The high costs and environmental concerns associated with using marine resources as sources of oils rich in polyunsaturated fatty acids have prompted searches for alternative sources of such oils. Some microorganisms, among them members of the genus Aurantiochytrium, can synthesize large amounts of these biocompounds. However, various parameters that affect the polyunsaturated fatty acids production of these organisms, such as the carbon and nitrogen sources supplied during their cultivation, require further elucidation. The objective of this investigation was to study the effect of different concentrations of carbon and total nitrogen on the production of polyunsaturated fatty acids, particularly docosahexaenoic acid, by Aurantiochytrium sp. ATCC PRA-276. We performed batch system experiments using an initial glucose concentration of 30g/L and three different concentrations of total nitrogen, including 3.0, 0.44, and 0.22g/L, and fed-batch system experiments in which 0.14g/L of glucose and 0.0014g/L of total nitrogen were supplied hourly. To assess the effects of these different treatments, we determined the biomass, glucose, total nitrogen and polyunsaturated fatty acids concentration. The maximum cell concentration (23.9g/L) was obtained after 96h of cultivation in the batch system using initial concentrations of 0.22g/L total nitrogen and 30g/L glucose. Under these conditions, we observed the highest level of polyunsaturated fatty acids production (3.6g/L), with docosahexaenoic acid and docosapentaenoic acid ω6 concentrations reaching 2.54 and 0.80g/L, respectively.
The search for nutraceutical products that can prevent and/or treat diseases has intensified during the last decade. Among these products, types ω3 and ω6 polyunsaturated fatty acids (PUFAs) have received a great deal of attention due to their health benefits and their extensive applications in the food and pharmaceutical industries.1 Docosahexaenoic acid (DHA, C22:6 ω3), for example, is necessary for the brain development of newborn children and contributes to increasing their intelligence and verbal and reasoning skills.2 Furthermore, DHA is helpful in treating atherosclerosis, rheumatoid arthritis, and Alzheimer's disease,3 as well as in preventing breast and colon cancer.4 Docosapentaenoic acid (DPA, C22:5 ω6) is another PUFA that is important for human health. DPA has been found to help prevent various diseases, such as cardiovascular disorders (such as myocardial infarction, thrombosis, and atherosclerosis), diabetes, asthma, inflammation and rheumatism (including arthritis and osteoporosis).5
The main commercial source of these compounds, particularly DHA, is oil obtained from marine fish.6 However, the widespread consumption of these oils is limited by marine chemical pollution, declining fish stocks, seasonal variations in the composition of fish oils, their poor oxidative stability, typical unpleasant odour and taste, and the high cost of their extraction and purification processes.7 This problems have inspired the development of new methods for the large-scale production of oils by safe and healthy sources.1,8
Heterotrophic microorganisms that are members of the Thraustochytrid group are alternative sources of these oils. These oleaginous microorganisms can accumulate more than 50% of their weight as lipids, with a high concentration of DHA of greater than 25% of the total lipids.9 Furthermore, the lipids of thraustochytrids contain a specific PUFA (DHA) instead of a mixture of PUFAs. Therefore, their oil has a higher level of oxidative stability than that of fish oil.10 The appropriate concentrations of carbon and nitrogen are essential for thraustochytrids to biosynthesize and accumulate polyunsaturated fatty acids. The concentration of the carbon source affects the synthesis of organic molecules and the availability of energy, whereas the concentration of the nitrogen source affects the synthesis of amino acids and nucleic acids. Therefore, understanding the effects of these substrates on cultivated microorganisms is crucial for optimizing their oil production. Herein, we present the results of a study of the effect of different concentrations of carbon and nitrogen on the PUFAs production of Aurantiochytrium sp. ATCC PRA-276.
Materials and methodsMicroorganismAurantiochytrium sp. ATCC PRA-276 cells were obtained from the American Type Culture Collection (Manassas, VA, USA).
