The history of laparoscopic surgery in gynaecological diseases progressed with the advances of Semm, as well as with the development of tools, equipment, and energy that led to its development in all surgical areas, including oncology.
ObjectiveTo present the initial experience in the laparoscopic treatment of benign and malignant gynaecological disease in Oaxaca Hospital Regional de Alta Especialidad.
Material and methodsAn analysis was performed on a total of 44 cases, distributed into: type III radical hysterectomy for invasive cervical cancer, hysterectomy type I cervical cancer in situ, extrafascial hysterectomy for benign disease, routine endometrium, ovary and routine salpingo-oophorectomy. The variables included age, BMI, surgical time, bleeding, intraoperative and postoperative complications, conversion, hospital stay, and pathology report.
ResultsHysterectomy type III; age 40.2 years, BMI 25.8kg/m2, 238ml bleeding, operative time 228min, 2.6-day hospital stay, intraoperative or postoperative complications, tumour size 1.1cm, 14 lymph nodes dissected, vaginal and negative parametrical edge. Type I hysterectomy cervical cancer in situ: 51 years, BMI 23.8kg/m2, 80ml bleeding, operative time 127minutes, uterus of 9cm, length of stay of 2 days, a conversion by external iliac artery injury, with bleeding of 1500ml. Routine endometrium: 50.3 years, BMI 30.3kg/m2, 83ml bleeding, operative time 180minutes, uterus 12.6cm, length of stay 2.3 days, no complications.
ConclusionThe management of benign and malignant pelvic diseases using laparoscopy is feasible and safe, with shorter hospital stays and a prompt recovery to daily activities.
La historia de la cirugía laparoscópica en afecciones ginecológicas creció con los avances de Semm, el perfeccionamiento de instrumentos, equipos y energías, que permitió el desarrollo en todas las áreas quirúrgicas, incluyendo la Oncología.
ObjetivoPresentar la experiencia inicial en el tratamiento de dolencias benignas y malignas ginecológicas por laparoscopia, en el Hospital Regional de Alta Especialidad de Oaxaca.
Material y métodosSe analizaron 44 casos distribuidos en: histerectomía radical tipo III por cáncer cervicouterino invasivo, histerectomía tipo I por cáncer cervicouterino in situ, histerectomía extrafascial por enfermedad benigna, rutina de endometrio, rutina de ovario y salpingooforectomía. Variables: edad, índice de masa corporal (BMI), tiempo quirúrgico, sangrado, complicaciones transoperatorias y postoperatorias, conversión, estancia hospitalaria y reporte de anatomía patológica.
ResultadosHisterectomía tipo III; edad 40.2 años, BMI 25.8kg/m2, sangrado 238ml, tiempo quirúrgico 228min, estancia hospitalaria 2.6 días, sin complicaciones transoperatorias o postoperatorias, tamaño del tumor 1.1cm, 14 ganglios disecados, borde vaginal y parametrios negativos. Histerectomía tipo I por cáncer cervicouterino in situ: 51 años, BMI 23.8kg/m2, sangrado 283ml, tiempo quirúrgico 127min, útero de 9cm, estancia hospitalaria 2 días, una conversión por lesión de arteria ilíaca externa con sangrado de 1,500ml. Rutina de endometrio: 50.3 años, BMI 30.3kg/m2, sangrado 83ml, tiempo quirúrgico 180min, útero 12.6cm, estancia hospitalaria 2.3 días, sin complicaciones.
ConclusiónEl manejo de afecciones benignas y malignas pélvicas por laparoscopia es factible, seguro, con menor estancia hospitalaria y una recuperación más pronta de las actividades cotidianas.
The human need to diagnose diseases more exactly led to the introduction of invasive means of examining abdominal organs.
The exploration of the inside of our organism commenced with the introduction of the cystoscope by Nitze in the Viennese Royal and Imperial Society of Medicine in 1879. This instrument was improved in 1886 by Leiter, who fitted it with a small Edison incandescent lamp.1
The development of abdominal laparoscopy was started by Kelling, in Dresden, who used the cystoscope described by Nitze. He inserted it through a hole opened in the abdominal wall of a dog to inspect the content of the intestines. He termed this technique of exploration “celioscopy” and presented the results in the Congress of the German Medical and Biological Society in Hamburg, in September 1901.2
Ott, a gynaecologist in San Petersburg, described “ventroscopy”, in which he viewed the inside of the cavity using a canula with a frontal light source. In 1910, Jacobeus3 in Stockholm used the cystoscope in humans, inserting it into the abdomen through a trocar after distending the cavity using water or air. He explored the inside of the cavity and termed this method “laparoscopy”, and he also used this technique in the thorax. In 1911 Berheim, of the United States, published a paper entitled: “Organoscopy: cystoscopy of the abdominal cavity.4 In 1916 Goetze developed a puncture needle to improve the insufflation of air; in 1920 Ordoff perfected the point and made it pyramidal to facilitate penetration; Stone developed a valvular device in the trocar to prevent gas from escaping. In 1929 Kalk invented 135° oblique optical vision; in 1934 Zollikofer used carbon dioxide instead of air for abdominal insufflation, thereby reducing the risk of a gas embolism and peritoneal irritation.5
In 1938 the Hungarian Veress, an internal medicine specialist in Vienna, designed an atraumatic needle for the creation of pneumothorax. This has an external sheath with a bevelled point and a blunt internal probe which emerges at the moment of penetration into the abdominal cavity, thereby avoiding damage to the internal organs. Due to this it was immediately adopted for the creation of pneumoperitoneum.6
Semm,6 a gynaecologist and engineer in Kiev, described the automatic insufflator, and in 1966 he started to perform carefully planned surgical procedures. He also designed a large number of instruments for cutting, coagulation, ligating and suturing to make this surgery possible, so that he is considered to be the father of laparoscopy. From this moment on laparoscopy fully entered the field of Gynaecology.6 The history of hysterectomy goes back to the 5th century BC, as there are references then to this procedure. In the 2nd century AD at the time of Hippocrates Soranus of Ephesia was said to have amputated a uterus through the vagina. In 1517 in Italy, Berengario da Carpi performed a vaginal hysterectomy, and the first information on attempted hysterectomies date from 1825. It was in 1846 that Bellinger performed the first elective abdominal hysterectomy.
