The rare incidence of primary malignant melanoma of the central nervous system and its ability to mimic other melanocytic tumours on images make it a diagnostic challenge for the neurosurgeon.
Clinical caseA 51-year-old patient, with a tumour located in the right forniceal callosum area. Total surgical excision was performed. Histopathological result was consistent with the diagnosis of primary malignant melanoma of the central nervous system, after ruling out extracranial and extraspinal melanocytic lesions.
ConclusionsThe primary malignant melanoma of the central nervous system is extremely rare. There are features in magnetic resonance imaging that increase the diagnostic suspicion; nevertheless there are other tumours with more prevalence that share some of these features through image. Since there is not an established therapeutic standard its prognosis is discouraging.
La rara incidencia del melanoma maligno primario del sistema nervioso central y su capacidad de mimetizar por imagen otros tumores melanocíticos lo hacen ser un reto diagnóstico para el neurocirujano.
Caso clínicoPresentamos el caso de una paciente femenina de 51 años de edad, con un tumor localizado en el área calloso forniceal derecha. Se realizó exéresis quirúrgica total, y el resultado histopatológico fue compatible con el diagnóstico de melanoma maligno primario del sistema nervioso central, habiéndose descartado lesiones extracraneales y extrarraquídeas mucotegumentarias melanocíticas.
ConclusionesEl melanoma maligno primario del sistema nervioso central es extremadamente raro. Existen características en imagen de resonancia magnética que incrementan la sospecha diagnóstica. Sin embargo, hay otros tumores más prevalentes que comparten algunas de esas características por imagen. No hay un estándar terapéutico establecido. Su pronóstico es desalentador.
Primary melanocytic tumours of the central nervous system are rare, histologically and clinically different from metastases from retinal or cutaneous malignant melanoma.1 They are classified into diffuse melanocytosis (diffuse melanosis) and meningeal melanomatosis, malignant melanoma and benign melanocytoma, with a small number of intermediate variants.1 This set of primary melanocytic neoplasms of the central nervous system have an extraordinary incidence, estimated at 0.9 per 10 million inhabitants.2 Of these, primary malignant melanoma of the central nervous system is even more unusual: it accounts for 1% of all cases of melanoma3,4 and 0.7% of all primary tumours of the central nervous system.5 Its incidence is specifically 0.005 cases per 100,000 individuals.6 It presents in an age range from 35 to 50.7 Bhandari et al.2 state that it was Virchow who, in 1859, described the first case of primary diffuse intracranial melanoma, and Oogle reported in 1899 the first case of primary solitary intracranial melanoma.7,8 These tumours arise from melanocytes present in the leptomeninges in the cerebral convexity, in the skull base, posterior fossa, cervical spinal canal, the pia mater covering vessels, reticular formation of the bridge and spinal cord, black substance and locus coeruleus.7 The most common sites within the central nervous system are: lobar (53.1%), posterior fossa (17.3%) and pineal region (13.6%).2 The diagnosis of these tumours is difficult; it has to be carried out by exclusion, with primary melanocytic tumours of the central nervous system, primary tumours of the central nervous system with a certain degree of melanisation, and mainly with metastases from primary extracranial melanoma, the most common neoplasm causing cerebral metastases after lung cancer and breast cancer.8,9 Although the prognosis of patients with primary malignant melanoma of the central nervous system is discouraging, especially in cases with leptomeningeal dissemination,6,10 and this being the most direct prognosis factor,11 it seems highly dependent on a full tumour resection.10,12
A current review of PubMed literature on primary malignant melanoma of the central nervous system was carried out. Due to its low incidence, report cases and small series of cases were found. Also, it must be considered that in Mexico there is only one case of primary malignant melanoma of the central nervous system, reported in 2008 by Avilés-Aguilar et al.,13 confirming the rare incidence of this tumour. In this article we document and present the radiological characteristics, macroscopic, histological and immunohistochemistry findings, and discuss the potential aetiological genotype, current management options and the prognosis of this infrequent entity. An illustrative case treated in our neurosurgery service is presented.
