased fixation.2,8
The synergistic effect between the symbionts is evident from the phosphorus concentration in the nodules, which is up to three times higher than in other organs.15 This link is also attributed to the number of genes and root exudates that the symbioses share. This evidence supports the hypothesis that the symbioses formed with legume family species were inherited from mycorrhizal fungi because two forms of symbiosis emerged at different evolutionary times during colonization by terrestrial plants.16 From a functional perspective, bacterial and AMF compatibility can also alter symbiotic efficiency because the combination of inoculating with AMF and bacterial strains can either reduce or increase efficiency in certain bacterial strains.17,8,9
This study is based on the hypothesis that the species P. gonoacantha depends on the mycorrhizal fungus for nodule formation, hypothesis described by the author Jesus et al.11 Thereby, the article aimed at investigate and confirm the dependence of the specie on arbuscular mycorrhizal fungi for nodulation and biological nitrogen fixation.
Materials and methodsThe experiments were conducted in a greenhouse located at Embrapa Agrobiology (Embrapa Agrobiologia), Seropédica, Rio de Janeiro (RJ), Brazil. The species P. gonoacantha (Mart.) Macbr. was used. The bacterial strains BR 4802, BR 4812, and BSP1 were obtained from the Centro de Recursos Biológicos Johanna Döbereiner at the Embrapa Agrobiology and were grown for two days in tryptone yeast (TY) liquid medium at 28°C and 150rpm. Thereafter, the cultures were centrifuged at 10,000rpm and 4°C for 10min. The pellet was resuspended in a 10mM manganese sulfate solution (MgSO4·7H2O). This centrifugation step was repeated three times. The optical density of the strains was adjusted to 1.0, which corresponds to 108cellsmL−1.
The AMF inocula were obtained from the Arbuscular Mycorrhizal Fungi Collection of the Embrapa Agrobiology (Coleção de Fungos Micorrízicos Arbusculares da Embrapa Agrobiologia – COFMEA), and two species were selected: Gigaspora margarita W.N. Becker & I.R. Hall (A1 CNPAB 001) and Dentiscutata heterogama T.H. Nicolson & Gerd. Sieverd (A2 CNPAB 02). The spores were extracted using the wet sieving method18 and centrifuged with sucrose.19,20 The spores were separated into Petri dishes, and the inocula purity was verified using a stereoscopic microscope. The spores were disinfected following the method described by Colozzi-Filho.21 The sterilized spores were maintained in the Petri dishes with 79 medium22 for one week to verify the disinfection efficiency. The spores were applied to pots by diluting the spores extracted in distilled water. The spore quantity was standardized to 50 spores per mL, and 1.0mL of this solution was applied to each pot. The P. gonoacantha (Mart.) Macbr. seeds were surface disinfected with 30% hydrogen peroxide for two minutes and then germinated in Petri dishes with filter paper and cotton for four days at 28°C in a germinating chamber under constant light.
Two experiments using P. gonoacantha (Mart.) Macbr. were performed. The first experiment featured a completely randomized design with eight treatments and six replicates. This experiment comprised treatments with the following inoculants: a mycorrhizal fungus, the bacterial strains, and a combination of both microorganisms (mycorrhizal fungus+bacterial strains). In addition to the treatments, control experiments were performed (an absolute control, a nitrogen control, and a nitrogen control with AMF). The Burkholderia sp strains BR 4802 and BR 4812 were used. The mycorrhizal fungus G. margarita (Gig.marg) was used.
The experiment was performed in a greenhouse in Magenta pots (pots transparent acrylic, square base and with the volume of 400mL) containing sterile sand and vermiculite at a 1:1 ratio (v:v) and a nitrogen-free nutrient solution. Every two weeks, each seedling was fertilized with 100mL of a nutrient solution containing the following (mgL−1): 2mM CaCl2(H2O)2, 1mM MgSO4(H2O)7, 3mM KCl, 0.9μM ZnSO4(H2O)7, 4μM H3BO3, 1μM CuSO4(H2O)5, 6μM MnSO4H2O, 0.1μM NaMoO4(H2O)2, and 1.66% Fe EDTA.23 The plants were watered to maintain a moisture content near the 70% field capacity of the containers. Each pot received two seeds. Before planting the seeds, each hole was inoculated with 108cells of each bacterial strain and 50 mycorrhizal fungal spores and then with seeds. Thinning was performed soon thereafter to homogenize the species’ development. The nitrogen controls received 100mg of N/plant (ammonium nitrate solution – NH4NO3) until the end of the experiment. The experimental plants were harvested 150 days after sowing.
