In order to obtain an arbuscular mycorrhizal fungi (AMF) native inoculum from Sierra de Moa and determine the most appropriate conditions for its big scale production, four light and temperature combinations were tested in three plant species (Calophyllum antillanum, Talipariti elatum and Paspalum notatum). Growth and development parameters, as well as the mycorrhizal functioning of the seedlings were evaluated. The natural light treatment under high temperatures (L-H) was the most suitable for the growth and development of the three plant species, showing the highest total biomass values, mainly of root, and a positive root-shoot ratio balance. This treatment also promoted higher values of root mycorrhizal colonization, external mycelium and AMF spore density. A total of 38 AMF species were identified among the plants and environmental conditions tested. Archaeospora sp.1, Glomus sp.5, Glomus brohultii and G. glomerulatum were observed in all the treatments. The L-H condition can be recommended for native inoculum production, as it promotes a better expression of the AM symbiosis and an elevated production of mycorrhizal propagules.
Arbuscular mycorrhizal fungi (AMF) (Phylum Glomeromycota) are present in almost all the terrestrial ecosystems.1 They associate with the roots of more than 80% of the vascular plants, giving place to a mutual symbiosis denominated arbuscular mycorrhiza (AM).2
The AM enhances the absorption of water and nutrients, mainly P.1 It also increases the tolerance of plants to biotic and abiotic stresses, as pathogens, drought and high salinity.3,4 Besides that, the AM plays a critical role in the functional and successional processes of plant communities as soil formation, management and nutrient cycling.5–7
In Cuba the production of previously selected AMF for their use as biofertilizers began in the decade of 1990 with good results in different agricultural crops.8–10 However, the use of AMF for the restoration of degraded forest ecosystems has received poor attention, requiring a different approach. Because of the high demands of AMF propagules as part of the functional strategies of forests, the use of native soil as inoculum has been proposed.11 In addition, this inoculum production strategy has been indicated in other parts of the world as an appropriate way to ensure the successful re-establishment of native plants in degraded soils.12,13
The Moa region (Holguín, Cuba) has one of the highest floristic diversity and endemism in Cuba.14,15 It is considered the main center of evolution of the flora and vegetation of the northeastern mountains of the country.16 These characteristics indicate the possibility of a high diverse AMF community in this region.
One of the main conservational problems in Moa results from the opencast exploration of mineral deposits, which ends up in the destruction and fragmentation of natural ecosystems. Loss of AM propagules is usually recorded following degradation of the plant cover, up to a level, that could further inhibit natural and/or artificial revegetation processes.17 Taking into account all the previously cited aspects and the necessity of restoration in these areas, the eco-technology proposed by Torres-Arias et al.18 represents a good alternative. It proposes the restoration of areas degraded by mining through the re-introduction of native AMF and plant species. This way, the present study pretends to determine the optimal conditions for AMF multiplication and propagule production using original soil from Sierra de Moa as fungal initial inoculum.
Materials and methodsThe soil used in the experiment, classified as Red Ferritic, was collected in Sierra de Moa, which belongs to the Nipe-Sagua-Baracoa massif, Holguin, Cuba. The sampling was made at a depth of 0–20cm. The chemical soil analyses were made at the Federal Biological Research Center for Agriculture and Forestry, Berlin, Germany (Table 1). A CHNS vario EL analyzer was used to determine the N content. For the rest of the elements the nitric acid extraction method was applied, using atomic absorption spectrometry (AAS) or inductively coupled plasma (ICP).
The AMF spore quantification in 100g of the collected soil, which resulted in 585 spores, was made by wet sieving and decanting.19,20 The rest of the soil was sieved (2mm) and mixed with sterilized quartz sand, at a proportion of three parts of soil for one part of sand, giving place to the substrate used in the experiment.
Three plant species with different growth and development characteristics were selected for the trial: Calophyllum antillanum Britton, Talipariti elatum (Sw.) Fryxell and Paspalum notatum Flüggé. The seeds of each plant species were processed depending on their characteristics and requirements.21 Then, they were planted in plastic pots with 1.4kg of the previously described substrate.
The treatments included four different combinations of light (L) and temperature (T) conditions: (a) full light and high temperature (L-H) (greenhouse with natural illumination and temperature between 25 and 38°C); (b) Shadow and high temperature (S-H) (Room temperature between 25 and 30°C, without artificial light); (c) Artificial light and low temperature (L-C) (Air conditioned room with temperature between 19 and 23°C); and (d) Shadow and low temperature (S-C) (Air conditioned room with temperature between 19 and 23°C). The total duration of the experiment was of 12 months. Plants were fertilized with Long Ashton standard solution (15mL/plant) at a 10% concentration and adjusted P content to 5mg/L.
At the end of the experiment the following variables were measured in C. antillanum and T. elatum: height (cm), diameter at the base of the stem (cm), number of leaves, and dry weight of roots, stem and leaves independently (g). In P. notatum, as herbaceous plant, it was just possible to measure dry weight of leaves, stem, roots and rhizomes (g). For the three plant species an approximate amount of 0.5g of fresh fine roots was colored to estimate the amount of external mycelium (EM) (dm3) and percentage of colonized roots.20,22,23
The spore quantification in 100g of soil from the pots was made using the previously cited methodology, followed by counting in stereoscopic microscope. AMF species identification was made based on the spores’ morphology. Spores were mounted on glass slides with PVLG (Polyvinyl Lacto Glycerol) and PVLG+Melzer's reagent.
