The effect of different modified atmosphere packaging regimes on the behavior of Salmonella spp. on minced meat was studied. Minced meat was experimentally contaminated with a Salmonella spp. cocktail (S. Enteritidis, S. Typhimurium, S. Infantis and S. Arizonae), packaged under vacuum or modified atmosphere with initial headspaces containing 20%O2/50%CO2/30%N2 and 20%O2/30%CO2/50%N2) and stored at 3±1°C for 12 days. Samples were analyzed for Salmonella spp., viable and lactic acid bacteria count every third day. Salmonella spp. counts decreased during storage in all packaging types, with reductions of about 1.5logCFU/g. A significant difference (p<0.01) was noted between Salmonella spp. counts in meat packaged in vacuum and modified atmospheres, although there was no significant difference in Salmonella spp. count between meat packaged in 50%CO2, and meat packaged in 30%CO2. At the end of the study, there were significant differences (p<0.01; p<0.05) in total viable and lactic acid bacterial counts between meat packaged in vacuum and modified atmosphere, and the lowest counts were noted in meat packaged in modified atmosphere with 50%CO2.
Pork and beef minced meat are widely consumed in Europe.1 In Serbia as well as in other Balkan and some Mediterranean countries, minced meat is an inseparable part of traditional dishes (e.g. moussaka, sarma), and meat products (e.g. sausages, ćevapčići, hamburger). The mincing process disrupts the meat cellular structure, releasing tissue fluids and making the minced meat a highly nutritious medium supporting bacterial growth; mincing also allows migration of surface bacteria throughout the product.2 Therefore, it presents a highly perishable product that need to be wrapped or packaged and chilled immediately to an internal temperature of not more than 2°C or frozen to -18°C during storage and transport (Regulation (EC) 853/2004).3
Despite measures to control foodborne pathogens from farm to fork the burden of diseases caused by foodborne pathogens remains important health and economic issue.4–8 Some of these pathogens, such as Salmonella spp., continue to cause major human public health and economic problems in both developed and developing countries.9Salmonella spp. are the second most often reported bacteria causing food-borne disease in humans, following Campylobacter spp.10,11 Meat can be contaminated with Salmonella during the slaughter, dressing and deboning processes, or during processing, transport, storage and household use, as a result of cross-contamination.12,13S. Enteritidis and S. Typhimurium are the most frequently reported serotypes causing human salmonellosis in both the EU and the United States, while the incidence of S. Infantis is increasing.14–18 This highlights the need for improved prevention and control of Salmonella spp. in food.
The food industry has developed numerous preservation techniques in order to prevent and control Salmonella and other food-borne pathogens and spoilage microorganisms in fresh meat products, making the meat safer for consumption and extending its shelf life. Vacuum packaging (VP) and modified atmosphere packaging (MAP) are the most commonly used packaging methods for meat and meat products. MAP is considered to be an effective technique for raw meat preservation.19–23 These methods are based on removal of the surrounding atmosphere (VP) or flushing it out and replacing it with a gas mixture (MAP) before sealing in gas barrier materials.20–23 Gases most often used in MAP are carbon dioxide, which inhibits bacterial growth, oxygen, which prevents anaerobic growth and retains meat color, and nitrogen, which avoids oxidation of fats and pack collapse. Depending on the type of food or effect desired, these gases can be used separately or in combination in various concentrations.19,21
Considering the prevalence of Salmonella in minced meat and the frequency of its consumption via many traditional products and, taking into account that packaging of meat is the most common method of food preservation, there is a need to explore the effect of packaging methods on Salmonella spp. survival, especially in mixed minced meat (pork and beef). Therefore, the aim of this study was to compare the effects of vacuum and two initial headspace-modified atmosphere conditions (20%O2/50%CO2/30%N2 and 20%O2/30%CO2/50%N2) on the survival of Salmonella spp., total viable bacteria and lactic acid bacteria in minced meat stored at 3±1°C.