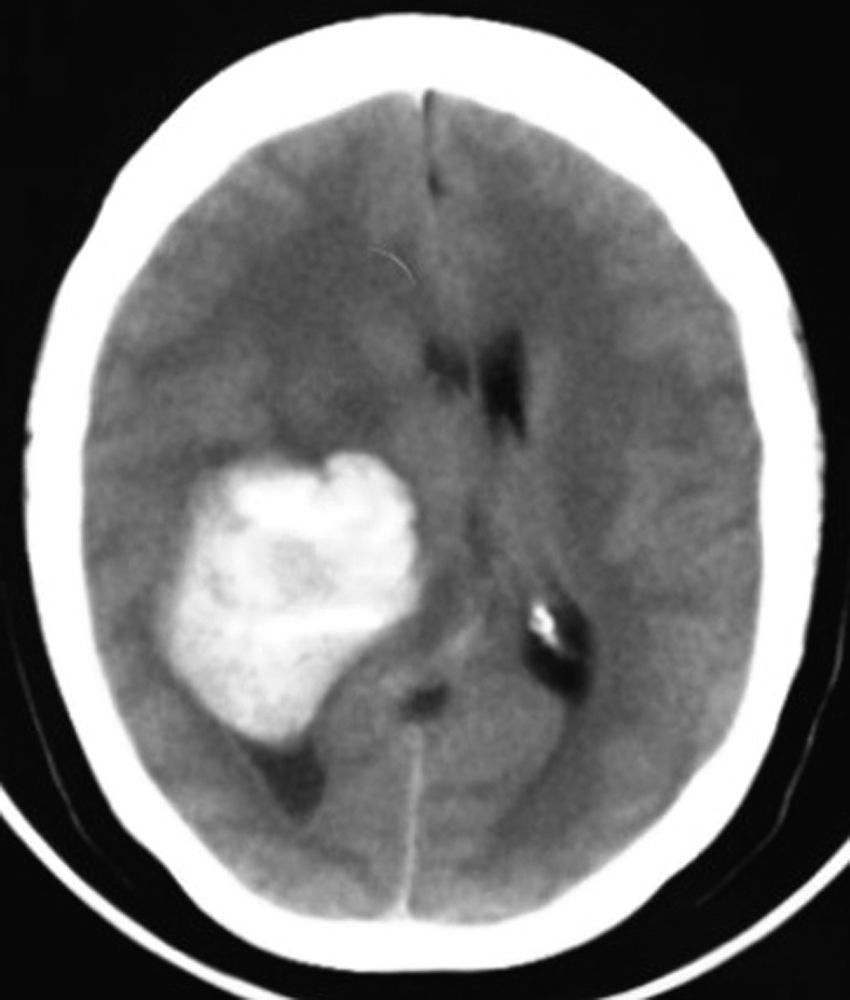
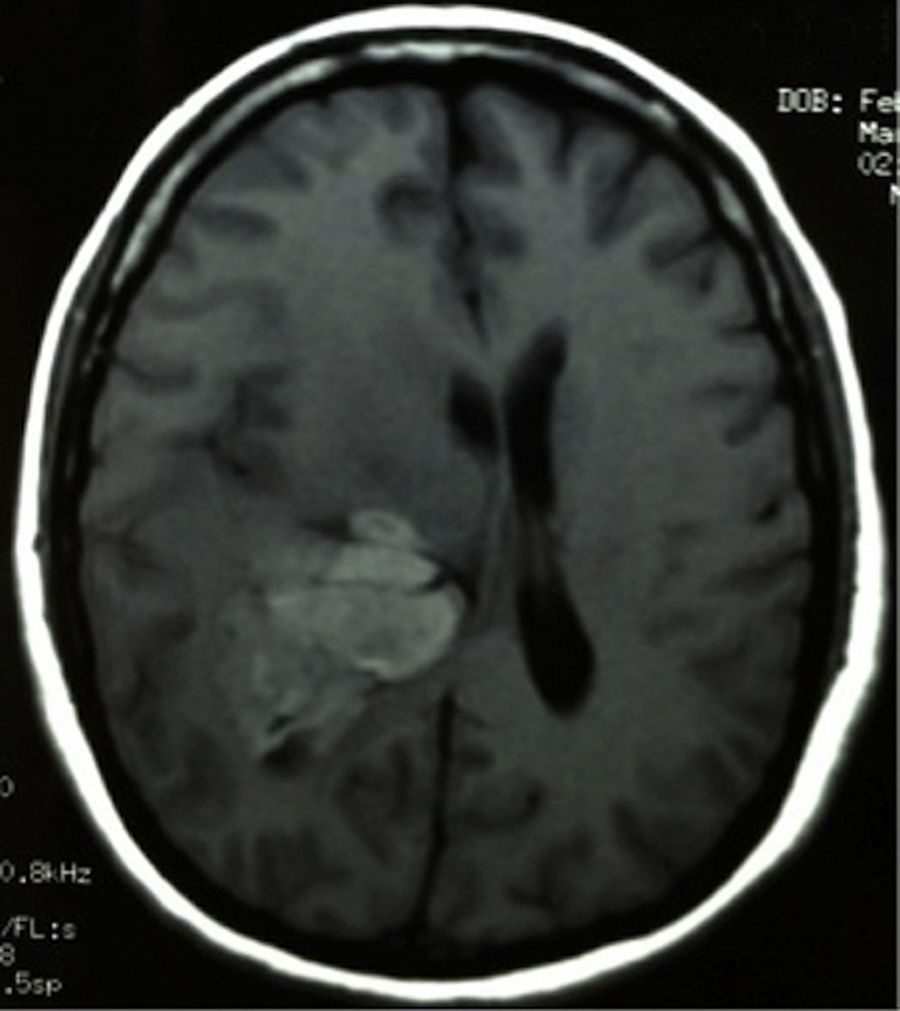
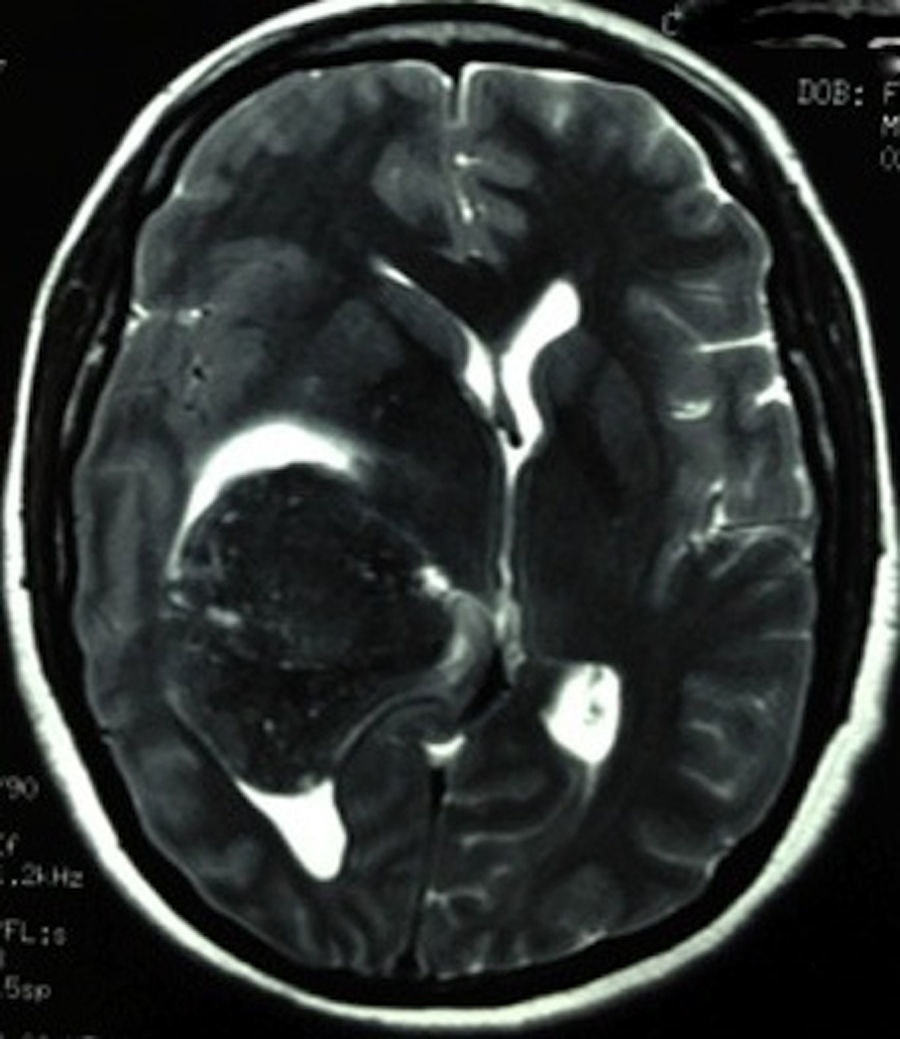
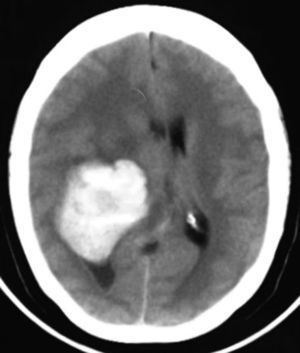
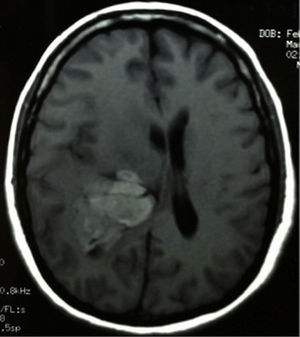
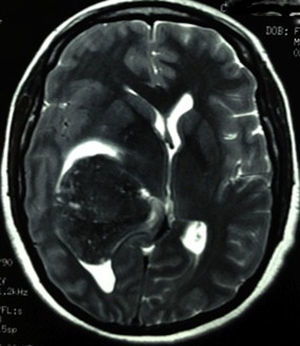
Clinical caseThis clinical case is of a female patient, 51 years of age, with no relevant history for her current condition. The symptoms described upon admission had one month of progression, with holocranial headache, mostly in the morning. Upon admission the patient mentioned intense holocranial headache, which ceased momentaneously with NSAID and was worsened with the Valsalva manoeuvre. She had difficulty in moving her left arm and leg, leading to ambulatory problems. In the physical examination we found: proper alertness, bradifrenia, 3/5 strength decrease in left arm and leg, and generalised hypertonia and hyperreflexia in the four limbs. Bilateral papillary oedema was observed in the ophthalmoscopy. The simple cranium axial tomography, carried out upon admission (Fig. 1), showed a hyperdense, well-defined image, right temporoparietal, apparently intraventricular, with collapse of the right lateral ventricle, mainly the atrium, and severe expansive effect. The lesion presented heterogeneous areas of density. It extends up to the right parietal lobe. Its medial limits are the right fornix callosum region, extending along the occipital horn. Compression of the callosum body is evident, as well as the fornix cross-sectional area on the right side. The left ventricular system is perceived as permeable, with no compensatory dilation. A magnetic resonance imaging study was also performed. In the axial T1 weighted image (Fig. 2), the craneodorsal extension of the tumour was observed; the tumour was intraaxial and isointense in relation to the cerebral parenchyma, and intratumoural hyperintense areas, with suspected intratumoural microhaemorrages. The expansive effect over the body of the fornix, the fornix cross-sectional area, the bulb of the corpus callosum and the splenium of the corpus callosum on the right side was evident, in addition to the complete collapse of the body and atrium of the right ventricular system and apparent occupation of the ipsilateral occipital horn. In the T1 weighted image with contrast material, the same hyperintense lesion was observed, homogeneously enhanced by the gadolinium. In the axial T2 weighted image (Fig. 3) a hypointense lesion was observed.