The comparisons between the treatments for each plant species were made through one-way ANOVA followed by Scott–Knott Test 5%. The statistical program used for the analyses was SISVAR 5.3.24
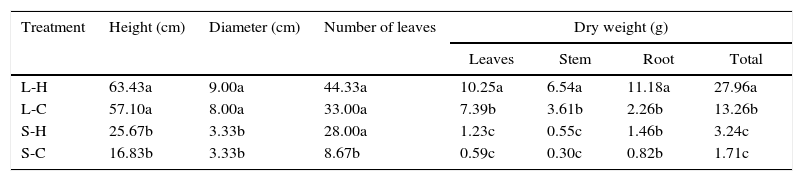
ResultsGrowth and development of the host plantsThe three plant species showed differential growing behaviors under variable light and temperature conditions. As showed in Fig. 1, C. antillanum and T. elatum grew under the four light and temperature combinations tested. However, the biomass production of these two species varied within the treatments. On the other hand, P. notatum was incapable of growing under reduced light sources (shadow treatments).
Seedlings of C. antillanum (I), T. elatum (II) and P. notatum (III) after 12 months. Letters indicate different light and temperature growing conditions: (A) L-H, full light and high temperature; (B) L-C, artificial light and low temperature; (C) S-H, shadow and high temperature and (D) S-C, shadow and low temperature.
The results indicate as the most proper conditions for the growing and development of C. antillanum those of plenty light (L-H and L-C), independently of the temperature (Table 2). Under the good illumination treatments the height and diameter were significantly superior compared to the shadow treatments (Table 2). With relation to the dry weight mean values, the best conditions were particularly those of natural light and elevated temperatures, where the results were significantly higher than in the other three treatments.
Mean values of height, diameter, number of leaves and dry weight of C. antillanum seedlings. Different letters indicate significant statistical differences (Scott Knott, 5%). L-H: full light and high temperature; L-C: artificial light and low temperature; S-H: shadow and high temperature; and S-C: shadow and low temperature.
| Treatment | Height (cm) | Diameter (cm) | Number of leaves | Dry weight (g) | |||
|---|---|---|---|---|---|---|---|
| Leaves | Stem | Root | Total | ||||
| L-H | 63.43a | 9.00a | 44.33a | 10.25a | 6.54a | 11.18a | 27.96a |
| L-C | 57.10a | 8.00a | 33.00a | 7.39b | 3.61b | 2.26b | 13.26b |
| S-H | 25.67b | 3.33b | 28.00a | 1.23c | 0.55c | 1.46b | 3.24c |
| S-C | 16.83b | 3.33b | 8.67b | 0.59c | 0.30c | 0.82b | 1.71c |
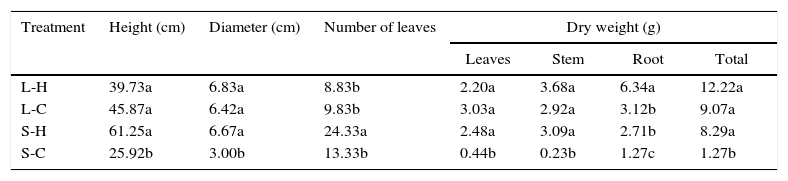
With a more homogeneous development, T. elatum had a similar behavior in three of the four environmental conditions tested. This species showed satisfactory results in the treatments L-H, L-C and S-H (Table 3). However, similarly to C. antillanum, in environments with natural light and high temperatures (L-H) T. elatum reached significantly higher total dry biomass, influenced by high levels of root production. Contrary to this, the worst treatment resulted that with conditions of shadow and low temperatures (S-C) with the lowest values for most of the variables analyzed.