Materials and methodsPork and beef muscles from leg of different carcasses used in the study were provided 48h post-slaughter by a local slaughterhouse (Pećinci-Subotište, Serbia). Connective tissues and visible fat were trimmed after which the pieces of meat were minced separately in a sterile grinder (4mm perforation diameter in the meat grinder plate), mixed in a 50:50 ratio of pork:beef and transported under refrigeration to the laboratory within an hour.
Four serovars of S. enterica (S. Enteritidis ATCC 13076, S. Typhimurium ATCC 14028, S. Arizonae ATCC 13314 and S. Infantis ATCC 51741) (www.atcc.org) were used in this study. The serovars were stored in Brain Heart Infusion (BHI; Merck, Germany) with 20% glycerol at −80°C until needed. One ml of each frozen Salmonella serovar was added to 10ml of BHI (Merck, Germany), incubated at 37°C for 24h, then were streaked on Xylose Lysine Tergitol-4 Agar (XLT4) (Merck, Germany) to verify their characteristics. In order to get a second subculture isolated, black colonies were picked from the XLT4 plates and inoculated into BHI tubes (1 colony per tube) and further incubated for another 24h at 37°C. After incubation, the cultures were centrifuged at 5000×g (Eppendorf, Hamburg, Germany) for 10min and suitable dilutions were prepared in BHI. A Salmonella cocktail was prepared by combining equal portions of standardized cell suspensions to yield approximately 8logCFU/ml of each serovar in the mixture. Salmonella counts were determined by serial dilution and subsequent enumeration on XLT4. This Salmonella cocktail (40ml of the cocktail) was used to inoculate 9kg of minced meat in the sterile mixer in the experimental laboratory of the Faculty of Veterinary Medicine, University of Belgrade. According to legal requirement for the absence of Salmonella in 25g of raw meat, meat used in the present study was not naturally contaminated with Salmonella. Minced meat was divided in portions of 100g, and packaged in three different conditions: VP, modified atmosphere package 1 (MAP1, containing 20%O2/50%CO2/30%N2) and modified atmosphere package 2 (MAP2, containing 20%O2/30%CO2/50%N2). MAP treatments were conducted considering ratio of 1:3 (v/w) between the volume of gas and weight of the minced meat (G/P ratio). A Variovac packaging machine (Variovac Primus, Zarrentin, Germany) was used for VP and MAP. Minced meat was packaged in a OPA/EVOH/PE foil (oriented polyamide/ethylene vinyl alcohol/polyethylene Dynopack, POLIMOON, Kristiansand, Norway), with low gas permeability (O2 – 3.2cm3/m2/day at 23°C, N2 – 1cm3/m2/day at 23°C, CO2 – 14cm3/m2/day at 23°C, water vapor – 15g/m2/day at 38°C). All minced meat samples weighed 100±5g and were refrigerated at 3±1°C.
Minced meat was analyzed for Salmonella spp., total viable count (TVC-mesophiles, 30°C), and lactic acid bacteria (LAB) count immediately and on days 3, 6, 9 and 12 of storage. For bacterial enumeration, approximately 10g of meat were weighed aseptically after package opening, transferred into sterile Stomacher bags and 90ml of Buffered Peptone Water (BPW) (Merck, Germany) was added to each sample. Meat samples were homogenized in a Stomacher blender (Stomacher 400 Circulator, Seward, UK) for 2min. Serial decimal dilutions were prepared in buffered peptone water (Merck, Germany) and 1ml or 0.1ml of appropriately diluted homogenized meat was inoculated directly on the surface of XLT4 (Merck, Germany) for Salmonella spp. enumeration24 and incubated for 24h at 37°C, Plate Count Agar (PCA; Merck, Germany) for TVC-mesophiles enumeration according to ISO 4833:2003,25 and incubated at 30°C for 72h and MRS Agar (Merck, Germany) for LAB enumeration according to ISO 15214:1998,26 and incubated at 30°C for 72h. After incubation, plates were examined visually for typical colonies and morphological characteristics associated with each growth medium, the number of colonies was counted and results were recorded as colony forming units per g (CFU/g). Suspect colonies of Salmonella spp. were tested using API 20e (BioMerieux Italia-Bagno a Ripoli, Florence), while suspect colonies of lactic acid bacteria were stained by Gram and catalase test was done.