In her first hospital stay, a loop diuretic was administered, steroid, anticonvulsant and semi-Fowler's position. Also, an elective surgical treatment was proposed. The presumptive diagnosis was meningioma, and a differential diagnosis was performed in the location between choroid plexus papilloma, metastases and glioma for ventricular atrium tumours, without taking into consideration primary malignant melanoma of the central nervous system. On the fourth day she presented sudden deterioration of alertness and consciousness, dilated right pupil and 2/5 tetraparesis, with accentuation of the superior motor neuron syndrome she had upon admission, and clinical signs of right uncal herniation were added. Therefore, an emergency decompressive right temporal craniectomy was performed. Pressure was released on the craniotomised temporal fossa. She was admitted into the intensive care unit in the postsurgical stage.
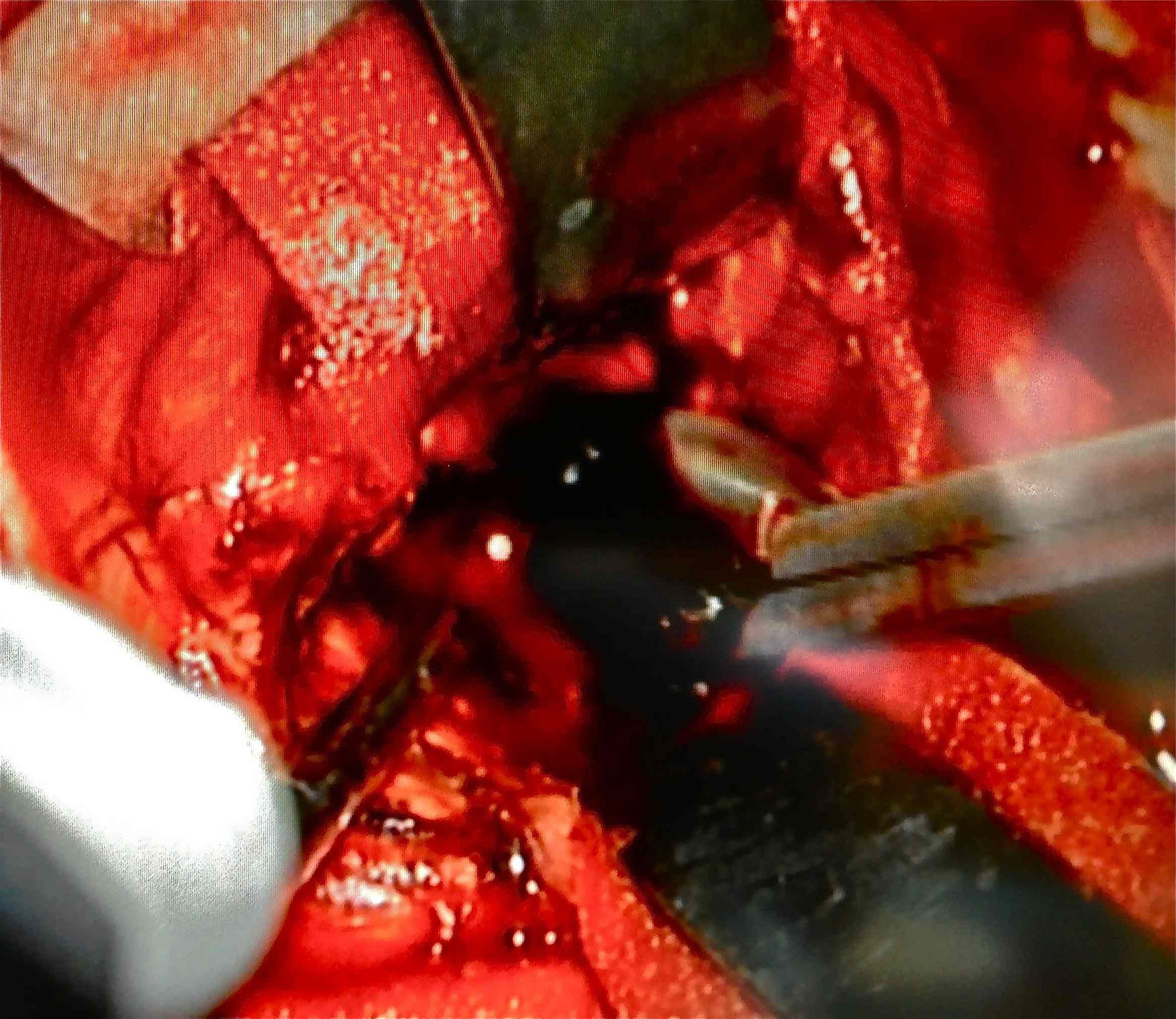
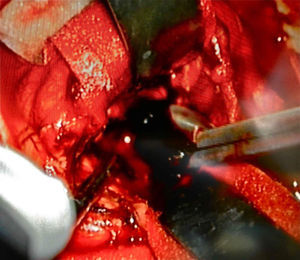