Mean values of height, diameter, number of leaves and dry weight of T. elatum seedlings. Different letters indicate significant statistical differences (Scott Knott 5%). L-H: full light and high temperature; L-C: artificial light and low temperature; S-H: shadow and high temperature; and S-C: shadow and low temperature.
| Treatment | Height (cm) | Diameter (cm) | Number of leaves | Dry weight (g) | |||
|---|---|---|---|---|---|---|---|
| Leaves | Stem | Root | Total | ||||
| L-H | 39.73a | 6.83a | 8.83b | 2.20a | 3.68a | 6.34a | 12.22a |
| L-C | 45.87a | 6.42a | 9.83b | 3.03a | 2.92a | 3.12b | 9.07a |
| S-H | 61.25a | 6.67a | 24.33a | 2.48a | 3.09a | 2.71b | 8.29a |
| S-C | 25.92b | 3.00b | 13.33b | 0.44b | 0.23b | 1.27c | 1.27b |
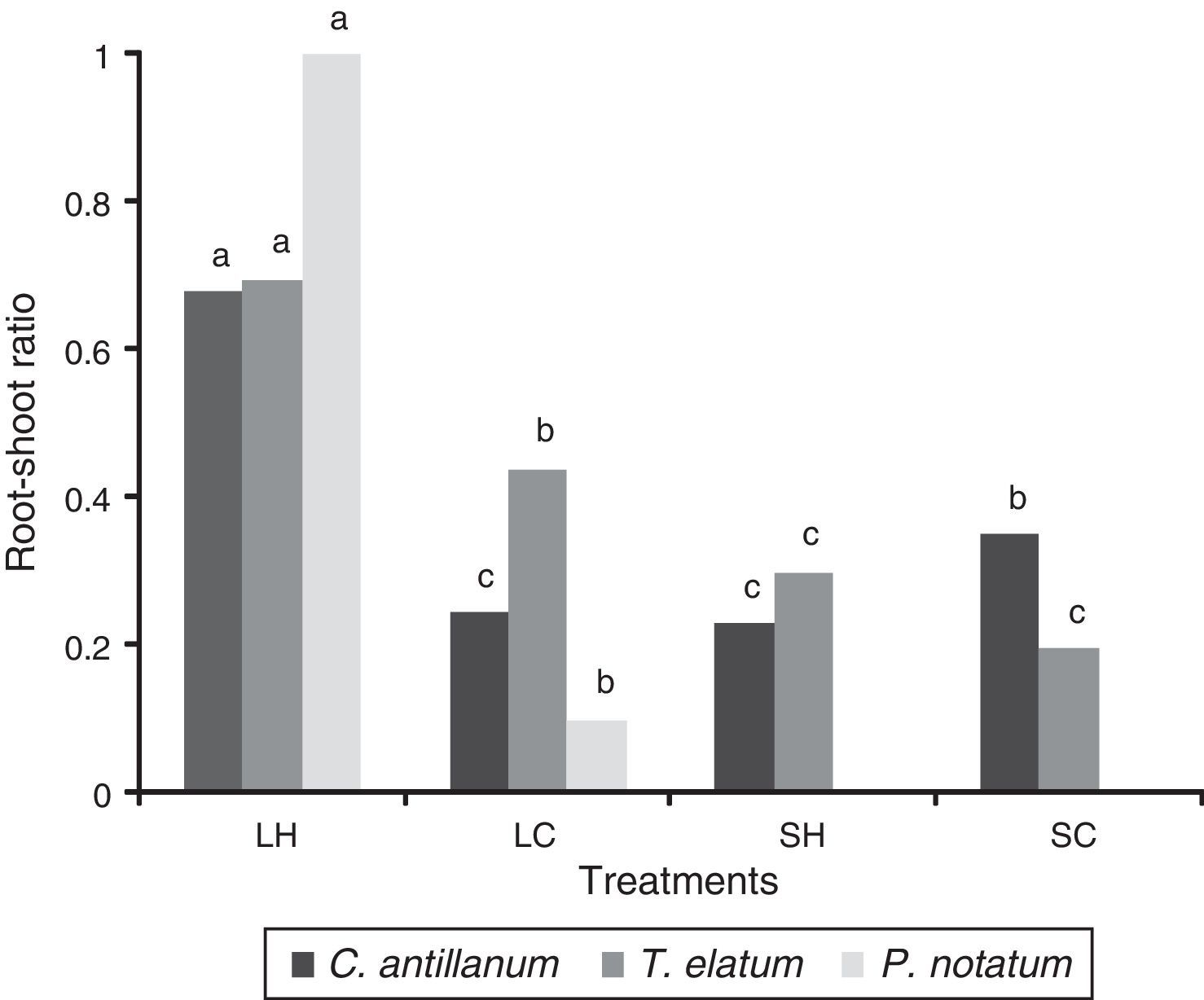
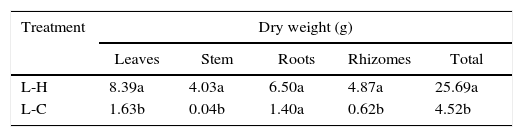
In general, P. notatum was less tolerant than the other two plant species, being incapable of growing under the shadow conditions, showing also a differential response to natural and artificial light (Fig. 1). Thus, P. notatum showed significantly higher values in all the analyzed variables under the L-H treatment (Table 4).The root-shoot ratio of the seedlings also varied as a function of the environmental conditions tested (Fig. 2). In the L-H treatment C. antillanum and T.elatum showed mean values of 0.69 and 0.68, respectively, which may be considered as high. On the other hand, P. notatum had an even higher root-shoot ratio mean value under these conditions (1.02).
Mean values of dry weight of P. notatum seedlings under two combinations of light and temperature conditions. Different letters indicate significant statistical differences (Scott Knott 5%). L-H: full light and high temperature and L-C: artificial light and low temperature.
| Treatment | Dry weight (g) | ||||
|---|---|---|---|---|---|
| Leaves | Stem | Roots | Rhizomes | Total | |
| L-H | 8.39a | 4.03a | 6.50a | 4.87a | 25.69a |
| L-C | 1.63b | 0.04b | 1.40a | 0.62b | 4.52b |
Root-shoot ratio of C. antillanum, T. elatum and P. notatum. Different letters indicate significant statistical differences (Scott Knott Test 5%). L-H: full light and high temperature; L-C: artificial light and low temperature; S-H: shadow and high temperature; and S-C: shadow and low temperature.
In general, regardless of their differential responses, conditioned by their growth habits, the three studied host species showed their best development under the natural full light combined with high temperature treatment (L-H). Was under these conditions that the higher biomass values were obtained, as well as the most appropriate balance between the root and the aerial part of the plant. These higher biomasses included a high root production and consequently a better association between the plant and the AMF.