The second experiment featured a fully randomized design with eleven treatments and six replicates. It comprised treatments with the following inoculants: mycorrhizal fungi, bacterial strains, and a combination of both microorganisms (mycorrhizal fungi+bacterial strains). In addition to the treatments, we also used two different controls (an absolute control and a nitrogen control with AMF). The Burkholderia sp strains BR 4802 and BSP1 were used, and the mycorrhizal fungi G. margarita (Gig.marg) and D. heterogama (Dent.het) were used.
The experiment was performed in a greenhouse in Magenta pots containing sterile sand and vermiculite at a 1:1 ratio (v:v) and a N-free nutrient solution. The seeds were inoculated with 108cells of each bacterial strain and 50 spores of each mycorrhizal fungal species. The nitrogen controls received 70mg of N/plant (ammonium nitrate solution – NH4NO3) throughout the experiment. The experimental plants were harvested 77 days after sowing. We added N to a separate solution at 5mg of N/plant. Initially, 20mg of N, 4mL of the solution, was applied.
The following were determined in both experiments: shoot dry weight, root dry weight, nodule dry weight, and mycorrhization rate. The number of replicates was divided among the variables root dry weight and mycorrhization rate, three for each analysis. The six replicates were used to evaluate shoot dry weight.
The data were transformed due to an absence of normality; we used the following formula: the square root of the variable plus one. The shoot dry weight of each inoculation treatment was compared with the absolute control weight that did not receive a treatment, which was used to calculate the efficiency. The shoot dry weight of each inoculation treatment was compared with the nitrogen control that induced the highest weight increase, which was used to determine the efficacy.
The efficiency and efficacy of each inoculation treatment were calculated using the following formulas11:
where Treat SDW=shoot dry weight of the inoculated treatment, and Abscontrol SDW=shoot dry weight of the absolute control;where Treat SDW=shoot dry weight of the inoculated treatment, and Nitrocontrol SDW=shoot dry weight of the nitrogen control.Next, the data were subjected to analyses of variance (ANOVA), and the means were compared with Tukey's test at a 5% probability using the SISVAR software.24
Root colonization was evaluated in fine root samples that were clarified and stained using the methods from Koske and Gemma25 and Grace and Stribley.26 Mycorrhization was evaluated using the root/grid intersect method from Giovannetti and Mosse,27 which was adapted from the root length measurement method in Newman.28
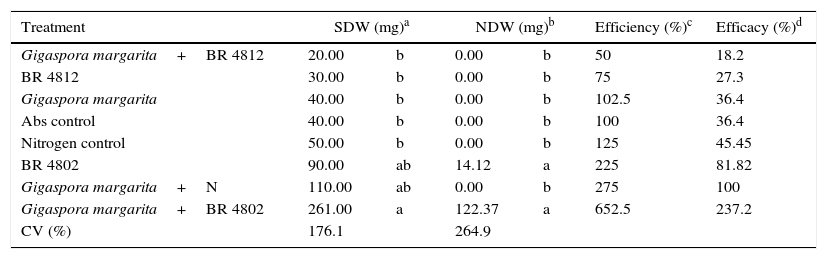
ResultsFirst experimentThe inoculated plants only nodulated with the BR 4802 strain, regardless of whether the mycorrhizal fungus was present. The highest accumulated shoot dry weight was measured when the plants were co-inoculated with G. margarita and the BR 4802 strain. This treatment also exhibited the highest symbiotic efficiency (Table 1). The plants only responded to nitrogen fertilizer in the presence of AMF. The colonization rates were 12, 18, and 28% for the G. margarita inoculation, with and without N, and G. margarita and BR 4802 strain co-inoculation treatments, respectively.