Once by 1900 the mortality rate was lower than 1%, hysterectomy started to become an option for the treatment of gynaecological diseases and symptoms. This broke the historical taboo imposed by Johnson, the Director of the London Medical Chirurgical Review, who had stated in 1825: “We consider the extirpation of the uterus not associated with previous protrusion or inversion to represent one of the cruellest and most impractical operations conceived or executed by man.7 It is not our intention to discourage audacious and new surgical procedures, but there is a limit which it would be imprudent to surpass”.
This surgical technique has undergone extremely rapid development. In 1984, Semm6 performed the first vaginal assisted laparoscopic hysterectomy. The first completely laparoscopic hysterectomy was performed in Pennsylvania in January 1988 by Reich et al., and the corresponding description was published in 1989.7 Later, the same doctor Semm, who was German, published the results of a supracervical technique known as “classical abdominal Semm hysterectomy”.8
Oncological surgery was rejected by some doctors at first, due to the risk of disseminating tumour cells and the need to shred the tumour so that it could be extracted. Nevertheless, this paradigm changed when in 1991 Coptcoal presented a radical nephrectomy due to carcinoma in the III Minimum Invasion Congress, Boston.9 Then in 1993 Ono et al.10 presented 2 cases of nephrectomy due to renal carcinoma renal without destroying the kidney, which was extracted through a small incision.
Hysterectomy is now the most frequently performed gynaecological surgery in the United States, with 555,000 operations, while in the United Kingdom 100,000 procedures are carried out per year.11
In the United States in 1997 63% of hysterectomies were open and only 9.9% were laparoscopic, and 1.5% were radical laparoscopic surgery due to malign disease; in 2001 the corresponding figures were 41% and 32%, respectively.12
Radical laparoscopic hysterectomy is currently the most common surgical procedure. It has rates of intraoperative complications such as bleeding and lesions to the bladder or urethra which are similar to those of open surgery. However, it has advantages including less postsurgical pain, a shorter recovery time13 and a lower rate of blood transfusions. Nevertheless, the duration of the operation has been reported to be longer.14 Respecting pathological results, the retrospective analysis by Taylor showed no differences in terms of the number of ganglia and positive surgical borders. Park et al.,15 in a retrospective analysis of 99 patients in clinical stages IA1 and IA2, found similar oncological results and intraoperative complications. Spirtos et al.16 evaluated 78 patients. Another author with Lee et al.17 evaluated 24 patients in clinical stages IA2 and IBI; in both studies type III total laparoscopic radical abdominal hysterectomy was performed, with pelvic and para-aortic lymphadenectomy and an oncological follow-up of from 3 to 5 years, with similar results to those of open surgery. On the other hand, Yan et al.,18 in a 12-year follow-up, showed that survival depends, as it does after open surgery, on the biology of the tumour and not the procedure used. The extension of radicality (parametrium, vaginal border) is not compromised according to the study by Ghezzi et al.19 which specifically covered complications of the urinary ducts such as lesions to the bladder or urethra. Nor was there a statistical difference between the laparoscopic vs the open groups in terms of acute retention.20 Even Choi et al., in Korea,21 carried out a study that compared totally laparoscopic radical abdominal hysterectomy with vaginally assisted radical laparoscopic hysterectomy and found similar results, although recovery was faster and with less bleeding for the totally laparoscopic procedure. The above point shows that the said procedure is a safe and viable option to perform.22,23
On the other hand, one study determined that 40 cases are required for a learning curve, while to reduce the duration of surgery 57 cases are required. This curve shortens if a basic training programme is applied,24 starting with benign procedures and with the experience of having performed open procedures beforehand, although the latter point is more debatable.25 The percentage of conversion to an open procedure from laparoscopy in an analysis of 260 consecutive patients, independently of their body mass index (BMI), age, previous surgery, size of uterus and tumour was 1.5%.26,27
In the Hospital Regional de Alta Especialidad, Oaxaca, complaints involving the female genital tract are the most common, and of these cervico-uterine cancer predominates. In August 2013 the laparoscopic (or minimally invasive) gynaecological surgery programme was launched, with the advice of an oncological surgeon from the National Cancer Institute and an expert in the field.
ObjectiveTo present our initial experience in 44 cases with benign and malign gynaecological disease, treated by minimally invasive surgery.
Material and methods44 cases of patients treated by laparoscopic surgery for gynaecological disease from August 2013 to October 2014 were evaluated consecutively. General patient data were gathered, including their age, weight, height and body mass index (BMI), preoperative diagnosis, days of hospitalisation, the duration of surgery, bleeding, size of uterus, conversion and the cause of conversion, when applicable, postoperative diagnosis, intraoperative and postoperative complications, with a follow-up period of at least 30 days as the minimum time for inclusion in the study, together with the pathology report on histology, number of ganglia and surgical parametrium and borders.