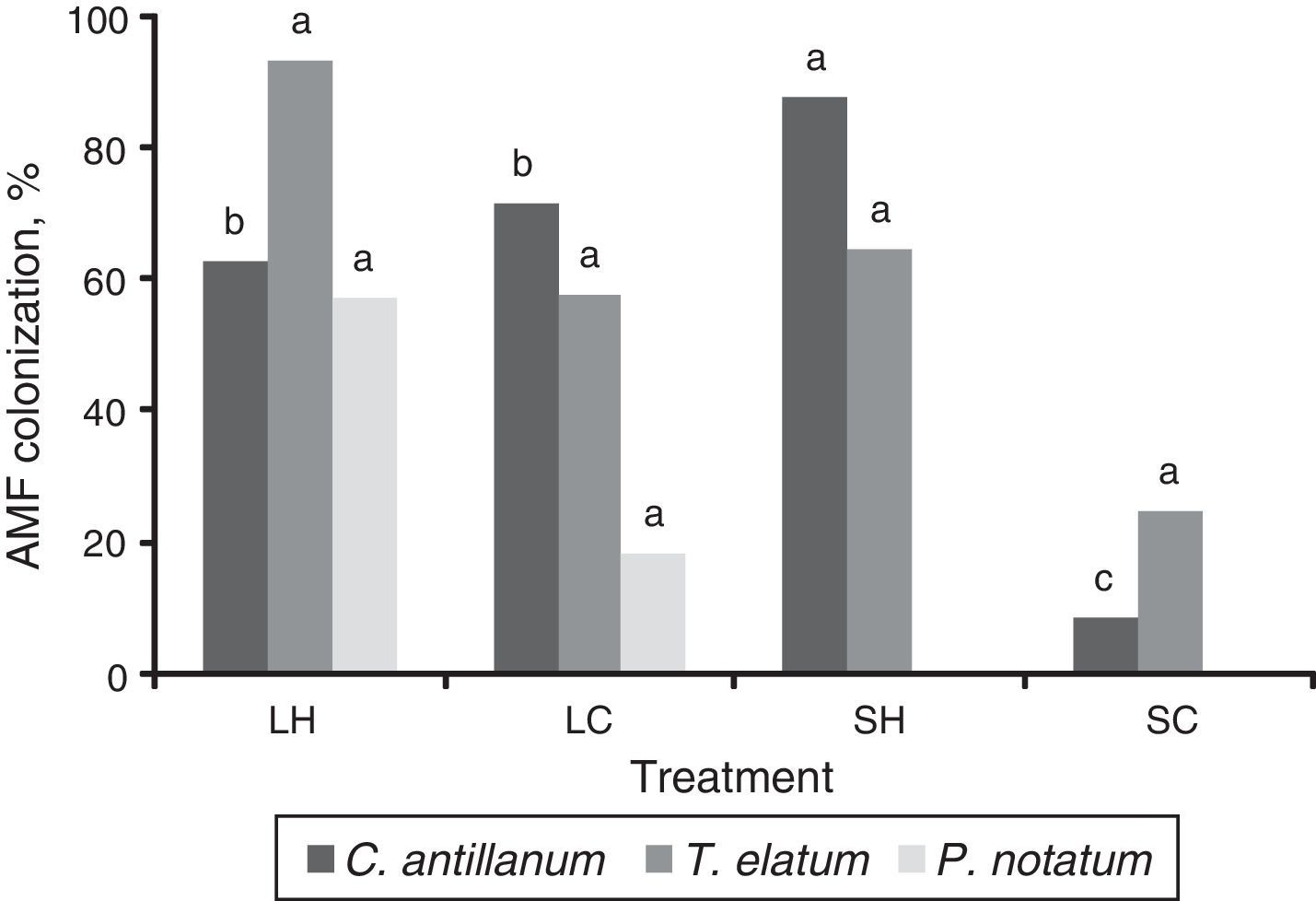
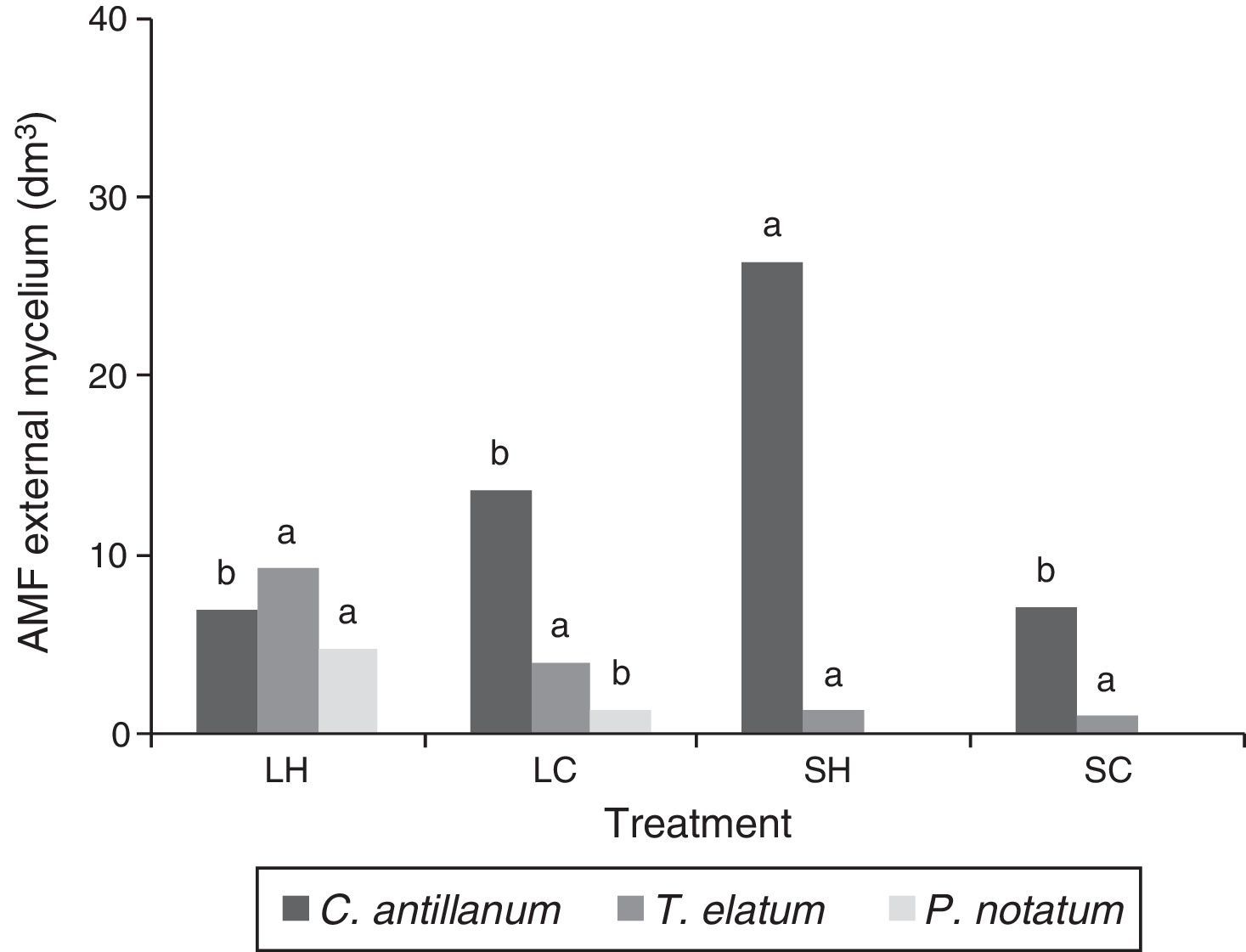
Mycorrhizal functioningThe highest values of mycorrhizal colonization and external mycelium were observed for the three plant species in the L-H treatment (Figs. 3 and 4). However, in general the results obtained for the mycorrhizal functioning in the plants do not correspond with the morphometric measurements of these.