The meat pH was measured after 10min at room temperature using a hand-held pH meter, Testo 205 (Testo AG, Lenzkirch, Germany), equipped with a penetrating glass electrode.
Measurement of headspace gas composition in the minced meat packaging was conducted using the gas composition tester, Oxybaby (WITT Gasetechnik GmbH & Co. KG, Witten, Germany). The measurement range of the instrument is 0–100% by volume (vol) for oxygen (O2) and carbon dioxide (CO2). The nitrogen content is calculated as the difference from 100% after the measured values of oxygen and carbon dioxide are deducted. The accuracy of the device is 0.1% for oxygen and carbon dioxide.
Statistical analysisThe study was conducted in a completely randomized design, six repetitions were carried out for each treatment and the treatments were arranged in a 3×5 factorial design (3 treatments, 5 sampling days). Numbers of bacteria (CFU/g) were transformed into logarithms (log) before statistical analysis. Statistical analyses of the results were conducted using the software GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com). The results were expressed as mean±standard error of the mean and are reported in tables. The effects of different treatments during the storage period were appraised by one-factor analysis of variance- ANOVA and Tukey's multiple comparison test (p<0.05).
ResultsMicrobiological status of the minced meatThe initial Salmonella spp. count in the inoculated minced meat was 8.8±0.04logCFU/g (Table 1). The Salmonella spp. count decreased until the day 6 in all groups, with significantly higher (p<0.05) counts in VP than in MAP1 minced meat. A significant reduction of Salmonella spp. count was found on day 6 (average reduction of 1.9logCFU/g). From day 9, slightly increasing Salmonella spp. counts were observed in all packaging types, except in packages with 50% CO2 where it decrease again from day 9 to day 12. Number of Salmonella spp. in VP meat was significantly higher (p<0.05) than in both MAP meat on day 12 (Table 1). Significant difference (p<0.05) also was noted between two MAP packaging, with lower count in MAP with 50% CO2.
Change in Salmonella spp. count, LAB count and TVC (logCFU/g) in packaged minced meat samples during storage at 3±1°C (mean±SEM).
| Parameter | Day of storage | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | F | df | p | |
| Salmonella spp. count | ||||||||
| VP | 8.8Aa±0.04 | 8.3Aa±0.01 | 6.9Ab±0.00 | 7.1Ab±0.01 | 7.4Ac±0.08 | 212.0 | 4 | <0.0001 |
| MAP1 | 8.8Aa±0.04 | 8.0Ab±0.02 | 6.7Bc±0.01 | 7.1Ad±0.00 | 6.9Be±0.01 | 569.6 | 4 | <0.0001 |
| MAP2 | 8.8Aa±0.04 | 8.0Ab±0.02 | 6.8ABc±0.02 | 7.1Ad±0.00 | 7.2Cd±0.02 | 344.6 | 4 | <0.0001 |
| F | – | 3.003 | 6.558 | 0.3535 | 57.68 | |||
| df | – | 2 | 2 | 2 | 2 | |||