Preparing the inoculumAurantiochytrium sp. ATCC PRA-276 cells grown on potato dextrose agar and stored at 4°C were transferred to 500mL flasks containing 100mL of culture medium consisting (g/L) of yeast extract (1.0), peptone (15.0) and glucose (20.0) dissolved in seawater (1.5% w/v). The glucose stock solution was sterilized separately. The cells were incubated in an orbital shaker (Ika, KS 260B) rotating at 150rpm at 30°C without light for 48h.11
Preparing the culture mediaWe cultivated the microorganism in a Biostat®Bplus bioreactor (Sartorius Stedim Biotech., Germany) containing a 5L borosilicate glass vessel and equipped with pressure flow meters and gas and liquid-flow controllers. We conducted batch and fed-batch experiments. For the batch system experiments we used a medium consisting of KH2PO4 (0.3g/L), MgSO4·7H2O (5.0g/L), NaCl (10.0g/L), NaHCO3 (0.1g/L), CaCl2·2H2O (0.3g/L), KCl (0.28g/L), glucose (30.0g/L) and different total nitrogen (TN) concentrations: 3.0g/L (1.36g/L (NH4)2SO4, 13.63g/L yeast extract and 13.63g/L monosodium glutamate), 0.44g/L (0.2g/L (NH4)2SO4, 2.0g/L yeast extract and 2.0g/L monosodium glutamate), and 0.22g/L (0.1g/L de (NH4)2SO4, 1.0g/L yeast extract and 1.0g/L monosodium glutamate). For the fed-batch system experiments, 0.14g/L of glucose and 0.0014g/L of total nitrogen were supplied hourly (6.66×10−4g/Lh of (NH4)2SO4, 6.66×10−3g/Lh of yeast extract and 6.66×10−3g/Lh of monosodium glutamate). The yeast extract, monosodium glutamate and glucose solutions were separately sterilized by treatment at 121°C for 15min in a CertoClav CV-EL-18L autoclave. The bioreactor was sterilized in an AJC Uniclave 77-127L autoclave for 60min. The other components of the medium were filtered-sterilized using 0.22μm membranes (Millipore).
After sterilization, the dissolved components were added to the bioreactor along with the following metal solutions: MnCl2·4H2O (8.6mg/L), ZnCl2 (0.6mg/L), CoCl2·4H2O (0.26mg/L), CuSO4·5H2O (0.02mg/L), FeCl3·6H2O (2.9mg/L), H3BO3 (34.2mg/L), and Na2EDTA (30.0mg/L) and the following vitamin solutions: thiamine (9.5mg/L) and calcium pantothenate (3.2mg/L), all of which had been sterilized using 0.22μm membrane filters (Millipore). The inoculum (350mL) was then added to the culture medium (10% v/v relative to the total volume of the culture medium).
Cultivation was performed at 23°C with agitation at 100rpm and the pH of the media adjusted to 6.0 using 4N NaOH. During the first 120h of cultivation, the culture medium was aerated at 2.5 vvm. After this period, air injection was discontinued.
Biomass concentrationThe biomass was determined every 24h using the method of Min et al.12 An aliquot of the culture medium was filtered using a previously weighed glass-microfiber filter paper (GF/C: 1.2μm, Whatman). The biomass retained in the filter was washed twice using distilled water and dried in an oven (Memmert) at 60°C for 24h. The biomass content was calculated as the difference between the initial and final weights.
Glucose concentrationThe glucose content of the culture supernatant was determined each 24h using the spectrophotometric method described by Miller,13 using a UV/Vis dual beam absorption spectrophotometer (Ati Unicam Helios Alpha, UK).
Total nitrogen concentrationThe total nitrogen (defined and complex sources) content of the culture supernatant was determined each 24h following the procedure described by Furlan et al.11
Fatty acid profileCulture samples collected at 24h intervals were centrifuged (Kubota, 6800) at 8742×g for 15min at 4°C, after which the biomass was washed with distilled water and centrifuged again. This process was repeated twice. The biomass was frozen at −20°C and dried for 48h using a freeze dryer (Heto PowerDry LL 3000).
Between 20 and 100mg of the lyophilized biomass was weighed and added to 50μL of the internal C21:0 standard solution (10mg/mL) to permit expressing the results as g of fatty acids/g of lyophilized biomass.
Methyl esters of the fatty acids were prepared by esterification using the acid catalysis method described by Cohen et al.14 A gas chromatography system (Varian, CP 3800) equipped with an autosampler, injector, and flame ionization detector (FID), both of the latter at 250°C, was used to identify the methyl esters in the samples. The methyl esters were separated using a DB-WAX polyethylene glycol capillary column (Agilent, 30m long, 0.25mm internal diameter and 0.25μm thick) using the following program: heating at 180°C (5min), gradually increasing at 4°C/min to 220°C (and holding for 25min), and then gradually increasing at 20°C/min to 240°C (and holding for 15min). The methyl esters were identified by comparing their retention times with those of chromatographic standards (Sigma–Aldrich Co, St. Louis, MO, USA).