Mycorrhizal colonization in roots of C. antillanum, T. elatum and P. notatum. Different letters indicate significant statistical differences (Scott Knott 5%). L-H: full light and high temperature; L-C: artificial light and low temperature; S-H: shadow and high temperature; and S-C: shadow and low temperature.
AMF external mycelium (dm3) in roots of C. antillanum, T. elatum and P. notatum. Different letters indicate significant statistical differences (Scott Knott Test 5%). L-H: full light and high temperature; L-C: artificial light and low temperature; S-H: shadow and high temperature; and S-C: shadow and low temperature.
In the C. antillanum seedlings the mycorrhizal colonization, although highly variable, reached a highest mean value of 62.7% in the L-H treatment (Fig. 3). This variable resulted highly sensitive to other light and temperature combinations showing marked decreases in the other three treatments, with the lowest mean colonization in the SH treatment (2.52%).
Contrary to this, T. elatum showed very high mycorrhizal colonization in the four tested treatments, ranging from 10-96%, and surpassing the values detected for C. atillanum. The treatment with the highest mycorrhizal colonization for these two species, once again, resulted that of natural light and high temperature (L-H), with values of 93.3% (T. elatum) and 96.1% (P. notatum). In shady treatments this variable ranged from 71% to 87.6%.
The estimated external mycelium, showed a different pattern compared to the mycorrhizal colonization, with variations among the three plant species and the four treatments. Under the L-H treatment, the highest values of external mycelium were observed for P. notatum and T. elatum, while C. antillanum had intermediate values. This last species was not influenced by the source of light (natural or artificial) being very similar in the L-H and L-C treatments (Fig. 4).
A considerable decrease in the external mycelium quantities was observed under the shady conditions treatments. C. antillanum showed extremely low values for this variable under the S-H and S-C treatments, while T. elatum's external mycelium was more affected by the S-C conditions.