The effect of co-inoculation on Piptadenia gonoacantha development. Mean of three replicates.
| Treatment | SDW (mg)a | NDW (mg)b | Efficiency (%)c | Efficacy (%)d | ||
|---|---|---|---|---|---|---|
| Gigaspora margarita+BR 4812 | 20.00 | b | 0.00 | b | 50 | 18.2 |
| BR 4812 | 30.00 | b | 0.00 | b | 75 | 27.3 |
| Gigaspora margarita | 40.00 | b | 0.00 | b | 102.5 | 36.4 |
| Abs control | 40.00 | b | 0.00 | b | 100 | 36.4 |
| Nitrogen control | 50.00 | b | 0.00 | b | 125 | 45.45 |
| BR 4802 | 90.00 | ab | 14.12 | a | 225 | 81.82 |
| Gigaspora margarita+N | 110.00 | ab | 0.00 | b | 275 | 100 |
| Gigaspora margarita+BR 4802 | 261.00 | a | 122.37 | a | 652.5 | 237.2 |
| CV (%) | 176.1 | 264.9 | ||||
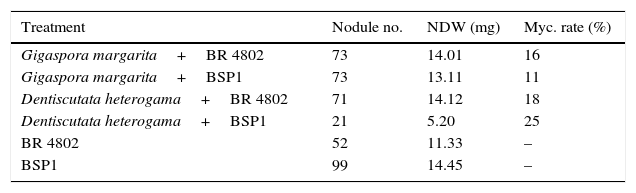
Root colonization by D. heterogama was greater when the bacterial strains were present. The fewest nodules were observed with the D. heterogama+BSP1 treatment. This strain exhibited variable behavior; it promoted greater nodulation without the mycorrhizal fungus. The G. margarita+BSP1 treatment promoted approximately three times more nodules than the D. heterogama+BSP1 treatment. This effect with the different fungal species was not observed for symbiosis with the other strain. The species G. margarita promoted different effects between the strains, reducing the number of nodules with the BSP1 strain and increasing the number of nodules with the BR 4802 strain upon co-inoculation (Table 2).
Nodule number, nodule dry weight (NDW), and mycorrhizal colonization rate in the inoculation treatments with without adding the mycorrhizal fungus of the second experiment using Piptadenia gonoacantha and the mycorrhization rate. Mean of three replicates.
| Treatment | Nodule no. | NDW (mg) | Myc. rate (%) |
|---|---|---|---|
| Gigaspora margarita+BR 4802 | 73 | 14.01 | 16 |
| Gigaspora margarita+BSP1 | 73 | 13.11 | 11 |
| Dentiscutata heterogama+BR 4802 | 71 | 14.12 | 18 |
| Dentiscutata heterogama+BSP1 | 21 | 5.20 | 25 |
| BR 4802 | 52 | 11.33 | – |
| BSP1 | 99 | 14.45 | – |
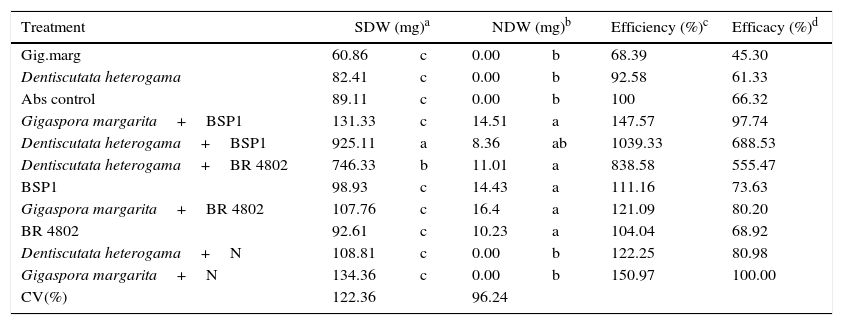
The co-inoculation treatments using the BSP1 and BR 4802 strains as well as D. heterogama increased the shoot dry weight; however, the nodule weights were low. The species D. heterogama (T.H. Nicolson & Gerd.) interacted positively with both bacterial strains, which suggests greater genetic compatibility. The treatments co-inoculated with the D. heterogama fungus and the bacteria, respectively, exhibited 10× and 8× more accumulated dry weight than the absolute control as indicated by the efficiency values and 7× and 6× more accumulated dry weight than the control upon inoculation with D. heterogama and the mineral N as indicated by the efficacy values (Table 3).