| p | – | 0.0800 | 0.0090 | 0.7079 | <0.0001 | |||
| LAB count | ||||||||
| VP | 3.1Aa±0.02 | 3.9Ab±0.01 | 4.0Abc±0.02 | 4.4Ac±0.09 | 5.0Ad±0.09 | 50.86 | 4 | <0.0001 |
| MAP1 | 3.1Aa±0.02 | 4.0Abd±0.05 | 3.7Bc±0.04 | 3.9Bb±0.03 | 4.0Bd±0.03 | 556.4 | 4 | <0.0001 |
| MAP2 | 3.1Aa±0.02 | 3.6Bb±0.03 | 3.9Cc±0.02 | 4.1ABd±0.05 | 4.9Ac±0.03 | 514.9 | 4 | <0.0001 |
| F | – | 25.45 | 155.9 | 4.529 | 66.67 | |||
| df | – | 2 | 2 | 2 | 2 | |||
| p | – | <0.0001 | <0.0001 | 0.0289 | <0.0001 | |||
| TVC | ||||||||
| VP | 7.0Aa±0.02 | 8.1Ab±0.01 | 7.9Ab±0.04 | 9.2Ac±0.03 | 9.5Ac±0.08 | 73.77 | 4 | <0.0001 |
| MAP1 | 7.0Aa±0.02 | 7.7Abc±0.07 | 7.2Ba±0.00 | 7.3Bab±0.00 | 7.9Bc±0.01 | 12.24 | 4 | <0.0001 |
| MAP2 | 7.0Aa±0.02 | 7.8Aab±0.00 | 8.8Cbc±0.02 | 8.2ABbc±0.03 | 9.1Ac±0.02 | 8.973 | 4 | 0.0001 |
| F | – | 2.364 | 118.9 | 11.65 | 9.907 | |||
| df | – | 2 | 2 | 2 | 2 | |||
| p | – | 0.1281 | <0.0001 | 0.0009 | 0.0018 | |||
Different lowercase lettera–e within lines indicate significant difference p<0.05 between different days of same treatment; different uppercase letterA–C within column, within same bacterial group, indicate significant difference p<0.05 between treatments of the same day.
The LAB count in minced meat increased during storage in all packaging types (Table 1). On day 12, the maximum detected LAB count was in VP meat, while the lowest LAB count was in MAP1, i.e. in meat packaged with modified atmosphere with a higher concentration of carbon dioxide. Significant differences (p<0.05) were detected between the LAB count in VP meat and MAP1, as well as between MAP1 and MAP2.
The maximum detected TVC was in the VP on day 12. The lowest TVC at the end of experiment was recorded in the MAP1, and it was significantly lower (p<0.05) than it other groups.
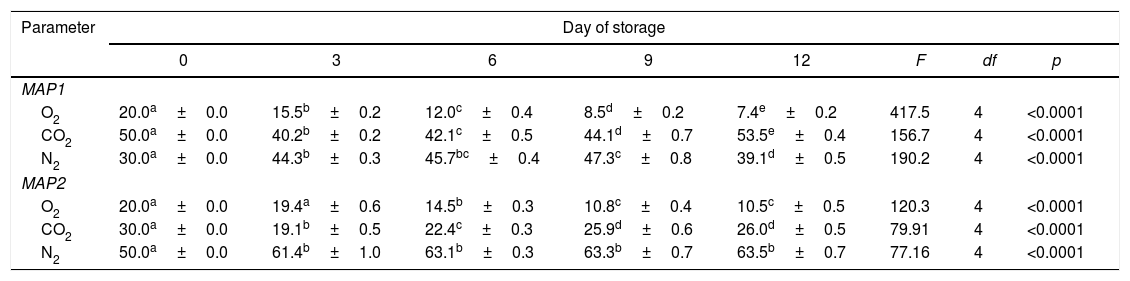
Headspace gasThe headspace gas data for the two MAP types are shown in Table 2.
Concentrations of CO2, O2 and N2 in headspace gas of packaged minced meat samples during storage at 3±1°C (%).
| Parameter | Day of storage | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | F | df | p | |
| MAP1 | ||||||||
| O2 | 20.0a±0.0 | 15.5b±0.2 | 12.0c±0.4 | 8.5d±0.2 | 7.4e±0.2 | 417.5 | 4 | <0.0001 |
| CO2 | 50.0a±0.0 | 40.2b±0.2 | 42.1c±0.5 | 44.1d±0.7 | 53.5e±0.4 | 156.7 | 4 | <0.0001 |
| N2 | 30.0a±0.0 | 44.3b±0.3 | 45.7bc±0.4 | 47.3c±0.8 | 39.1d±0.5 | 190.2 | 4 | <0.0001 |
| MAP2 | ||||||||
| O2 | 20.0a±0.0 | 19.4a±0.6 | 14.5b±0.3 | 10.8c±0.4 | 10.5c±0.5 | 120.3 | 4 | <0.0001 |
| CO2 | 30.0a±0.0 | 19.1b±0.5 | 22.4c±0.3 | 25.9d±0.6 | 26.0d±0.5 | 79.91 | 4 | <0.0001 |
| N2 | 50.0a±0.0 | 61.4b±1.0 | 63.1b±0.3 | 63.3b±0.7 | 63.5b±0.7 | 77.16 | 4 | <0.0001 |
Different lowercase lettera–e within lines indicate significant difference p<0.05 between different days of the same headspace gas.