Inclusion criteriaAll patients over the age of 18 years old were included, with any benign disease (adnexal masses, uterine myomatosis with small, medium and large elements), malign disease (cervico-uterine cancer in situ, microinvasive cervico-uterine cancer [IA1, IA2] invasive cancer [IB1] with a tumour size of 2cm or less, cancer of the endometrium in early stages, uterine sarcoma) or complaints with a suspicion of malignity (pelvic tumour: ovarian, simple or complex endometrial hyperplasia with or without atypicality).
Exclusion criteriaTumours larger than 2cm in the neck of the uterus, neuropathy or cardiopathy which contraindicated general anaesthesia.
Elimination criteriaPatients in which a laparoscopic procedure had been started but which was converted due to finding advanced disease, retroperitoneal adenopathies, peritoneal carcinomatosis or implants in the supramesocolic epiploon.
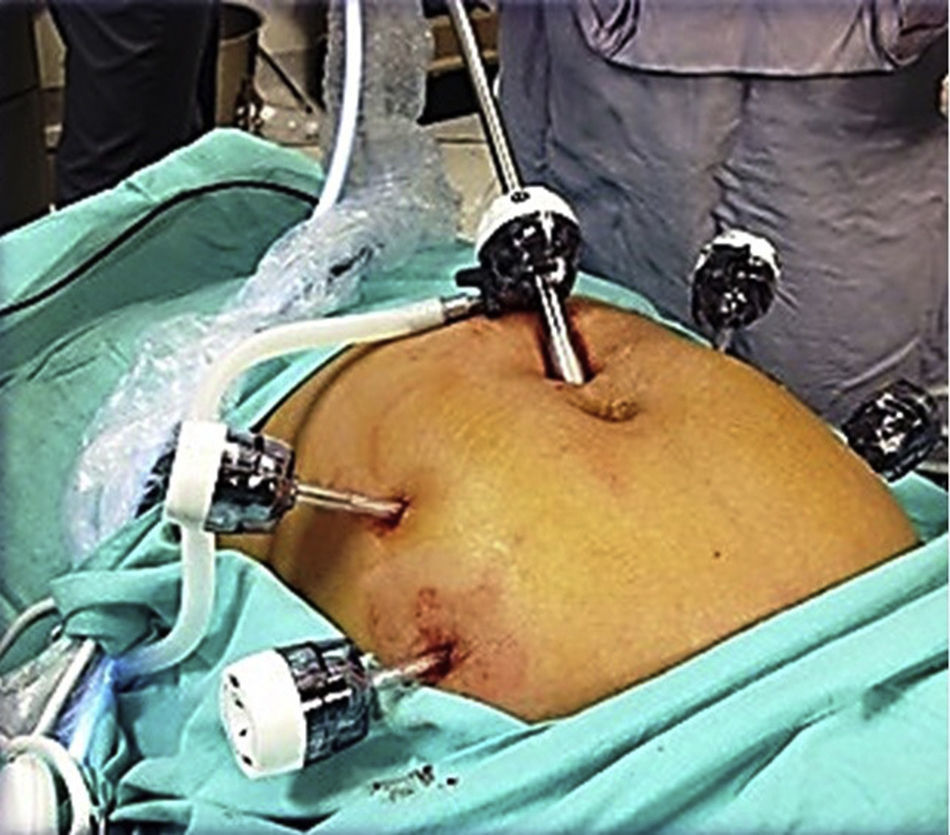