Afterwards, in better clinical condition and having improved the right pupillary diameter, on her first day in the intensive care unit, she went into surgery for a tumoral resection. A posterosuperior craniotomy was performed, as well as a superior intersulcal, right parietal approach. There was total resection of the tumour. The macroscopic characteristics of the tumour were soft neoplastic, aspirable, highly vascularised and bleeding, with vessel clusters, of low quality and difficult coagulation, parenquimatose and leptomeningeal infiltration. During surgery, an intraaxial lobar and extraventricular tumour was confirmed, with severe expansive effect on the right lateral ventricular system. The main characteristic of this tumour is that it was “black” in colour (Fig. 4).
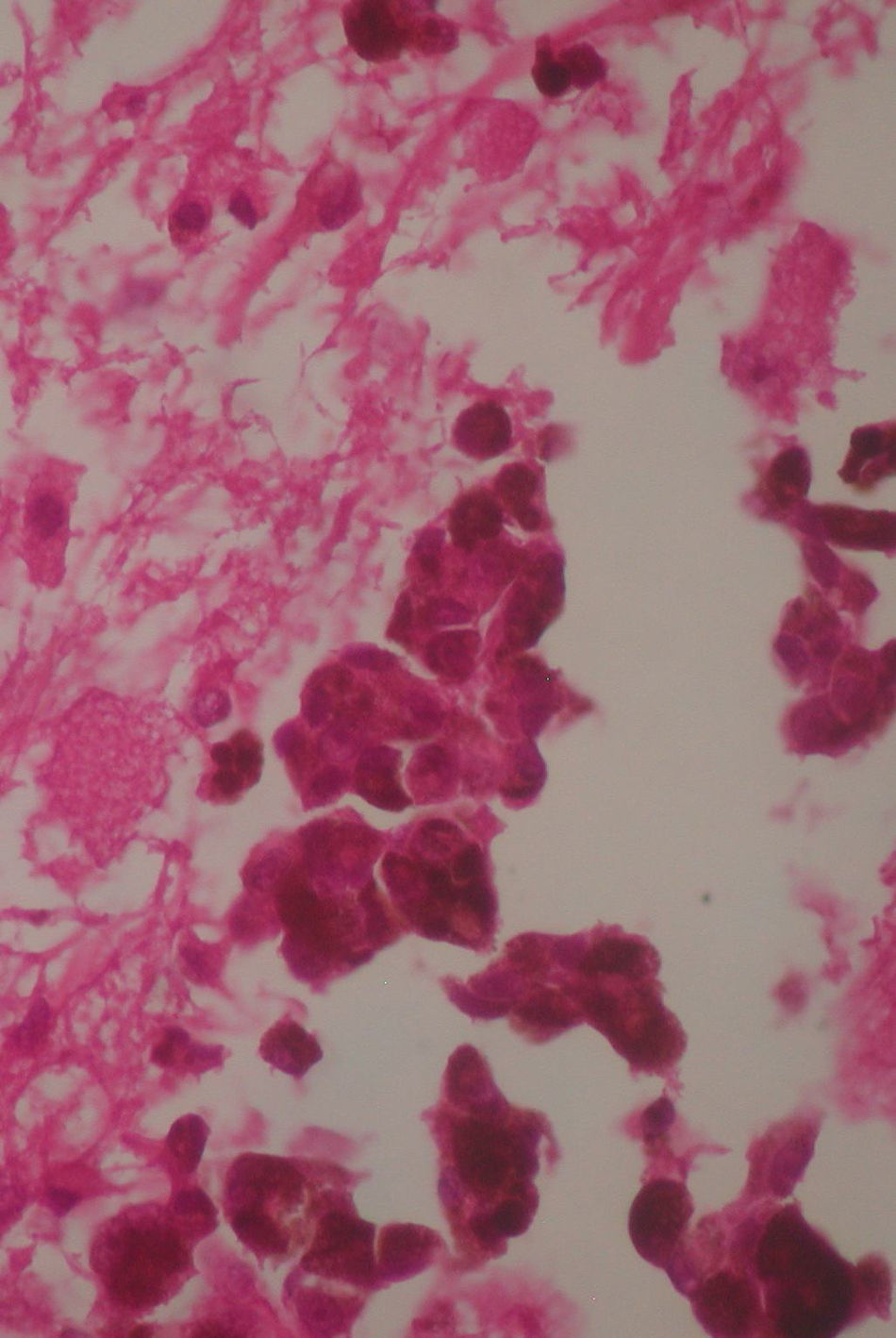
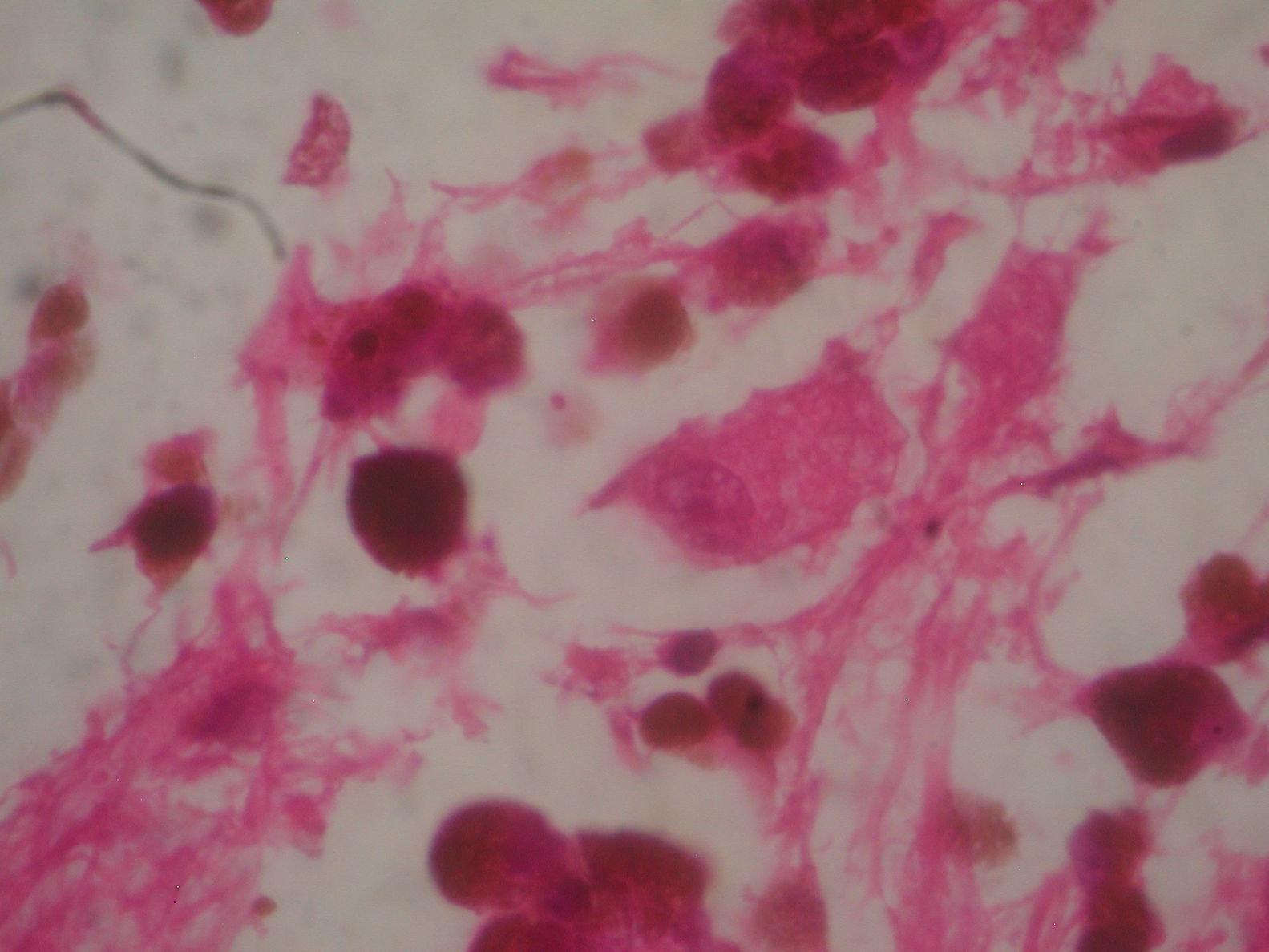
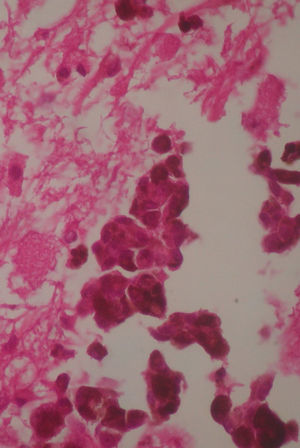
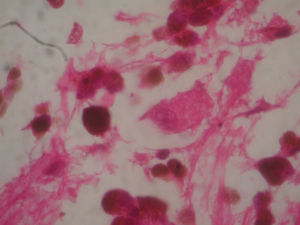
Two weeks after the surgery, a simple tomography of the head was taken, where midbrain was observed with no evidence of compression, an area of right temporoparietal encefalomalacia, secondary to the total tumour resection. The right lateral ventricular system with no collapse, permeable right occipital horn and atrium, with no transependimary communication, because the tumour was extraventricular and those structures were not injured during resection. The review of the histopathology sample revealed a highly cellular neoplasia, organised in nests of epithelial cells, with pleomorphic nuclei and a variable amount of cytoplasmic melanin, in addition to prominent nucleoli (Figs. 5 and 6), and positive for HMB45 and S-100 protein. With the postsurgical findings mentioned and the previous immunohistochemistry, a directed anamnesis was performed, regarding the family history of skin neoplasms in the patient, which were denied. The case was formalised by the dermatology and ophthalmology service, and an exhaustive review was carried out to exclude mucotegumentary lesions and primary retinal neoplastic lesions.