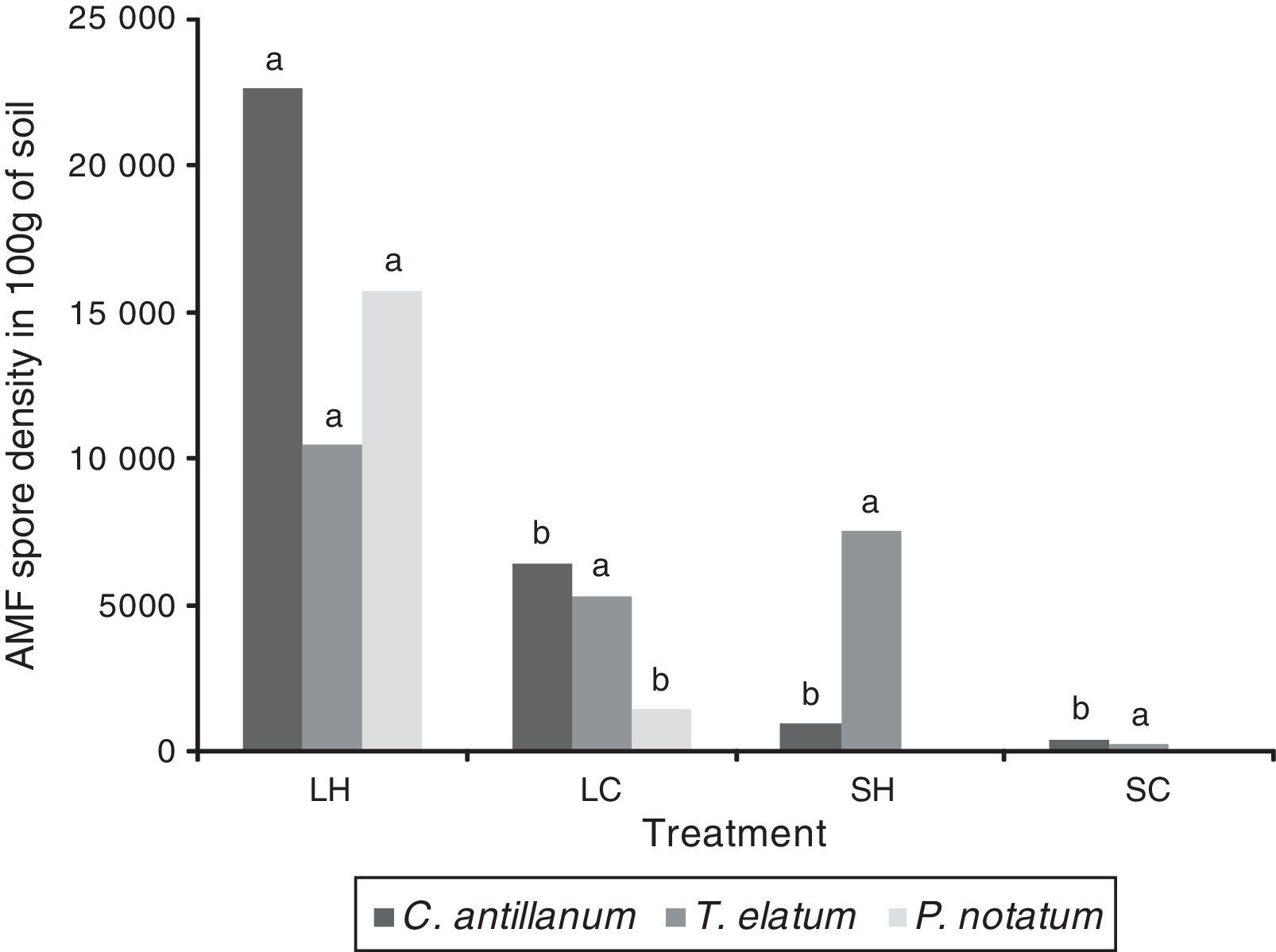
AMF spore densityThe mean spore density among the four treatments ranged between 260 and 22,610 spores per 100g of soil (Fig. 5). In general, the mean spore density under most of the tested conditions increased compared to the initial number of spores found in the soil. The highest mean values for this variable were obtained under the L-H treatment: C. antillanum (22,610spores/100g of soil), T. elatum (10,473spores/100g of soil) and P. notatum (15,720spores/100g of soil).
Mean number of AMF spores in 100g of rizospheric soil from C. antillanum, T. elatum and P. notatum. Different letters indicate significant statistical differences (Scott Knott 5%). L-H: full light and high temperature; L-C: artificial light and low temperature; S-H: shadow and high temperature; and S-C: shadow and low temperature.
Under these conditions the three plant species had an exponential increase in the number of spores, reaching values up to ten times higher than those initially detected in the soil. In the shadow treatments (S-H and S-C) the mean spore density was lower. In both cases, C. antillanum showed decreases in the spore density compared to the native soil used as inoculum, while in T. elatum this just happened under the S-C treatment.
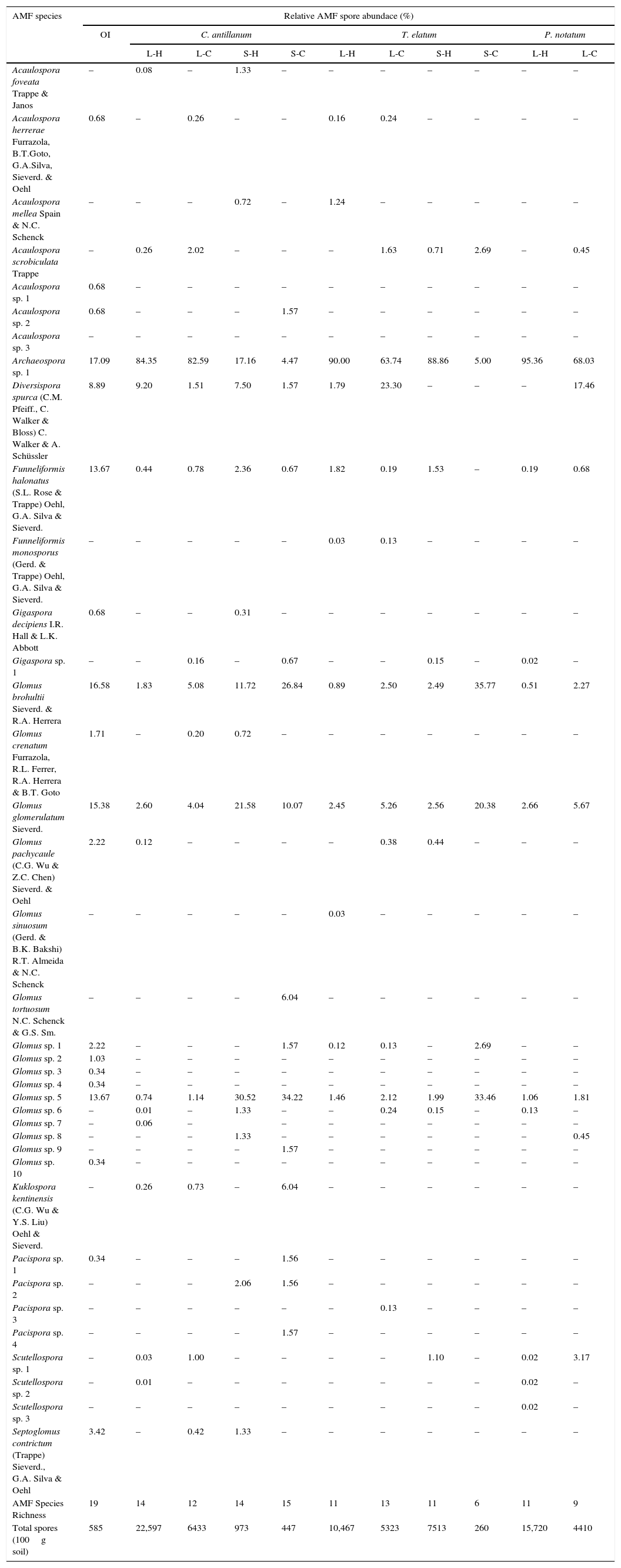
At the native soil, used as natural inoculum, 19 AMF species were detected. In general, a total of 38 species were verified under the different host and experimental conditions (Table 5). The identified species were distributed among six families: Acaulosporaceae, Archaeosporaceae, Diversisporaceae, Gigasporaceae, Glomeraceae and Pacisporaceae. The better represented genera were Glomus with 16 species; followed by Acaulospora, Pacispora, Scutellospora, Funneliformis and Gigaspora, with seven, four, three and two species, respectively. Other genera like Archaeospora, Diversispora, Kuklospora and Septoglomus were just represented by one species. The most frequent species were Archaeospora sp. 1, Gomus brohultii, Glomus sp. 5, G. glomerulatum, Diversispora spurca and Funneliformes halonatus.