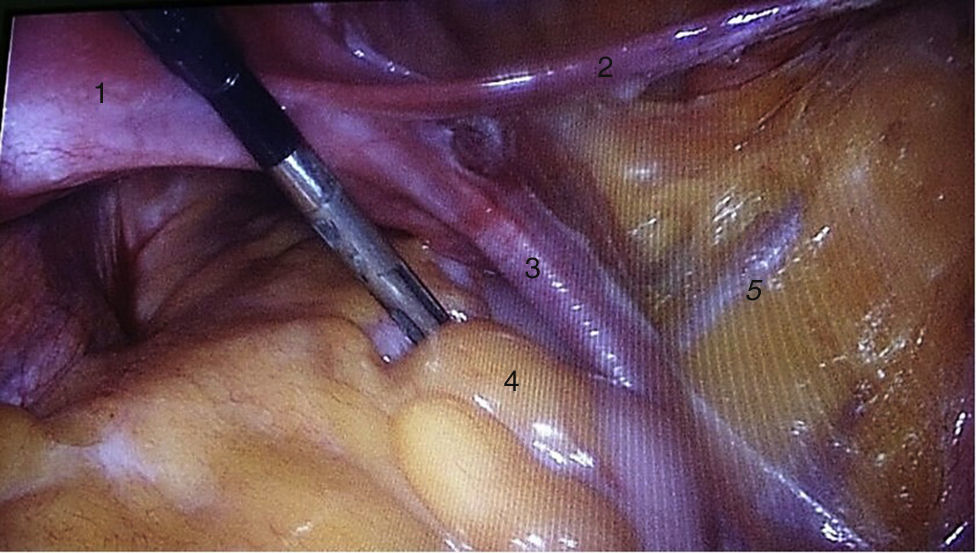
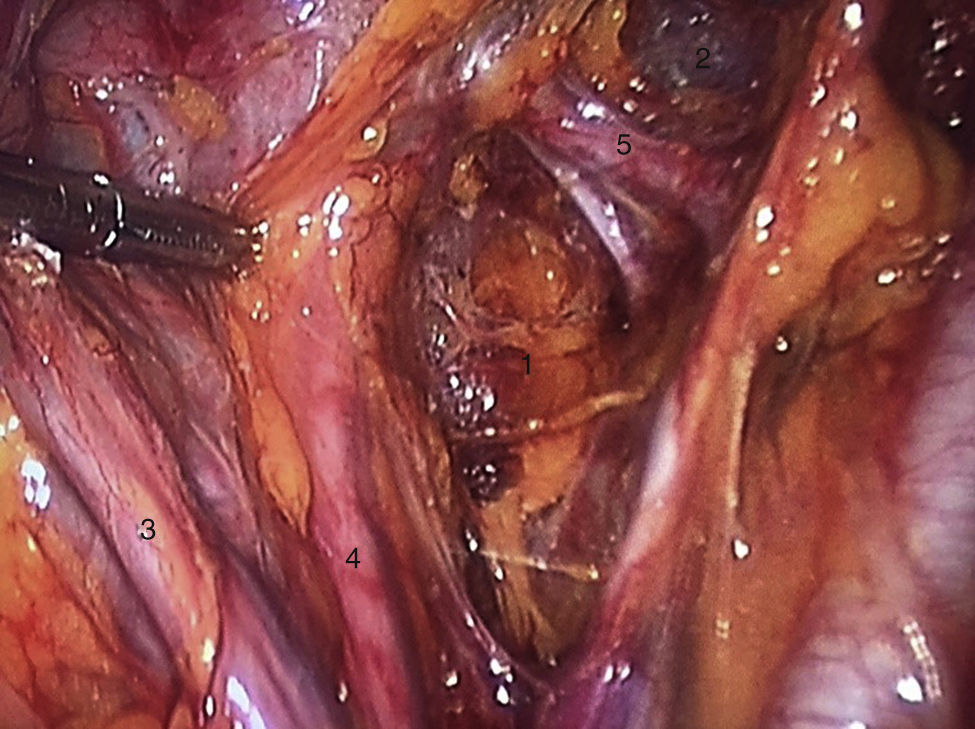
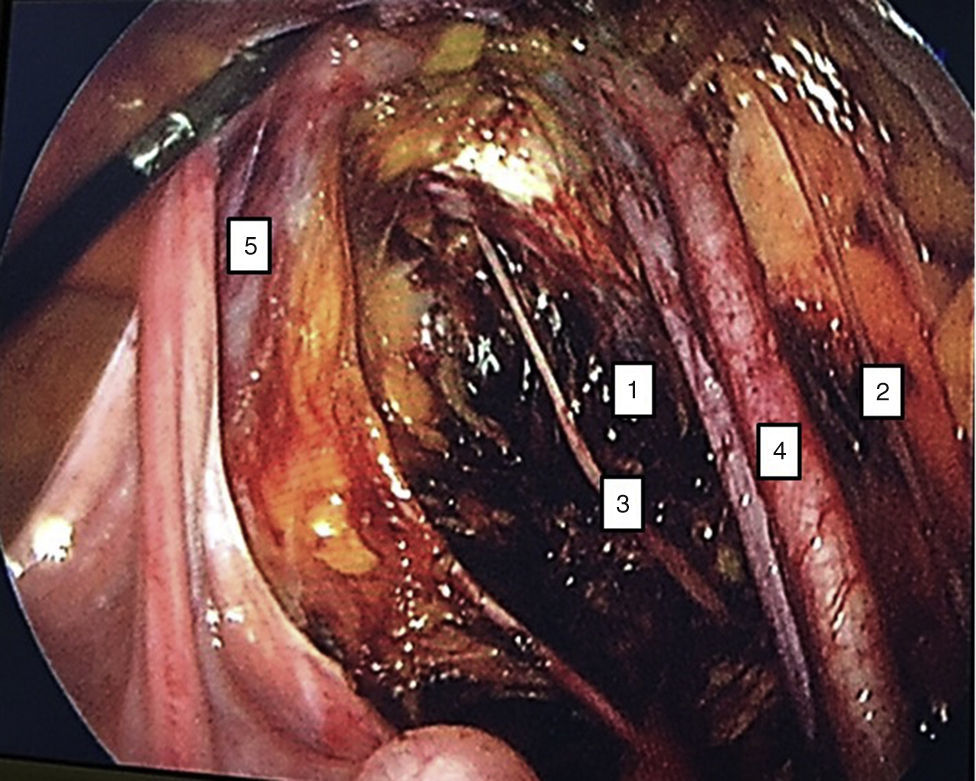
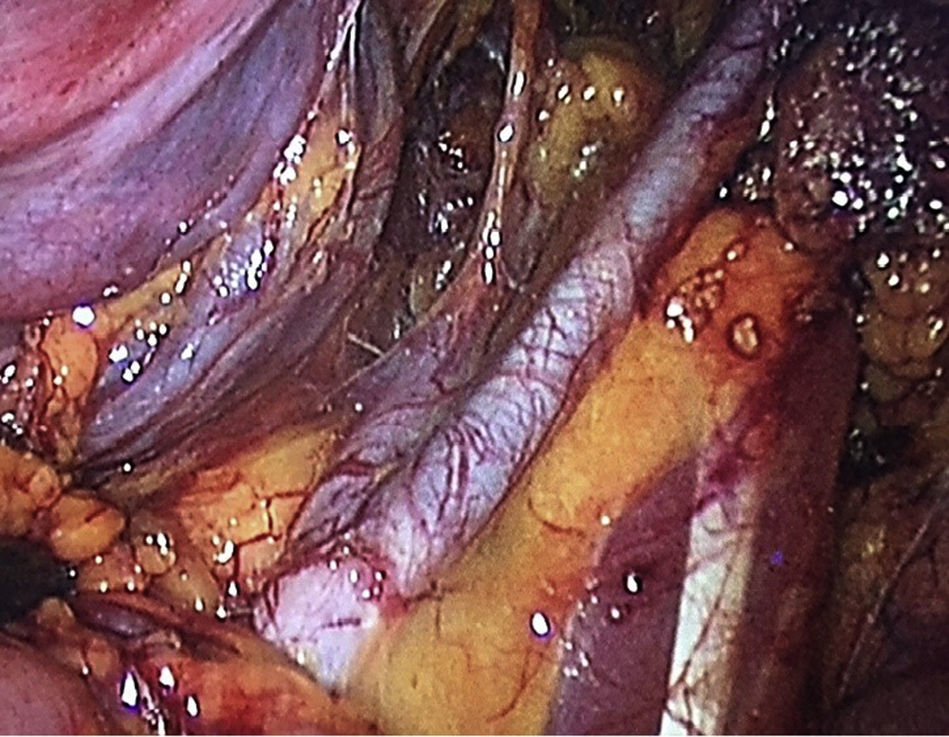
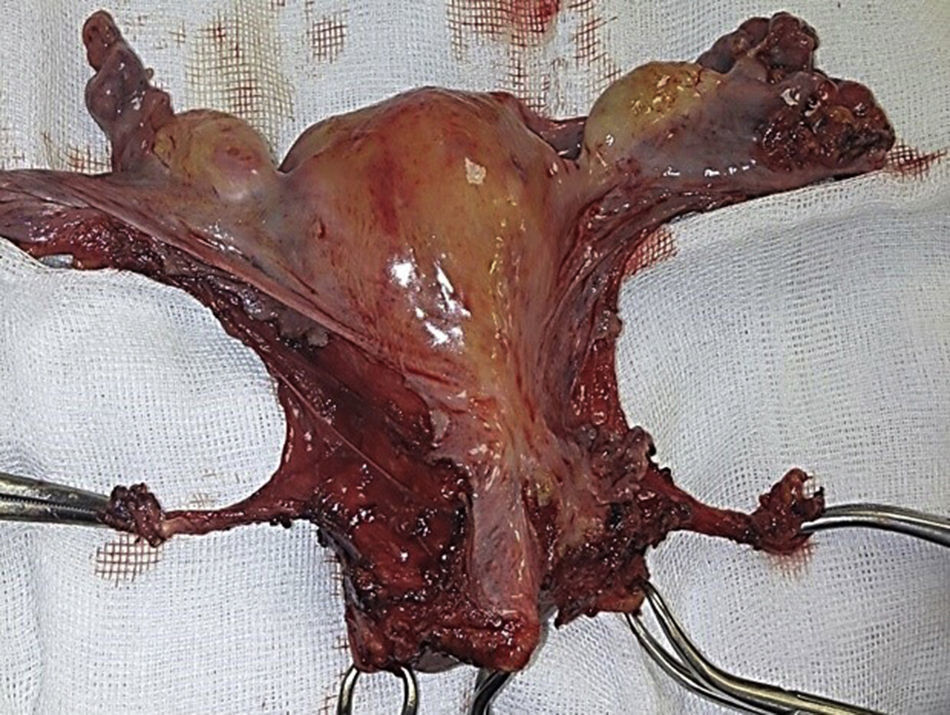
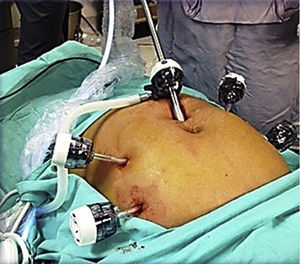
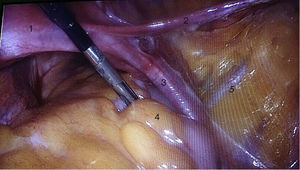
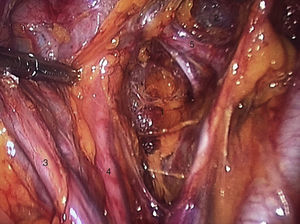
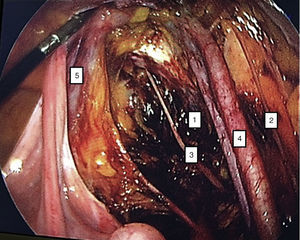
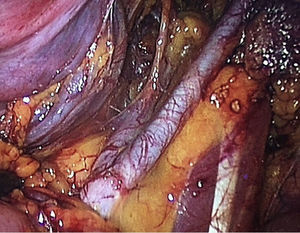
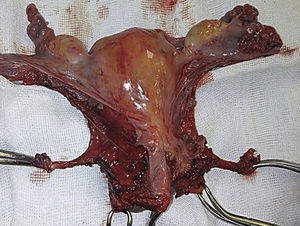
To standardise the surgical technique 2 initial procedures were performed under the supervision of an oncological surgeon with experience in laparoscopy. The surgical technique of total laparoscopic abdominal hysterectomy uses 5 trocars in the following positions: supraumbilical opening at 2cm from the umbilical scar, 10mm wide for the camera with 30° vision, 4 5mm trocars, 2 in the iliac fossae at 2cm from the anterior and superior iliac spine, and 2 in the right and left flanks (Fig. 1). The energy used in the first 5 cases was Ligasure Atlas, while in the rest of the cases monopolar energy and Enseal were used, helped by an Ascleaup or Clermont Ferrand uterine manipulator. The procedure started with the identification of anatomical structures (Fig. 2), the creation of a space around the bladder and rectum (Fig. 3), and evaluation of the parametrium (Fig. 4). Pelvic lymphadenectomy uses monopolar energy, respecting the conventional limits (circumflex vein, obturator nerve, urethra and 2cm above the bifurcation of the common iliac vessels) (Fig. 5). The surgical piece is extracted through the vagina, and the vaginal vault is closed by continuous suture with 0 or 1 intracorporeal vicryl (Fig. 6). In the case of adnexal masses, they are initially approached using 3 trocars, a 10mm umbilical one and 2 5mm ones in the region of the right and left iliac fossae.