The results were analyzed using an analysis of variance (ANOVA) and the mean values were compared using the Tukey test, with the significance level set at 5%. Before performing the ANOVA, the normality of the data distributions were evaluated using the Kolmogorov–Smirnov test and the homoscedasticity of the data was evaluated using the Cochran test.15
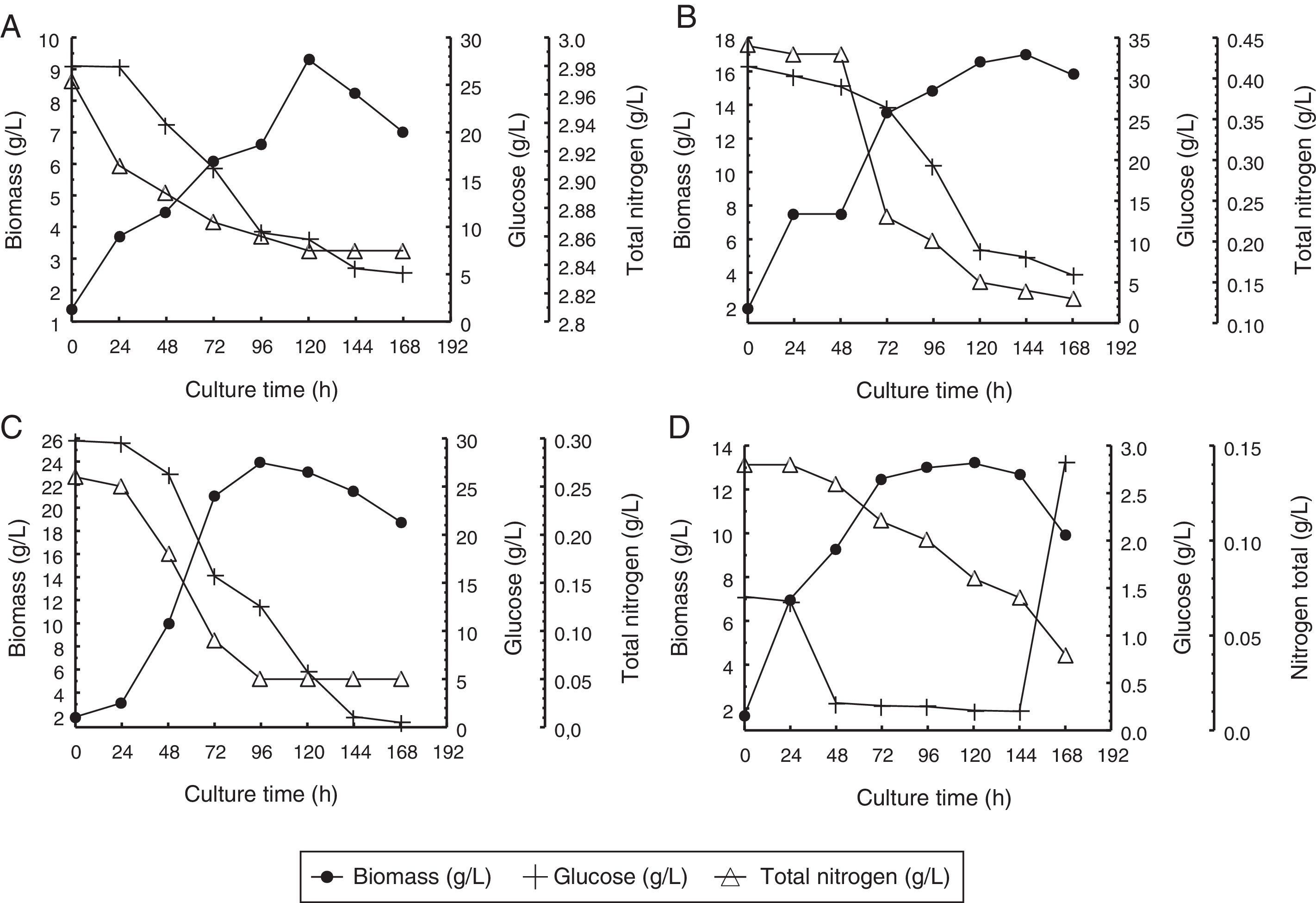
ResultsGrowth kinetics, glucose and total nitrogen consumptionFig. 1 shows the average biomass, glucose and total nitrogen concentrations over time in cultures of Aurantiochytrium sp. ATCC PRA-276 growing under four different conditions.
Concentrations of biomass, glucose and total nitrogen over time in the culture media of Aurantiochytrium sp. ATCC PRA-276. The graphs show the data obtained using different treatments, as follows: a batch system with an (A) initial nitrogen concentration of 3.0g/L, (B) initial nitrogen concentration of 0.44g/L or (C) initial nitrogen concentration of 0.22g/L or (D) a fed-batch system with 0.14g/L of glucose and 0.0014g/L of nitrogen supplied hourly.
As shown in Fig. 1(A), in the experiments using an initial total nitrogen concentration of 3.0g/L, the maximum biomass concentration (9.3g/L) was reached at 120h, for an average yield of 0.07g/Lh of biomass. The average glucose consumption rate in these experiments was 0.13g/Lh and 0.43g of biomass was produced per gram of glucose consumed (YBiomass/Glucose). The average total nitrogen consumption rate was 0.0010g/Lh, resulting in a substrate to biomass conversion factor (YBiomass/nitrogen) of 65.8.