Relative spore abundance (%) of the AMF species identified in the native soil used as inoculum (OI); and in the rizosphere of C. antillanum, T. elatum and P. notatum under four different light and temperature conditions. L-H: full light and high temperature, S-H: shadow and high temperature, L-C: artificial light and low temperature and S-C: shadow and low temperature.
| AMF species | Relative AMF spore abundace (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OI | C. antillanum | T. elatum | P. notatum | ||||||||
| L-H | L-C | S-H | S-C | L-H | L-C | S-H | S-C | L-H | L-C | ||
| Acaulospora foveata Trappe & Janos | – | 0.08 | – | 1.33 | – | – | – | – | – | – | – |
| Acaulospora herrerae Furrazola, B.T.Goto, G.A.Silva, Sieverd. & Oehl | 0.68 | – | 0.26 | – | – | 0.16 | 0.24 | – | – | – | – |
| Acaulospora mellea Spain & N.C. Schenck | – | – | – | 0.72 | – | 1.24 | – | – | – | – | – |
| Acaulospora scrobiculata Trappe | – | 0.26 | 2.02 | – | – | – | 1.63 | 0.71 | 2.69 | – | 0.45 |
| Acaulospora sp. 1 | 0.68 | – | – | – | – | – | – | – | – | – | – |
| Acaulospora sp. 2 | 0.68 | – | – | – | 1.57 | – | – | – | – | – | – |
| Acaulospora sp. 3 | – | – | – | – | – | – | – | – | – | – | – |
| Archaeospora sp. 1 | 17.09 | 84.35 | 82.59 | 17.16 | 4.47 | 90.00 | 63.74 | 88.86 | 5.00 | 95.36 | 68.03 |
| Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & A. Schüssler | 8.89 | 9.20 | 1.51 | 7.50 | 1.57 | 1.79 | 23.30 | – | – | – | 17.46 |
| Funneliformis halonatus (S.L. Rose & Trappe) Oehl, G.A. Silva & Sieverd. | 13.67 | 0.44 | 0.78 | 2.36 | 0.67 | 1.82 | 0.19 | 1.53 | – | 0.19 | 0.68 |
| Funneliformis monosporus (Gerd. & Trappe) Oehl, G.A. Silva & Sieverd. | – | – | – | – | – | 0.03 | 0.13 | – | – | – | – |
| Gigaspora decipiens I.R. Hall & L.K. Abbott | 0.68 | – | – | 0.31 | – | – | – | – | – | – | – |
| Gigaspora sp. 1 | – | – | 0.16 | – | 0.67 | – | – | 0.15 | – | 0.02 | – |
| Glomus brohultii Sieverd. & R.A. Herrera | 16.58 | 1.83 | 5.08 | 11.72 | 26.84 | 0.89 | 2.50 | 2.49 | 35.77 | 0.51 | 2.27 |
| Glomus crenatum Furrazola, R.L. Ferrer, R.A. Herrera & B.T. Goto | 1.71 | – | 0.20 | 0.72 | – | – | – | – | – | – | – |
| Glomus glomerulatum Sieverd. | 15.38 | 2.60 | 4.04 | 21.58 | 10.07 | 2.45 | 5.26 | 2.56 | 20.38 | 2.66 | 5.67 |
| Glomus pachycaule (C.G. Wu & Z.C. Chen) Sieverd. & Oehl | 2.22 | 0.12 | – | – | – | – | 0.38 | 0.44 | – | – | – |
| Glomus sinuosum (Gerd. & B.K. Bakshi) R.T. Almeida & N.C. Schenck | – | – | – | – | – | 0.03 | – | – | – | – | – |
| Glomus tortuosum N.C. Schenck & G.S. Sm. | – | – | – | – | 6.04 | – | – | – | – | – | – |
| Glomus sp. 1 | 2.22 | – | – | – | 1.57 | 0.12 | 0.13 | – | 2.69 | – | – |
| Glomus sp. 2 | 1.03 | – | – | – | – | – | – | – | – | – | – |
| Glomus sp. 3 | 0.34 | – | – | – | – | – | – | – | – | – | – |
| Glomus sp. 4 | 0.34 | – | – | – | – | – | – | – | – | – | – |
| Glomus sp. 5 | 13.67 | 0.74 | 1.14 | 30.52 | 34.22 | 1.46 | 2.12 | 1.99 | 33.46 | 1.06 | 1.81 |
| Glomus sp. 6 | – | 0.01 | – | 1.33 | – | – | 0.24 | 0.15 | – | 0.13 | – |
| Glomus sp. 7 | – | 0.06 | – | – | – | – | – | – | – | – | |
| Glomus sp. 8 | – | – | – | 1.33 | – | – | – | – | – | – | 0.45 |
| Glomus sp. 9 | – | – | – | – | 1.57 | – | – | – | – | – | – |
| Glomus sp. 10 | 0.34 | – | – | – | – | – | – | – | – | – | – |
| Kuklospora kentinensis (C.G. Wu & Y.S. Liu) Oehl & Sieverd. | – | 0.26 | 0.73 | – | 6.04 | – | – | – | – | – | – |
| Pacispora sp. 1 | 0.34 | – | – | – | 1.56 | – | – | – | – | – | – |
| Pacispora sp. 2 | – | – | – | 2.06 | 1.56 | – | – | – | – | – | – |
| Pacispora sp. 3 | – | – | – | – | – | – | 0.13 | – | – | – | – |
| Pacispora sp. 4 | – | – | – | – | 1.57 | – | – | – | – | – | – |
| Scutellospora sp. 1 | – | 0.03 | 1.00 | – | – | – | – | 1.10 | – | 0.02 | 3.17 |
| Scutellospora sp. 2 | – | 0.01 | – | – | – | – | – | – | – | 0.02 | – |
| Scutellospora sp. 3 | – | – | – | – | – | – | – | – | – | 0.02 | – |
| Septoglomus contrictum (Trappe) Sieverd., G.A. Silva & Oehl | 3.42 | – | 0.42 | 1.33 | – | – | – | – | – | – | – |
| AMF Species Richness | 19 | 14 | 12 | 14 | 15 | 11 | 13 | 11 | 6 | 11 | 9 |
| Total spores (100g soil) | 585 | 22,597 | 6433 | 973 | 447 | 10,467 | 5323 | 7513 | 260 | 15,720 | 4410 |
Considering the proposal of Alarcón,25 which indicates mycorrhizal colonization as moderately high when close to 50% under controlled experimental conditions, the values obtained here for this variable may be classified as high. This is in correspondence which what have been reported for other tree plant species in several works. Orozco et al.26 reported values of colonization of up to 84% under greenhouse conditions in six leguminous trees. More recently, Goetten et al.27 found levels of colonization that ranged between 44.8 and 74.8% in seedlings of six woody plant species inoculated with Claroideoglomus etunicatum and Rhizophagus clarus.