44 patients were analysed. They were treated using several gynaeco-oncological procedures, and grouped in the following way: 9 radical hysterectomies due to invasive cervico-uterine cancer, 11 simple hysterectomies due to benign disease of the uterus (myomatosis), 7 type I hysterectomies due to cervico-uterine cancer in situ, 12 oophorectomies due to benign adnexal masses, 3 endometrium routines, one hysterectomy plus bilateral pelvic lymphadenectomy due to uterine sarcoma, and one complementary ovarian routine.
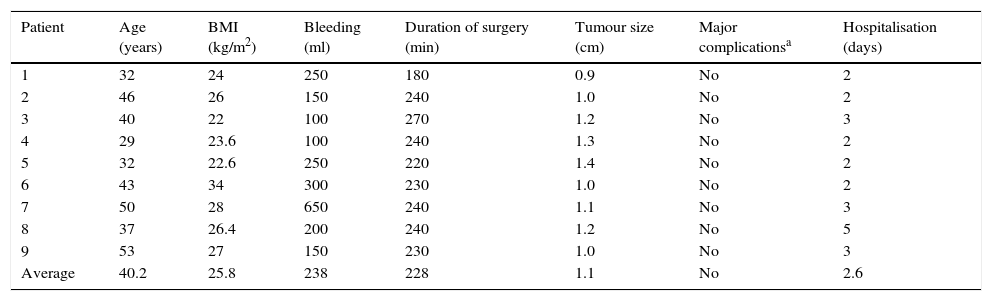
The average age in the radical hysterectomy group was 40.2 years old, with averages of a BMI of 25.8kg/m2, bleeding of 238ml, duration of surgery 228min, size of uterus 8.4cm, hospitalisation during 2.6 days, while there were no transoperative or postoperative complications. The histological report was of 4 patients with invasive cancer and tumour size of from 1 to 1.2cm, histological type 2 epidermoid, 2 adenocarcinomas of from 12 to 14 dissected pelvic ganglia, 0.8/1.1cm vaginal border negative, negative parametriums. Only one patient required adjuvant treatment due to a positive ganglion, 2 patients had microinvasive cervico-uterine IA2 cancer, 13 dissected pelvic ganglia, negative vaginal border and parametriums, 3 patients with diagnosis of in situ cervico-uterine cancer (patients with an external diagnosis of invasive cancer by biopsy or external cone, and in those for whom it was not feasible to revise the slides, and with a cytological-colposcopic of invasive carcinoma) (Table 1).
Radical laparoscopic hysterectomy.
| Patient | Age (years) | BMI (kg/m2) | Bleeding (ml) | Duration of surgery (min) | Tumour size (cm) | Major complicationsa | Hospitalisation (days) |
|---|---|---|---|---|---|---|---|
| 1 | 32 | 24 | 250 | 180 | 0.9 | No | 2 |
| 2 | 46 | 26 | 150 | 240 | 1.0 | No | 2 |
| 3 | 40 | 22 | 100 | 270 | 1.2 | No | 3 |
| 4 | 29 | 23.6 | 100 | 240 | 1.3 | No | 2 |
| 5 | 32 | 22.6 | 250 | 220 | 1.4 | No | 2 |
| 6 | 43 | 34 | 300 | 230 | 1.0 | No | 2 |
| 7 | 50 | 28 | 650 | 240 | 1.1 | No | 3 |
| 8 | 37 | 26.4 | 200 | 240 | 1.2 | No | 5 |
| 9 | 53 | 27 | 150 | 230 | 1.0 | No | 3 |
| Average | 40.2 | 25.8 | 238 | 228 | 1.1 | No | 2.6 |
BMI: body mass index.
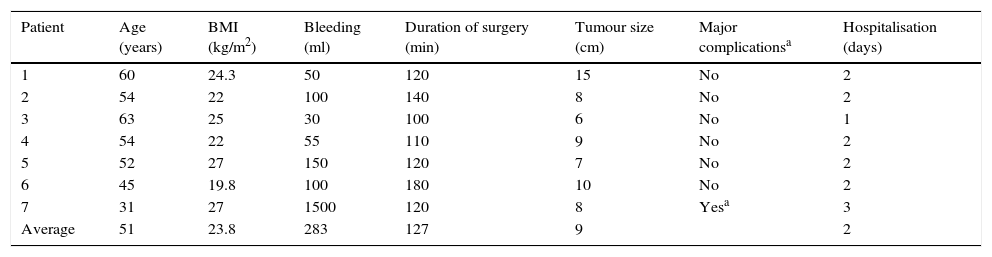
The average age in the group of type I hysterectomy due to diagnosis of cervico-uterine cancer in situ was 51 years old, with averages of a BMI of 23.8kg/m2, 283ml bleeding, duration of surgery 127min, size of uterus 9cm and hospitalisation during 2 days. One patient underwent conversion due to injury to the external iliac artery with 1500ml bleeding. There were no complications with injuries to the bladder, rectum or urethra. In all cases the reported disease confirmed the in situ diagnosis (Table 2).