The effect of co-inoculation on Piptadenia gonoacantha development. Mean of three replicates. Means followed by the same letter in the column do not differ at a 5% probability level by Tukey's test.
| Treatment | SDW (mg)a | NDW (mg)b | Efficiency (%)c | Efficacy (%)d | ||
|---|---|---|---|---|---|---|
| Gig.marg | 60.86 | c | 0.00 | b | 68.39 | 45.30 |
| Dentiscutata heterogama | 82.41 | c | 0.00 | b | 92.58 | 61.33 |
| Abs control | 89.11 | c | 0.00 | b | 100 | 66.32 |
| Gigaspora margarita+BSP1 | 131.33 | c | 14.51 | a | 147.57 | 97.74 |
| Dentiscutata heterogama+BSP1 | 925.11 | a | 8.36 | ab | 1039.33 | 688.53 |
| Dentiscutata heterogama+BR 4802 | 746.33 | b | 11.01 | a | 838.58 | 555.47 |
| BSP1 | 98.93 | c | 14.43 | a | 111.16 | 73.63 |
| Gigaspora margarita+BR 4802 | 107.76 | c | 16.4 | a | 121.09 | 80.20 |
| BR 4802 | 92.61 | c | 10.23 | a | 104.04 | 68.92 |
| Dentiscutata heterogama+N | 108.81 | c | 0.00 | b | 122.25 | 80.98 |
| Gigaspora margarita+N | 134.36 | c | 0.00 | b | 150.97 | 100.00 |
| CV(%) | 122.36 | 96.24 | ||||
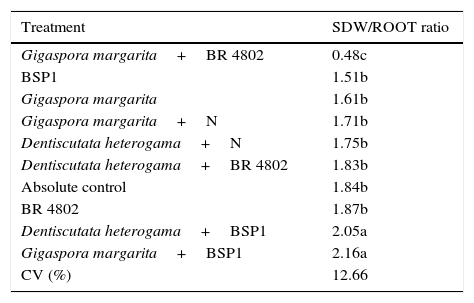
The SDW/ROOT ratios significantly differed, ranging between 0.48 and 2.16. The treatments that included BSP1 strain inoculation combined with the two mycorrhizal fungal species exhibited the highest SDW/ROOT ratios. The G. margarita+BSP1 treatment exhibited a ratio close to 2, which suggests that co-inoculation favors greater shoot development. This same fungal species yielded lower ratios than the absolute control when co-inoculated with the BR 4802 strain. When this same strain was co-inoculated with the two mycorrhizal fungal species, we observed development equivalent to that of the absolute control (Table 4).
Comparative analysis between the SDW/ROOT ratios and between the treatments that yielded the greatest weight gain with the species Piptadenia gonoacantha. Mean of three replicates. Means followed by the same letter in the column did not differ at a 5% probability level using the Scott Knott test.
| Treatment | SDW/ROOT ratio |
|---|---|
| Gigaspora margarita+BR 4802 | 0.48c |
| BSP1 | 1.51b |
| Gigaspora margarita | 1.61b |
| Gigaspora margarita+N | 1.71b |
| Dentiscutata heterogama+N | 1.75b |
| Dentiscutata heterogama+BR 4802 | 1.83b |
| Absolute control | 1.84b |
| BR 4802 | 1.87b |
| Dentiscutata heterogama+BSP1 | 2.05a |
| Gigaspora margarita+BSP1 | 2.16a |
| CV (%) | 12.66 |
Jesus et al.11 indicated that P. gonoacantha is highly dependent on AMF, especially for nodulation. However, the present study suggests otherwise because plants from this species nodulated without the mycorrhizal fungus, which was also observed by Bornaud et al.8 Most likely, the different substrates in the studies affected the results; however, the phosphorus source may have been more crucial to the results than the substrate. Jesus et al.11 used a small quantity of a slightly soluble source, rock phosphate; however, a nutrient solution with readily available phosphorus was used herein.