However, the levels of mycorrhizal colonization found in the present study for P. notatum and T. elatum, which surpassed 90% in the L-H treatment may be considered as very high. These amounts of colonization in forest species have only been reported in natural ecosystems with highly competitive conditions and where the dominant plants are strongly mycorrhized. Levels of root colonization of 94 and 98% for Lysiloma latisiliquum (L.) Benth. and Bucida palustris Borhidi & O. Muñiz, respectively under natural forest conditions have been reported.28
These results reaffirm that pioneer species, unlike climax ones, exhibit high colonization rates and susceptibility to associate with AMF.29,30 The first tropical forest successional stages are characterized by high light and low nutrient levels. Under these conditions, P. notatum and T. elatum showed higher values of mycorrhizal colonization in comparison to climax species as C. antillanum. This explains why the latter, although having a high root biomass, showed lower percentage of mycorrhizal colonization compared to the other two species under the L-H treatment. It is also well established that any impact on host plant photosynthesis indirectly affects AMF growth, and thus carbon allocation to the rhizosphere via the extraradical mycelium.31
The levels of mycorrhizal colonization have also been associated with the amount of seed reserves of each plant species as well as the growth strategies and development of the seedlings in their first stages.26,32 In this case, P. notatum and T. elatum have small seeds with low reserves, and epigeous seedlings containing leaves and showing a fast growth rate. Contrary to this, C. antillanum has big seeds with high reserve levels, and hypogeous plantlets with a lower growth rate.33
The external mycelium (EM) might also be associated with the amount of seed reserves.33 The higher values of this variable in the illuminated environments in comparison to the shady ones, indicates a relation between the EM, the successional strategies of the plant species and their mycorrhizal dependency.11
The number of spores under the illuminated conditions was much higher than that reported for other natural Cuban ecosystems.28,34,35 The lower values under the shadow treatments are probably associated to the lower biomass production, mainly of roots, of these species under these conditions. These shady treatments might have stimulated decreases in the photosynthetic rates of the plants. Consequential reductions in the total biomass might have resulted in the establishment of a less efficient arbuscular mycorrhizal symbiosis.36
Among the identified AMF species Archaeospora sp.1, Glomus. sp.5, Gl. brohultii and G. glomerulatum seem to be “generalists” being detected at all the hosts and environmental conditions (Table 5). These high values of unknown, and possibly new species, have been reported for other Cuban natural reserves, e.g. San Ubaldo-Sabanalamar, Pinar del Rio and Ciénaga de Zapata, Matanzas.34,35 These results might represent and indicative of high AMF diversity in the Cuban island, which is not surprising if considered that Cuba possesses the highest plant diversity in the Caribbean and is among the four islands with more plant species number in the World.37
Inoculation with AMF has proved to increase plant resistance to environmental stresses.13 Among the mechanisms through which this occurs are a higher robustness of the root system and an increased probability of the plant being colonized by a well-adapted and specific AMF community (the inoculated one). This represents a way of reducing the colonization by “cheaters” or not efficient AMF species when seedlings are transferred to the natural environments.38
Independently of the different successional strategies of the studied plant species the best conditions for their growth and development, as well as for the AMF native inoculum production are those L-H. They stimulate an optimal plant growth (higher biomass production) amd a positive balance of the root-shoot ratio, important aspects for an efficient AM symbiosis. Under these conditions the highest number of mycorrhizal propagules is obtained. Native AMF species besides beeing better adapted to the edaphoclimatic conditions of the area, ensuring the success of the symbiosis and a better development of the plnats, potentiates the in situ conservation of the AMF community.
Conflicts of interestThe authors declare no conflicts of interest.
To PEC-PG CNPq and CAPES-MES to finantial support and grant to ROF.