Simple hysterectomy.
| Patient | Age (years) | BMI (kg/m2) | Bleeding (ml) | Duration of surgery (min) | Tumour size (cm) | Major complicationsa | Hospitalisation (days) |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 24.3 | 50 | 120 | 15 | No | 2 |
| 2 | 54 | 22 | 100 | 140 | 8 | No | 2 |
| 3 | 63 | 25 | 30 | 100 | 6 | No | 1 |
| 4 | 54 | 22 | 55 | 110 | 9 | No | 2 |
| 5 | 52 | 27 | 150 | 120 | 7 | No | 2 |
| 6 | 45 | 19.8 | 100 | 180 | 10 | No | 2 |
| 7 | 31 | 27 | 1500 | 120 | 8 | Yesa | 3 |
| Average | 51 | 23.8 | 283 | 127 | 9 | 2 |
BMI: body mass index.
The average age in the group of type I hysterectomy due to diagnosis of uterine myomatosis was 47 years old, with average of a BMI of 27kg/m2, 305ml bleeding, duration of surgery 197min, size of uterus 14cm, hospitalisation during 1.9 days. There were 2 conversions in this group: due to perforation of the uterine body by the uterine manipulator, which made it impossible to move, and due to a tear in the uterine artery with 900ml transoperative bleeding. One patient had a partial tear of the bladder, without total breakage, and this was repaired laparoscopically. Finally, one patient had an extensive haematoma of the abdominal wall 2 weeks after the operation, and 4 weeks afterwards had prolapse of intestinal loops through the vagina. There were no complications involving organs such as the bladder, rectum or urethra (Table 3).
Benign tumour of the uterus body (myomatosis, hyperplasia, polyp).
| Patient | Age (years) | BMI (kg/m2) | Bleeding (ml) | Duration of surgery (min) | Uterus size (cm) | Hospitalisation (days) | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 41 | 22.5 | 550 | 240 | 10×13 | 3 | AB |
| 2 | 47 | 31 | 100 | 180 | 30×220 | 2 | No |
| 3 | 44 | 27 | 900 | 180 | 9×5 | 2 | Yes, VL |
| 4 | 46 | 28 | 360 | 240 | 13×6 | 1 | No |
| 5 | 35 | 24 | 500 | 180 | 10×7 | 1 | No |
| 6 | 71 | 26.6 | 100 | 280 | 10×9 | 2 | Yes, AWH |
| 7 | 44 | 28 | 350 | 255 | 1×9 | 2 | C |
| 8 | 45 | 22.5 | 100 | 155 | 11×5 | 2 | No |
| 9 | 45 | 28.5 | 100 | 1800 | 10×6 | 2 | No |
| 10 | 47 | 31 | 100 | 180 | 18×114 | 2 | No |
| 11 | 52 | 28 | 200 | 100 | 15×10 | 2 | No |
| Average | 47 | 27 | 305 | 197 | 14 | 1.9 | 4.4% |
C: conversion; AB: abrasion of the bladder; AWH: abdominal wall haemorrhage; BMI: body mass index; VL: vascular lesion.
The average age in the group of salpingo-oophorectomy due to adnexal mass was 50 years old, with averages of a BMI of 29kg/m2, 30ml bleeding, duration of surgery 77min, size of cyst 9.5cm and hospitalisation during one day. There were no transoperative complications in this group, and no postoperative complications after 30 days of follow-up. In all cases the histopathological report was benign, and there was only one case of ovarian tube abscess (Table 4).
Laparoscopic salpingo-oophorectomy.
| Patient | Age (years) | BMI (kg/m2) | Bleeding (ml) | Duration of surgery (min) | Tumour size (cm) | Hospitalisation (days) | Major complications |
|---|---|---|---|---|---|---|---|
| 1 | 66 | 24 | 50 | 60 | 11 | 1 | No |
| 2 | 47 | 37 | 10 | 85 | 7 | 1 | No |
| 3 | 62 | 31 | 10 | 70 | 10 | 1 | No |
| 4 | 36 | 27 | 20 | 60 | 10 | 1 | No |
| 5 | 31 | 21 | 20 | 90 | 12 | 1 | No |
| 6 | 70 | 31 | 110 | 120 | 25 | 1 | No |
| 7 | 62 | 26 | 10 | 50 | 5 | 1 | No |
| 8 | 39 | 22 | 10 | 60 | 4 | 1 | No |
| 9 | 61 | 23.7 | 10 | 70 | 7 | 1 | No |
| 10 | 40 | 45 | 100 | 145 | 9 | 1 | No |
| 11 | 73 | 27 | 10 | 60 | 10 | 1 | No |
| 12 | 21 | 28 | 10 | 60 | 5 | 1 | No |
| Average | 50 | 29 | 30ml | 77 | 9.5 | 1 | 0% |
BMI: body mass index.