In the experiments using an initial nitrogen concentration of 0.44g/L, the highest biomass concentration (17.0g/L) was obtained at 144h, foran average yield of 0.11g/Lh of biomass (Fig. 1(B)). The average glucose consumption rate was 0.15g/Lh, resulting in a glucose to biomass conversion factor (YBiomass/glucose) of 0.65. The average total nitrogen consumption rate was 0.0018g/Lh. Each gram of nitrogen that was consumed was converted into 50.7g of biomass (YBiomass/Nitrogen).
In the experiments using an initial nitrogen concentration of 0.22g/L, the highest biomass concentration (23.9g/L) was observed at 96h (Fig. 1(C)), for a maximum yield of 0.23g/Lh of biomass. In these experiments, the average glucose consumption rate was 0.17g/Lh and the glucose to biomass conversion factor (YBiomass/Glucose) was 1.28. The average nitrogen consumption rate was 0.0022g/Lh, resulting in a YBiomass/Nitrogen conversion factor of 104.7, meaning that 104.7g of biomass was produced per gram of nitrogen consumed.
When the fed-batch cultivation process was used, the highest biomass concentration (13.2g/L) was reached at 120h (Fig. 1(D)), for a maximum yield of 0.10g/Lh of biomass. The average glucose consumption rate was 0.13g/Lh, resulting in a glucose to biomass conversion factor (YBiomass/Glucose) of 0.64. Using this cultivation process, the average total nitrogen consumption rate was 0.0016g/Lh and the YBiomass/Nitrogen conversion factor was 49.15, meaning that 49.15g of biomass was produced per gram of nitrogen consumed.
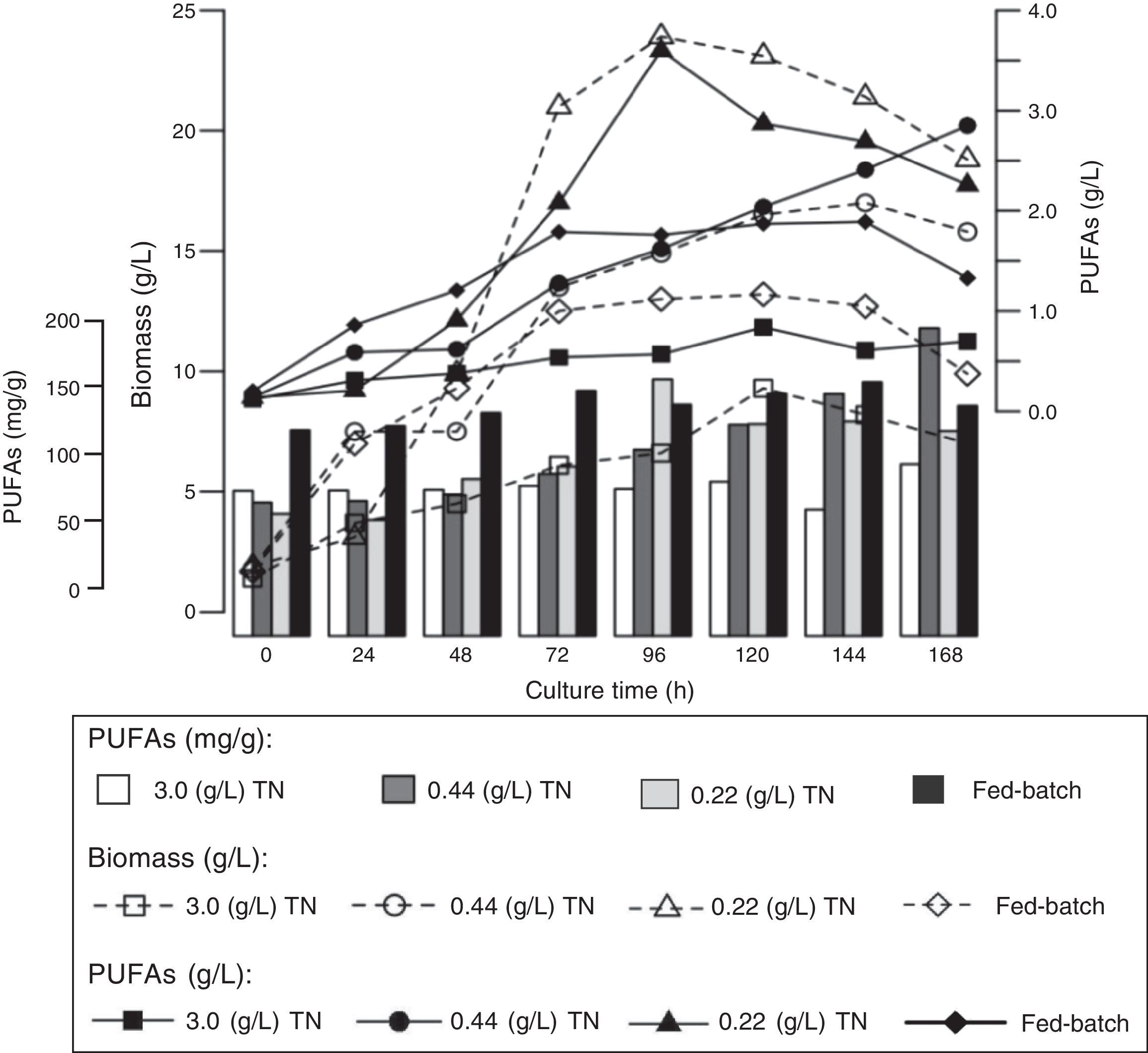
Fatty acid profileFig. 2 shows the average PUFAs concentrations in the biomass that were reached throughout Aurantiochytrium sp. ATCC PRA-276 cultivation in the different experiments.