Having ruled out metastatic tumours of the central nervous system with the use of negative tumour markers and scintigram, the final conclusion was a diagnosis of primary malignant melanoma of the central nervous system. The patient was discharged 9 days after her postsurgical status, the oncology service determined total cerebral radiotherapy with 3.000centigray in 10 fractions, concomitant with temozolamide for 6 weeks. She is currently under oncology treatment.
DiscussionMelanosomic melanin is different from neuromelanin, which is only found on neurons.9 Leptomeningeal melanocytes are derived from the neural crest, consisting of a population of multipotential cells arising from the lateral margin of the neural tube during early embryo development, on day 22 of the embryogenesis, differentiating in leptomeningeal cells, glial cells, medullar adrenal cells and melanocytes.12 The higher concentration of melanocytes in the nervous system is in the pia mater, around the spinal cord and the high cervical cord.2,14 However, it is possible for the primary melanoma to arise from heterotopic masses of melanocytes in the central nervous system.2
Several theories have been proposed, such as: (1) Mesodermal theory, stating that the cell pigment arises from the mesoderm and reaches the brain or spine through pial blood vessels. (2) Ectodermal theory, where only epithelial cells can produce pigment; therefore, the primary malignant melanoma of the central nervous system derive from aberrant embrionary ectodermal cells. (3) Neurogenic theory: pigmented cells are derived from the neural crest and may cause tumours.3 Chromosome abnormalities have also been reported, such as the deletion or rupture in the long and short arm of chromosome 6, with a potential loss in the function of tumour suppressor genes in primary malignant melanoma of the central nervous system,15 and experimental models have been designed on mice showing the role of oncogenes16,17 and the appearance of primary malignant melanoma of the central nervous system on mice when the oncogene NRAS is expressed in melanocytes during embryogenesis through an endogenous NRAS promoter.18 It is known that this oncogene induces proliferation of melanocytes and congenital melanocytic lesions in humans. The acquisition of somatic mutations in the NRAS oncogene in melanocytes of the central nervous system is a risk factor predisposing to primary melanoma of the central nervous system in children.16 Other somatic mutations, such as that of the GNAQ gen, at codon 209, are frequent events in primary melanocytic neoplasms of the central nervous system, with relevance in the future immunotherapy of these tumours.17 Histopathology findings commonly found in the primary malignant melanoma of the central nervous system consist of pigmented tumour cells, cytologically atypical, with invasion to the central nervous system, pleomorphic nuclei; these are highly cellular neoplasms, organised in a syncytial pattern, epithelial, or irregular conglomerates of pigmented cells with leptomeningeal infiltration.12 However, a discouraging prognosis has been observed when these lesions are large, indurated, with deep invasion, epithelioid cellular pattern and marked mitotic activity.10,19
The peak of incidence of primary malignant melanoma of the central nervous system is in the fourth and fifth decade of life, and has a preponderance in males.2,4 Presentation symptoms include intracranial hypertension and hydrocephalus (43.2%), focal neurologic deficit due to cerebral and spinal compression effect (34.6%), subarachnoid haemorrhage (17.3%) and convulsions (11.1%). It may be found as solitary or in the context of neurocutaneous melanosis,12,20 and associations have been described between primary malignant melanoma and giant congenital nevo, although it is rare,21 and transoperatory findings between leptomeningeal melanosis and primary melanoma of the central nervous system.22 The beginning of symptoms may be acute, potentially associated to intratumoural haemorrhages, common in this tumour.15 70% of these lesions are black as surgical finding.12
Typical radiologic characteristics in cranial computed tomography are those of a hyperdense lesion, in images with no contrast material.2 In the magnetic resonance we observe a hyperintense tumour in T1 and hypointense in T2.2 This is due to the paramagnetic effect of stable free radicals of melanin.2,23 Odd electrons of melanin and protons of water produce a dipole-dipole interaction causing hyperintensity in T1 and hypointensity in T2.9 However, there are variants in relation to these findings: hypointensity in T1 and hyperintensity in T2, predominantly found in tumours with less than 10% of melanin in its cells,23 and in tumours with intralesional haemorrhage.4 The only imaging sign for diagnosis is leptomeningeal dissemination.2 Other primary tumours must be taken into consideration in the differential diagnosis with the primary malignant melanoma of the central nervous system, such as meningioma, meduloblastoma, astrocytoma, melanotic schwannoma, pituitary tumours, choroid plexum papillomas.2