The average age in the group of endometrial cancer was 50.3 years old, with averages of a BMI of 30.3kg/m2, 83ml bleeding, duration of surgery 180min, size of uterus 12.6cm, hospitalisation during 2.3 days and no conversions or complications. Histopathology reported 2 patients with endometriode-type histology, one with disease limited to the endometrium and 12 negative pelvic ganglia; another with minor invasion of 50% involvement in the myometrium and 14 negative pelvic ganglia. The last type was carcinosarcoma with involvement of more than 50% of the myometrium and 7 of 14 pelvic ganglia were positive (Table 5).
Endometrium routine.
| Patient | Age (years) | BMI (kg/m2) | Bleeding (ml) | Duration of surgery (min) | Uterus size (cm) | Hospitalisation (days) | Major complicationsa |
|---|---|---|---|---|---|---|---|
| 1 | 47 | 43 | 150 | 240 | 14×7 | 3 | No |
| 2 | 40 | 24 | 50 | 120 | 12×10 | 2 | No |
| 3 | 64 | 24 | 50 | 180 | 12×8 | 2 | No |
| Average | 50.3 | 30.3 | 83 | 180 | 12.6×8.3 | 2.3 | 0% |
BMI: body mass index.
One patient with a preoperative diagnosis of uterine sarcoma was 36 years old, with a BMI of 27kg/m2, 100ml bleeding, duration of surgery 200min, size of uterus 13cm, hospitalisation during 2 days, with no complications or conversion. The histological report indicated high grade uterine sarcoma, with 15 negative pelvic ganglia.
Finally, one patient diagnosed complementary ovary routine was 36 years old, with a BMI of 27kg/m2, 50ml bleeding, duration of surgery 210min and size of uterus 8×7cm. The pathology report was of inframesocolic epiploon, peritoneal washing, peritoneum and pelvic ganglia (14 ganglia) biopsies negative for malignity, size of uterus 8cm, duration of hospitalisation 2 days (Table 6).
It should be pointed out that no antibiotic prophylaxis or intestinal or vaginal preparation were used, without signs of infection at the surgical site after a follow-up of one month.
DiscussionWith the arrival of all the infrastructure that centres on laparoscopic surgery, it is now used for increasingly complex procedures. On example of this is the pelvic cavity, where procedures such as types I, II or III hysterectomy according to Piver's classification are performed: pelvic, para-aortic or para-caval lymphadenectomy27,28 or series of more than 500 patients with endometrial cancer treated using laparoscopy29 with results at 4 years similar to those of the open procedure. This benefit is even maintained in obese patients with a BMI higher than 40kg/m2 and endometrial cancer,30 although there are reports that obesity is associated with major postoperative complications due to the metabolic alterations which arise.31 Nevertheless, relapse and overall survival over the long term are still being evaluated. To date, and over the mid-term (37 months) the ASTEC Trial shows no differences in comparison with the open procedure. Manchana et al.32 evaluated and compared the open procedure with the robotic and laparoscopic procedures for cancer of the endometrium. The results were positive for the latter two procedures, with less bleeding,33 shorter hospitalisation and a faster recovery.34
Respecting ovarian cancer, Zhang et al.35 analysed patients in early stages of the disease and found favourable results for laparoscopy, with less morbidity and shorter hospitalisation. It is also a useful tool for staging, given that it has a lower rate of complications than laparotomy.36,37 The challenge for laparoscopy in the treatment of cancer was overcome a long time ago with the performance of laparoscopic procedures such as Whipple's operation or the biliodigestive shunt. Respecting cervico-uterine cancer, Mendivil et al.38 found in a retrospective study of 5 years’ experience that laparoscopic procedures for the treatment of the said complaint were associated with shorter hospitalisation (2.9 days). These data are compatible with those of our study, together with an overall survival of 89.7% at 60 months.38,39 In Oaxaca Hospital Regional de Alta Especialidad minimally invasive surgery commenced with benign diseases (uterine myomatosis and adnexal masses). The majority of complications were present in this group (bleeding greater than 500ml, abrasion of the bladder, vascular lesion). Minimally invasive surgery then continued to be used for malign gynaecological diseases, with similar or shorter durations of hospitalisation than open surgery and no greater complications such as intestinal lesion, injury to the bladder or urethra,40 and with similar or smaller amounts of blood loss to open procedures.41 The duration of surgery tended to be longer and fewer ganglia were harvested than in open surgery, although patients returned to their normal activities sooner. According to international literature the above-mentioned major complications reduced significantly after the surgeon had performed more than 30 laparoscopic hysterectomies,42 and these results agree with those obtained in our unit.
Although the treatment of benign and malign pelvic disease using simple or radical laparoscopic hysterectomy and pelvic lymphadenectomy is viable, safe and requires shorter hospitalisation with an earlier recovery of normal activities than is the case with open surgery,42 the institutional challenge will be to increase the number of ganglia dissected and reduce the duration of surgery.
ConclusionsThe treatment of benign and malign pelvic disease using laparoscopy is viable, safe and requires shorter hospitalisation with an earlier recovery of normal activities.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Vásquez-Ciriaco S, Isla-Ortiz D, Palomeque-Lopez A, García-Espinoza JA, Jarquín-Arremilla A, Lechuga-García NA. Experiencia inicial en el tratamiento de enfermedad ginecológica benigna y maligna por laparoscopia en el Hospital Regional de Alta Especialidad de Oaxaca. Cir Cir. 2017;85:12–20.