Evaluating the PUFAs concentrations in the biomass (g/L) showed that there were significant differences among the values at different times throughout cultivation in all of the experiments. The highest PUFAs concentration (3.6g/L) was observed at 96h of cultivation in the experiments using an initial nitrogen concentration of 0.22g/L. The second highest PUFAs concentration (2.85g/L) was obtained at 168h of cultivation in the experiments using an initial nitrogen concentration of 0.44g/L. In the fed-batch culture, the maximal PUFAs concentration of 1.89g/L was reached at 144h of cultivation. In the experiments using an initial nitrogen concentration of 3g/L, the maximal PUFAs concentration of 0.84g/L was reached at 120h of cultivation (Fig. 2).
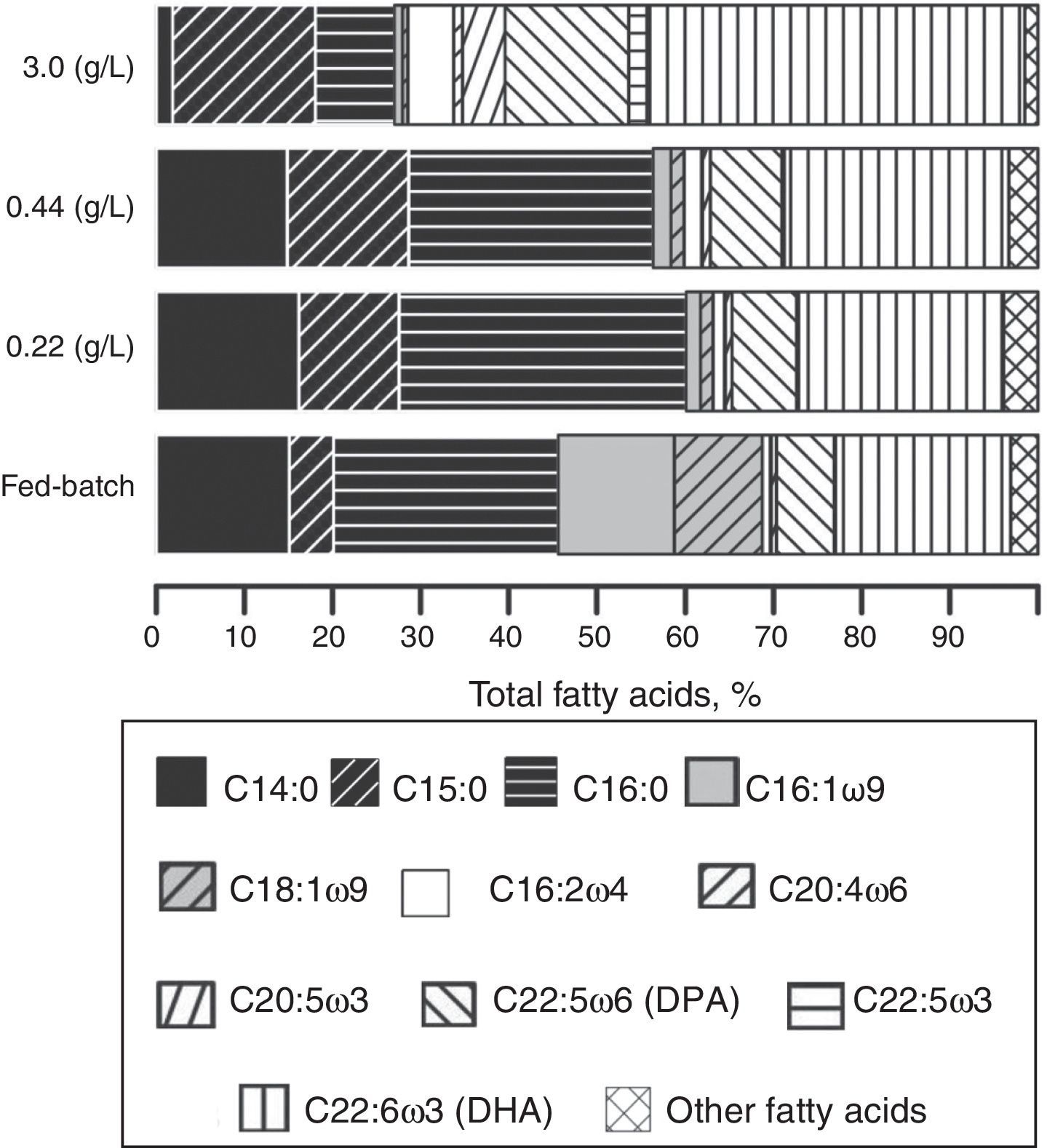
Fig. 3 shows the fatty acid profile at the time point when the highest PUFAs yield was obtained in each experiment (g/L).