There is much controversy if upon diagnosis of a melanoma this is primary of the central nervous system or metastatic; however, Hayward24 proposed in 1976 the following classifications: (1) Primary malignant melanoma of the central nervous system. (2) Secondary malignant melanoma of the central nervous system. (3) Variants of other intracranial tumours with melanin. For the purposes of classifying these tumours, the factors to look for are as follows: (a) with no finding of malignant melanoma outside the central nervous system; (b) cranial or spinal leptomeninges taken by the tumour; (c) intramedullary spinal lesions; (d) hydrocephalus; (e) tumour in the pineal or pituitary gland, and (f) solitary cerebral lesion.24 These criteria are still relevant and used currently, because the immunohistochemical diagnosis or differential histological diagnosis between the primary and secondary malignant melanoma of the central nervous system is difficult.25
The final diagnosis has to be performed by a pathologist, taking into consideration the result of immunohistochemical markers used, such as the S100 protein (expressed in cells originated in the neural crest), HMB45 (detected in melanosomas) and Melan.2 Generally this tumour is negative for glial fibrillary acidic protein (GFAP) and epithelial membrane antigen (EMA).26
Surgical treatment is the main option.2 The use of combined chemotherapy, such as decarbazine together with 1-(4-amino-2-methyl-5-pyrimidinyl) methyl-3-(2-chloroethyl) 3-nitrosourea hydrochloride (ACNU) and vincristine with OK 432 (picibanil), has proven promising results in neoplasms of metastatic and primary origin.2 In 2011 the FDA approved new medication for malignant metastatic melanoma of the central nervous system; a new immunotherapy with ipilimumab and vemurafenib, blocker of mitogen-activated protein (MAP) kinase,27 which may be used in cerebral primary malignant melanoma; however, there are still no clinical trials or clinical evidence to support its use as a sole therapy. Most authors agree that there is no defined therapy standard, and the efficacy of radiotherapy and chemotherapy on cerebral primary malignant melanoma is still controversial.28 The prognosis of cerebral primary malignant melanoma is better than that of metastatic malignant melanoma, especially with full resection,12 since, despite the aggressive multimodal management, in cerebral secondary malignant melanoma a survival of 3–6 months is reported,15 compared to that of patients with diagnosis of solitary primary malignant melanoma of the central nervous system, which is 20.7 months.11
ConclusionThe diagnosis of primary malignant melanoma of the central nervous system is a diagnosis by exclusion. However, it represents a challenge, since it first has to be suspected, and then try to exclude primary melanotic lesions with higher incidence and prevalence. The difficulty in establishing this diagnosis is secondary to the variants of behaviour presented in imaging studies of this tumour. In the case we presented, an intraventricular meningioma was suspected, and the signs observed in the magnetic resonance were not taken into consideration, in accordance with the malanotic pattern of iso/hyperintensity in T1 and hypointensity in T2.
The extremely low incidence of this tumour has not allowed the development of management and diagnostic guidelines; therefore, the prognosis is still discouraging in general, only supported by the fact of a resection as extensive as possible of the lesion. It is necessary to perform a deep study of the genesis of primary melanoma of the central nervous system in order to use specific therapeutic approaches with immunotherapy, chemotherapy and radiotherapy. Further, the differential diagnosis between primary and metastatic malignant melanoma of the central nervous system continues to be complex. Hayward's criteria are used to perform the differential diagnosis between these two diseases.28 An extracranial lesion has to be ruled out scrupulously before making a diagnosis of primary melanoma of the central nervous system.
Conflict of interestThe authors declare that there are no conflicts of interest.
We thank Doctors Ernesto Gómez Limón and Eduardo Zambrano Velarde, both affiliated to the neurosurgery service, for their guidance and opinion which were very important to prepare this article.
Please cite this article as: Quillo-Olvera J, Uribe-Olalde JS, Alcántara-Gómez LA, Rejón-Pérez JD, Palomera-Gómez HG. Melanoma maligno primario del sistema nervioso central: un reto diagnóstico. Cir Cir. 2015; 83: 129–134.