Analyzed together, the results of these two experiments indicate that “pau-jacaré”(P. gonoacantha (Mart.) J. F. Macbr) greatly depends on mycorrhizae for growth and requires AMF when phosphorus is a limiting factor. However, when phosphorus is readily available, the plant no longer depends on the fungus to grow and can nodulate without it. However, this does not mean that co-inoculation is not important because visual observations of the nodules from plants not inoculated with AMF indicate that these nodules are amorphous. This amorphous characteristic indicates that the fungus leads to morphological changes and altered efficiency in these nodules, as demonstrated by the acetylene reduction assay in plants with and without the mycorrhizal fungus. Further, this characteristic is evident in the scientific literature, which indicates a positive interaction between the fungus and bacteria; bacteria favor colonization of P. gonoacantha roots by AMF,8 and the fungus favors bacterial efficiency.29
The different results for the different bacteria-AMF combinations indicate that certain combinations are more efficient than others, which suggests a certain specificity between the symbiotic microorganisms. These data corroborate Bournaud,8 who observed that the mycorrhizal colonization rate of P. gonoacantha roots varied due to a co-inoculated rhizobial strain. The colonization rate in the different experiments may reflect strategic mycorrhizal colonization because D. heterogama should promote higher colonization of the root system compared with the fungal species with larger diameter spores relative to the duration of each experiment (G. margarita). The short duration of the experiments may have produced the different results for the different strategies. Fungal species under the K strategy establish in the root system over a longer time period due to the longer duration required for germination and development as well as the low number of spores produced, which was observed for G. margarita (W.N. Becker & I.R. Hall), exhibiting the lowest colonization rates.
According to the efficiency and efficacy results in the second experiment, the D. heterogama and BSP1 strain combination promoted an approximately 10× higher gain in shoot weight, which demonstrates a high recommendation potential.30 These results corroborate results from Bournaud et al.,8 who observed that the BSP1 strain provides the highest shoot growth, nodulation, and efficiency in co-inoculated P. gonoacantha plants. However, the author used Rhizophagus clarus (T.H. Nicolson & N.C. Schenck) C. Walker & A. Schübler. The plants inoculated with the BSP1 strain exhibited slightly higher root mycorrhization than plants inoculated with the same fungus plus the BR 4802 strain. These data also corroborate the results from Bournaud et al.,8 who observed that the BSP1 strain encourages root colonization by AMF and consequently increases nodule efficiency. Here, the effect observed for R. clarus was observed for D. heterogama. However, the accumulated dry weight and mycorrhizal colonization values were lower in plants inoculated with G. margarita, which indicates that the different fungus-bacteria combinations may not necessarily exhibit a positive effect on P. gonoacantha plant development.
Co-inoculation does not ensure better development as observed herein. For example, Patreze et al.31 and Carneiro et al.3 observed low colonization for the species Anadenanthera colubrina (0–14%), A. falcata (20–49%), and A. peregrina (1–19%). In addition to nutritional factors, the variable infection rate results may be associated with specificity between the host plants and AMF.
An analysis of the nitrogen controls (NC and AMF+NC) indicates the importance of the mycorrhizal fungus for better absorption of the added nitrogen. These results were confirmed during the second experiment, where the gain in shoot dry weight of the plants inoculated with G. margarita (W.N. Becker & I.R. Hall) was twice the weight gain as in plants that only received N. These observations highlight the mycorrhizal dependence of the P. gonoacantha species, not only for phosphorus absorption but also for other nutrients.32,33
Three groups of means formed upon analyzing the SDW/ROOT ratio in the species P. gonoacantha; the BSP1 strain without co-inoculation exhibited inferior behavior compared with the strain that contacts the two AMF species in the experiment. This contact altered the ratio in favor of greater shoot development. The analysis allows us to discern a range of possible responses between the microorganisms and their respective combinations.
ConclusionsThis article concluded that mycorrhizal fungi are not necessary for nodulation in the species P. gonoacantha, which highlights the effect of a substrate on forming any symbiotic association because the substrate used herein was different.
The co-inoculated P. gonoacantha plants produced a higher shoot weight with D. heterogama and BSP1 strain. This combination was very promising for P. gonoacantha inoculation in the future for seedlings.
These results stress the relevance of studies of inoculation in tree species used in reforestation and land reclamation.
Conflicts of interestThe authors declare no conflicts of interest.