Fatty acid profiles of the biomass of Aurantiochytrium sp. ATCC PRA-276 when batch-cultured for120h using an initial nitrogen concentration of 3.0g/L total nitrogen, at 168h when batch-cultured using an initial nitrogen concentration of 0.44g/L, at 96h when batch-cultured using an initial nitrogen concentration of 0.22g/L, and at 144h when cultured using the fed-batch process.
In the experiments using an initial nitrogen concentration of 3.0g/L, 9% (w/w) of the biomass was composed of PUFAs at 120h of cultivation, of which 20% was DPA ω6 (Fig. 3), which represented 1.8% of the biomass (0.17g/L). As shown in Fig. 3, DHA accounted for 61.3% of the PUFAs present, i.e., 5.5% of the biomass (0.51g/L).
Using an initial nitrogen concentration of 0.44g/L, 18% (w/w) of the biomass was composed of PUFAs at 168h of cultivation, of which 22% was DPA ω6, i.e., 3.9% of the biomass (0.63g/L). We also observed that DHA accounted for 69.2% of the PUFAs, which was12.5% of the total biomass (1.97g/L) (Fig. 3).
In the experiments using an initial nitrogen concentration of 0.22g/L, 15% (w/w) of the biomass was composed of PUFAs at 96h of cultivation, of which 22.3% was DPA ω6 (Fig. 3), accounting for 3.34% of the total biomass (0.80g/L). We also observed that DHA comprised 70.5% of the PUFAs (Fig. 3), i.e., 10.62% of the biomass (2.54g/L).
After 144h of cultivation using the fed-batch system, 14.9% (w/w) of the biomass was composed of PUFAs, of which 23.1% was DPA ω6 (Fig. 3), i.e., 3.43% of the total biomass (0.44g/L). In addition, DHA accounted for 70.5% of the PUFAs (Fig. 3), i.e., 10.5% of the total biomass (1.33g/L).
DiscussionIn the experiments using an initial nitrogen concentration of 3g/L, the cells consumed the least amount of TN (0.0010g/Lh), resulting in the lowest cell yield (0.07g/Lh). This result could be due to the low C/N substrate ratio in the culture (4). An excess of nitrogen may have inhibited the growth of the evaluated strain. Chen et al.16 conducted a study in which they optimized the nitrogen sources for Aurantiochytrium sp. BR-MP4-A1 and obtained a maximum biomass concentration (9.27g/L) by supplying 2.4g/L of TN (as monosodium glutamate, yeast extract, and tryptone), which resulted in a C/N ratio (5). In the experiments in which we used an initial nitrogen concentration of 3g/L, a similar maximum biomass concentration of 9.3g/L was reached (Fig. 1(A)).
Using an initial TN concentration of 0.44g/L to cultivate Aurantiochytrium sp. BR-MP4-A1, Li et al.17 obtained a maximum biomass concentration of 14.5g/L, whereas we observed a maximum biomass concentration of 17.0g/L in cultures of Aurantiochytrium sp. ATCC PRA-276 (Fig. 1(B)). This difference is due to the substrate to biomass conversion rate (YBiomass/Glucose) being 0.53 in the previous study, whereas in this study, each gram of glucose consumed was concerted to 0.65g/L of biomass. The difference in the conversion rate can be attributed to the use of different species in the studies.
Cultivation using an initial TN concentration of 0.22g/L (Fig. 1(C)) resulted in higher substrate consumption rates (0.17g/Lh glucose and 0.0022g/Lh TN) and higher cell biomass productivity (0.23g/Lh) compared with those obtained using the other TN concentrations tested in this study. In addition, the glucose to biomass conversion factor (1.28) was higher under these conditions than under the other tested conditions, which also resulted also in a higher cell concentration (23.9g/L). Ganuza and Izquierdo18 used Schizochytrium sp. G13/2S to study the effect of substrate levels on lipid accumulation. These authors found that using initial concentrations of 0.30g/L of TN and 40g/L of glucose resulted in a biomass yield of 15.7g/L.