In all meat sampled, the pH was 5.7 at the beginning of the study and then increased during storage (Table 3). A significant difference (p<0.05) in pH was observed between VP and MAP1 on day 9.
Change of pH in packaged minced meat samples during storage at 3±1°C (mean±SEM).
| Group of sample | Day of storage | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | F | df | p | |
| VP MAP1 MAP2 | 5.7Aa±0.004 | 5.8Ab±0.003 | 5.8Abc±0.003 | 5.9Acd±0.003 | 5.9Ad±0.009 | 28.18 | 4 | <0.0001 |
| 5.7Aa±0.004 | 5.8Aab±0.002 | 5.8Abc±0.008 | 5.8Bcd±0.006 | 5.9Bd±0.006 | 13.04 | 4 | <0.0001 | |
| 5.7Aa±0.004 | 5.8Aab±0.005 | 5.8Abc±0.009 | 5.9Bc±0.005 | 5.9ABc±0.009 | 15.46 | 4 | <0.0001 | |
| F | – | 1.98 | 2.58 | 7.51 | 3.99 | |||
| df | – | 2 | 2 | 2 | 2 | |||
| p | – | 0.1728 | 0.1091 | 0.0055 | 0.0409 | |||
Different lowercase lettera–d within lines indicate significant difference p<0.05 between different days of same treatment; different uppercase letterA–B within column, within same bacterial group, indicate significant difference p<0.05 between treatments of the same day.
The decrease of Salmonella spp. count until day 6 in the MAP packaging in the present study partly can be attributed to the inhibitory action of carbon dioxide, especially in the MAP1 with high CO2 content (50%), which was more effective than VP in the reducing Salmonella count (Table 1). Gram-negative bacteria, including Salmonella are highly sensitive to CO2 because its interact with membrane lipids, causing changes in ion membrane transport, penetrates the membrane and causes cytoplasmic acidification, disordered synthesis of specific enzymes, reduces cell metabolism extended the lag phase of microbial growth and reduced the growth rate during the logarithmic phase.22,27–30 More sensitive Gram-negative Salmonella compare to LAB, Gram-positive bacteria, as is the case in the present study, can be explained due to the denser cell wall and higher peptidoglycan content of Gram-positive bacteria compared to Gram-negative bacteria.31,32
Carbon dioxide is highly soluble in high moisture and fatty foods such as meat.30 In addition to the level of solubility of carbon dioxide an important factor affecting bacterial growth is storage temperature of the packaged meat. Storage temperatures below 5°C, as in the current study where meat samples were stored at 3±1°C, increase the solubility of carbon dioxide, whose antimicrobial activity increases, but also increase the sensitivity of bacterial cells to the effects of carbon dioxide.27,30,33,34 In the present study, the CO2 concentration was 50% (MAP1) and 30% (MAP2) at the beginning of the experiment. Decrease of CO2 during first tree days of storage is attributed to its absorption in meat and was fallowed by its increase until the end of the storage period. Increase of CO2 was caused by bacterial activity. Similar results were reported by Goulas.35 The decrease of O2 concentration observed in both MAP can be attributed to the growth of aerobic bacteria and microbial respiration, which utilize O2 and produce CO2 that contributes to spoilage.