In the experiments using fed-batch system (Fig. 1(D)), the concentration of TN decreased over time because its consumption rate was higher (0.0016g/Lh of TN) than its supply rate (0.0014g/Lh of TN). The concentration of glucose in the culture medium decreased until 144h of cultivation and subsequently increased. This phenomenon can be explained by the glucose supply being greater after 144h than that required for cell development and maintenance.
The fatty acid profiles observed in our experiments (Fig. 3) are similar to those previously obtained by Zhu et al.19 and Furlan et al.20 using Schizochytrium limacinum OUC88 and Thraustochytrium sp. ATCC 26185, respectively. Zhu et al.19 found C14:0 (3.8–9.6%), C15:0 (2.1–10.1%), C16:0 (32.6–43.3%), DPA ω6 (7.1–8.2%) and DHA (29.8–36.5%) as the main fatty acids. Furlan et al.20 found C14:0 (1.5–9%), C15:0 (21–35%), C16:0 (5–33%), DPA ω6 (7–9%) and DHA (20–31.5%) as the main fatty acids. These results are similar to our results: C14:0 (1.8–16%), C15:0 (5–16%), C16:0 (9–32.5%) and the polyunsaturated, including DPA ω6 (6.5–14%) and DHA (20–43%). Therefore, the fatty acids produced by members of the Thraustochytriidae family are likely to be mainly C14:0, C15:0, C16:0, DPA ω6 and DHA.
Li et al.17 studied the composition of the Aurantiochytrium sp. BR-MP4-A1 biomass and concluded that DPA ω6 and DHA comprised 6.6% and 28.9%, respectively, of the total fatty acids. These authors also observed that the DPA ω6 (18%) and DHA (78%) levels were higher than those of the other PUFAs that were quantitated, similar to the results obtained in this study (Fig. 3).
We also observed a reduction in PUFAs production relative to that of the total fatty acids when the TN concentration decreased. In contrast, C16:1 ω9 and C18:1 ω9 production was increased because these fatty acids are the precursors used for PUFAs synthesis. Therefore, the smaller the fractions of C16:1 ω9 and C18:1 ω9, the higher the fraction of PUFAs (Fig. 3).
Among the major fatty acids that are PUFAs, the content of DPA ω6 (20–23.1%) varied little, whereas the content of DHA (61.3–70.5%) varied greatly. High concentrations of available total nitrogen in the culture medium facilitate the synthesis of other fatty acids in addition to DHA and DPA ω6, which form a significant fraction of the PUFAs present. For example, C16:2 ω4, C20:5 ω3 and C22:5 ω3 comprised 5.19%, 4.84% and 2.06%, respectively, of the fatty acids observed in the biomass obtained in cultures grown using an initial nitrogen concentration of 3g/L TN and C16:2 ω comprised 1.95% of the fatty acids observed in cultures grown using an initial nitrogen concentration of 0.44g/L (Fig. 3).
The main commercial sources of PUFAs are species of fatty fish, such as herring, mackerel, salmon and sardines. The Aurantiochytrium strain used in this study accumulated higher concentrations of PUFAs (28–70%) than those reported in sardine oil (31.1%) by Morais.21 The DHA concentration (20–43%) produced by this oleaginous microorganism was 2- to 4-fold higher than that found in the sardine oil (11%).21 Cultivated Aurantiochytrium sp. ATCC PRA-276 is a promising alternative source of oil rich in PUFAs. Using fish for the large-scale production of such oil is limited by the changes in the lipid compositions and contents and the fatty acid profiles of fish, which are affected by the seasons and their species, sex, size, reproductive status, catch location, diet and nutritional status.22 Moreover, fish oil exhibits a great diversity of fatty acids with different chain lengths and degrees of unsaturation and thus, requires expensive extraction and purification processes.8
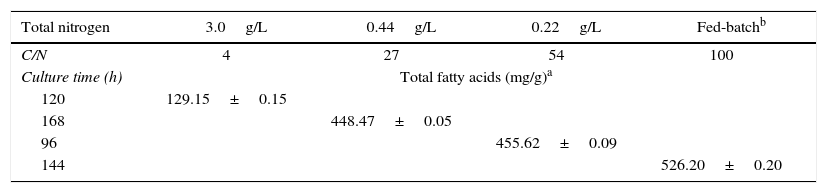
Cultivating an oleaginous microorganism in the laboratory under controlled environmental conditions reduces the risk of contamination and can increase fatty acid production at a low cost. In this study, we observed that the total fatty acid content of the biomass (%, w/w) increased as the TN concentration was decreased. This phenomenon occurred because lipids generally accumulate in oleaginous microorganisms when the medium contains an excess of the carbon source and a limited amount of nitrogen (high C/N ratio). In the presence of low-level nitrogen, the synthesis of proteins and nucleic acids is limited by the enhanced conversion of carbon to oil.23,24 This process was observed in the experiments using a fed-batch system, in which C/N ratio was high, eventually leading to the accumulation of a high level of total fatty acids (526.20mg/g) in the biomass (Table 1). However, fed-batch cultures exhibited lower PUFAs production (1.89g/L) and consequently lower DPA ω6 (0.44g/L) and DHA (1.33g/L) production than those of the batch cultures because the yields of these fatty acids are dependent on the accumulation of PUFAs in the total lipids as well as on the accumulation of oils in the biomass. Additionally, the fatty acid yield was also related to the cell concentration at a given time.
Total fatty acid content of the Aurantiochytrium sp. ATCC PRA-276 biomass with the highest PUFAs concentration under each experimental condition.
For example, in the experiments using an initial TN concentration of 0.22g/L, there was a higher substrate consumption rate, increased biomass productivity and a higher C/N ratio (54), which resulted in higher yields of DPA ω6 (0.80g/L) and DHA (2.54g/L).
Ganuza and Izquierdo18 observed greater DPA ω6 (3.85% w/w) and DHA (15.4% w/w) accumulation by Schizochytrium sp. G13/2S cells grown using initial nitrogen and glucose concentrations of 0.30g/L and 40g/L, respectively, than was observed in our experiments (3.34% w/w DPA ω6, and 10.62% w/w DHA) using an initial nitrogen concentration of 0.22g/L. However, their DPA ω6 (0.6g/L) and DHA (2.42g/L) production rates were lower than ours, which can attributed to the higher maximum biomass concentration (23.9g/L) obtained in our experiments compared with that reported by Ganuza and Izquierdo18 (15.7g/L).
Using a similar culture medium with an initial TN concentration of 0.44g/L to grow Schizochytrium sp. ATCC 20889, Jiang et al.8 observed that DHA accounted for 26% of the total fatty acids after 120h of cultivation, which is very similar to the 25.5% DHA level in the total fatty acids observed in our study. In our study, the accumulated biomass consisted of approximately 12.5% (w/w) DHA, which is higher than the value (8.8%) reported by Jiang et al.8
Burja et al.24 evaluated the effect of different concentrations of nitrogen on fatty acid production by Thraustochytrium sp. ONC-T18. Using an initial TN concentration of 0.75g/L, these authors obtained 0.04g/L of DHA, corresponding to 0.53% (w/w) of the biomass, and reported a low biomass concentration (7.5g/L) and a low level of DHA in the biomass. Burja et al.24 also observed that using a higher initial TN concentration (1.23g/L), 1.56g/L of DHA was obtained, which is approximately 3 times the DHA concentration (0.51g/L DHA) observed in our experiments using an initial TN concentration of 3.0g/L.
ConclusionsHerein, we presented the results of a study of the effect of different concentrations of carbon and nitrogen on PUFAs production by Aurantiochytrium sp. The PUFAs production of this microorganism depends on the accumulation of total fatty acids and on the concentration of the biomass. Therefore, the culture medium should facilitate the growth of this microorganism and provide an adequate nitrogen supply with respect to the C/N ratio because it accumulates oils when the total nitrogen supply is limited.
The main polyunsaturated fatty acids found in the Aurantiochytrium sp. ATCC PRA-276 biomass were DPA ω6 (20–23.1%) and DHA (61.3–70.5%). The maximum cell concentration of 23.9g/L (with 45.5% of its weight consisting of fatty acids) was observed at 96h of cultivation using initial concentrations of 30g/L of glucose and 0.22g/L of total nitrogen. Under these conditions, the highest PUFAs concentration (3.6g/L) was reached, with the DHA and DPA ω6 concentrations being 2.54 and 0.80g/L, respectively.
The results of this study showed that the growth of Aurantiochytrium sp. ATCC PRA-276 and its accumulation of PUFAs, particularly DHA, are dependent on the concentrations of the carbon and nitrogen substrates. The results also demonstrated that the cultivation period is an important variable for PUFAs production by Aurantiochytrium sp. ATCC PRA-276.
Aurantiochytrium sp. ATCC PRA-276 is capable of producing high levels of PUFAs. Therefore, developing new techniques for cultivating this microorganism could reduce the cost and increase the production of oils for use in food and medicines.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior of Brazil (CAPES) and developed at the Portuguese Institute of Sea and Atmosphere (IPMA) in Lisbon, PT, with the aid of a scholarship grant awarded to the first author by the Doctoral in the Country with Internship Abroad Programme (PDEE) (grant no. 6906/10-9). The authors also thank the ALGAENE Project and Depsiextracta Biological Technologies, Lda. for their support.