Competitive microbiota, as well as the initial concentration of bacterial cells, has an influence on the growth of Salmonella spp. Although Salmonella spp. are able to grow and compete with other microorganisms,36 these bacteria are described in the literature as a relatively weak competitor.30,37–40 Because of these reason it is supposed that this group of bacteria are inhibited in MAP in the present study until day 6 (Table 1) by LAB, dominant bacteria in packaged meat stored at refrigeration temperatures (below 10°C).41–45 LAB are an integral part of the natural microbiota of meat.46 During present study, the LAB increase in the minced meat during 12 days at 3±1°C was greater than increases of the other bacterial groups studied, which could be due to the better adaptation of LAB to these conditions. During refrigeration of packaged raw meat, the mostly used method in order to extend shelf life, there is a pronounced growth of psychrotrophic and strictly or facultative anaerobic microbes like LAB.46 Although were expected increased number of LAB under high concentrations of CO2, LAB counts were higher in VP (Table 1), which is in agreement with the results of Li et al.,40 who reported higher LAB counts for raw pork packaged under vacuum than in MAP (40%O2/40%CO2/20%N2). In the present study the LAB count reached around 5logCFU/g (Table 1), which was less than the usual limit of acceptability at levels 6logCFU/g.45,47–49 Increasing LAB counts, as the dominant microorganisms in packaged meat stored at refrigeration temperatures were reported by Pexara et al.,50 Santos et al.,51 Martinez et al.,52 and Ruiz-Capillas and Jimenez-Colmenero.53 Based on the results of present study which showed that CO2 is effective against high Salmonella count (8logCFU/g), it is supposed that will be effective against lower levels of naturally-occurring Salmonella as well.
TVC is a parameter which also determines meat shelf-life. The initial microbial load is one of the most important parameters determining the shelf life of meat.39 The biggest proportions of the initial microbiota on fresh meat are mesophilic and psychrotrophic bacteria, and this latter group of bacteria is mainly responsible for meat spoilage. For these reasons TVC is used as an important microbiological quantitative indicator of production process hygiene, and for safety evaluation, as well as a spoilage indicator of raw meat.2,54,55 Based on numerous investigations, a TVC value of 107CFU/g in meat is considered as a critical value for assessment of spoilage.56–59 The number of microorganisms including bacteria depends on the intrinsic and extrinsic factors including pH, meat surface morphology, O2 availability, temperature and the presence and development of other bacteria.60 Changes in these factors and bacterial competition could influence the changes in the TVC in the present study. At the end of experiment, lower TVC was present in meat samples packaged with modified atmosphere, especially MAP with 50% CO2. Results from the present study showed lower values of TVC in meat packaged in the higher concentration of carbon dioxide, which can be attributed to antibacterial effect of modified atmosphere, especially carbon dioxide are consistent with the results of other authors.27,61
Many factors can affect the pH of packaged meat, but it is considered that a major factor responsible for its decline is the LAB population.62 Microorganisms’ growth as well as chemical reactions occurring during proteolytic processes throughout storage cause increases in meat pH (Table 3). These processes create alkali compounds (ammonia, trimethylamine, dimethylamine) responsible for pH increases.63 The increasing pH in all meat packaging regimes can be explained due to the high concentration of bacteria, resulting in production of alkali compounds. Furthermore, the pH increases could also be due to proteolysis, causing the production of free amino acids and leading to the formation of NH3 and amines.64 The results obtained in the present study are consistent with those obtained by Milijašević,65 Bozec et al.,66 and Cachaldora et al.45 while in the results obtained by Schirmer and Langsrud44 and Babić et al.,67 meat pH remained consistent during the storage period.
ConclusionsAll types of packaging used in present study decreased the Salmonella spp. count during first days of storage. This pathogen was best inhibited by MAP containing higher CO2 concentration (50%), followed by MAP with 30% CO2 and VP. Furthermore, MAP with a higher CO2 level exhibited greater antibacterial activity against TVC and LAB.
Conflicts of interestThe authors declare no conflicts of interest.
This paper was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia, through the funding of the project Selected biological hazards to the safety/quality of food of animal origin and the control measures from farm to consumer (No. 31034).