Between 1991 and 2013, 1000 liver transplantations were performed at Virgen del Rocio Hospital (Seville, Spain). A retrospective study was conducted, analyzing the characteristics of recipients and donors, indications, surgical technique, complications and survival in 2 different stages (1991–2002 vs 2003–2013) coinciding with the implementation of the MELD scale as a prioritization model. The most frequent indication was of hepatopathy of hepatocellular origin in 48.8%. There was a significant increase in the indications for hepatocarcinoma (8.6% and 24.1% P=.03), and the rate of retransplantation (5.9% vs 9.6%, P=.04). There was a change in the age of donation, going from 27.7 years in 1990 to 62.9 years in 2012 (P=.001). The percentage of patients who did not require blood transfusion doubled (6.16% vs 14.31%, P=.001). Survival of all patients after 1, 5 and 10 years was 77%, 63.5% and 51.3%, respectively.
Desde 1991 a 2013 se realizaron en el Hospital Virgen del Rocío 1.000 trasplantes hepáticos. Se realizó un estudio retrospectivo, en el que se analizaron las características de los donantes y los receptores, las indicaciones, la técnica quirúrgica, las complicaciones y la supervivencia en 2 etapas diferentes (1991-2002 vs 2003-2013), coincidiendo con la implantación del MELD como modelo de priorización. La indicación más frecuente fue la hepatopatía de origen hepatocelular en 48,8%. Hubo un incremento significativo en las indicaciones por hepatocarcinoma (8,6% y 24,1% p=0,03), y de la tasa retrasplantes (5,9% Vs 9,6%, p=0,04). Se apreció un cambio en la edad de donación, pasando de 27,7 años en 1990 a 62,9 años en 2012 (p=0,001). El porcentaje de pacientes que no precisaron transfusión de hemoderivados se duplicó (6,16 vs 14,31%, p=0,001). La supervivencia de todos los pacientes a uno, 5 y 10 años fue del 77, 63,5 y 51,3%, respectivamente.
Since the first liver transplantation (LT) was performed in 1963 by Starzl et al.,1 the management of terminal liver disease has undergone major changes, and LT is currently considered the treatment of choice for this disease.2,3
In the late eighties and early nineties, the development of surgical and anesthetic techniques, preservation solutions and new immunosuppressive agents, as well as improved candidate selection, led to an increase in the survival rates of LT recipients.4,5
Spain accounts for only 0.7% of the world's population. However, 10% of the world's LT are conducted in this country thanks to the higher donor rates per inhabitant. These donation rates and, therefore, LT make it possible to develop and maintain 24 transplant centers nationwide that carry out an important annual activity in the field of LT.6
The objective of this study was to analyze the first 1000 LT performed at the Virgen del Rocío Hospital in Seville, Spain, and evaluate the changes experienced over time with regard to donors, grafts, recipients and surgical technique, as well as the survival results obtained over the course of the first 22 years of the program.
MethodsBetween 1991 and 2013, the first 1000 LT were carried out at the Virgen del Rocío Hospital in Seville. The analysis was conducted throughout the first quarter of 2017, to ensure a follow-up of at least 3 years in all patients included and to be able to study the survival adequately. In order to evaluate the changes after the implementation of the MELD model, we divided the study period into 2 phases: the first from the initial LT (1991) until the year 2002, with a total of 406 LT; and the second from 2003 until LT number 1000 (2013), with 596 LT. During this period, the patient variables were collected in a prospective database: demographic aspects of the donor and recipient, indication for LT, ischemia time, type of anastomosis, complications, retransplantation (reLT), hospital stay, readmissions and mortality.
An observational, descriptive study was carried out for the most part, although we analyzed the changes produced between the stages in a retrospective manner. The categorical variables are expressed in percentages. Continuous data have been reported as mean±SD or median (range). To test the hypothesis, the chi-squared test, Student's t test and ANOVA were used if the conditions for their application were met; or its non-parametric alternatives if they were not met. Survival estimates were calculated using the Kaplan–Meier method, and the comparison of results between the groups was performed using the log-rank test. A P-value less than .05 was considered statistically significant. The statistical analysis was completed using the SPSS® 24.0 statistical package (SPSS, Inc., Chicago, IL, USA).
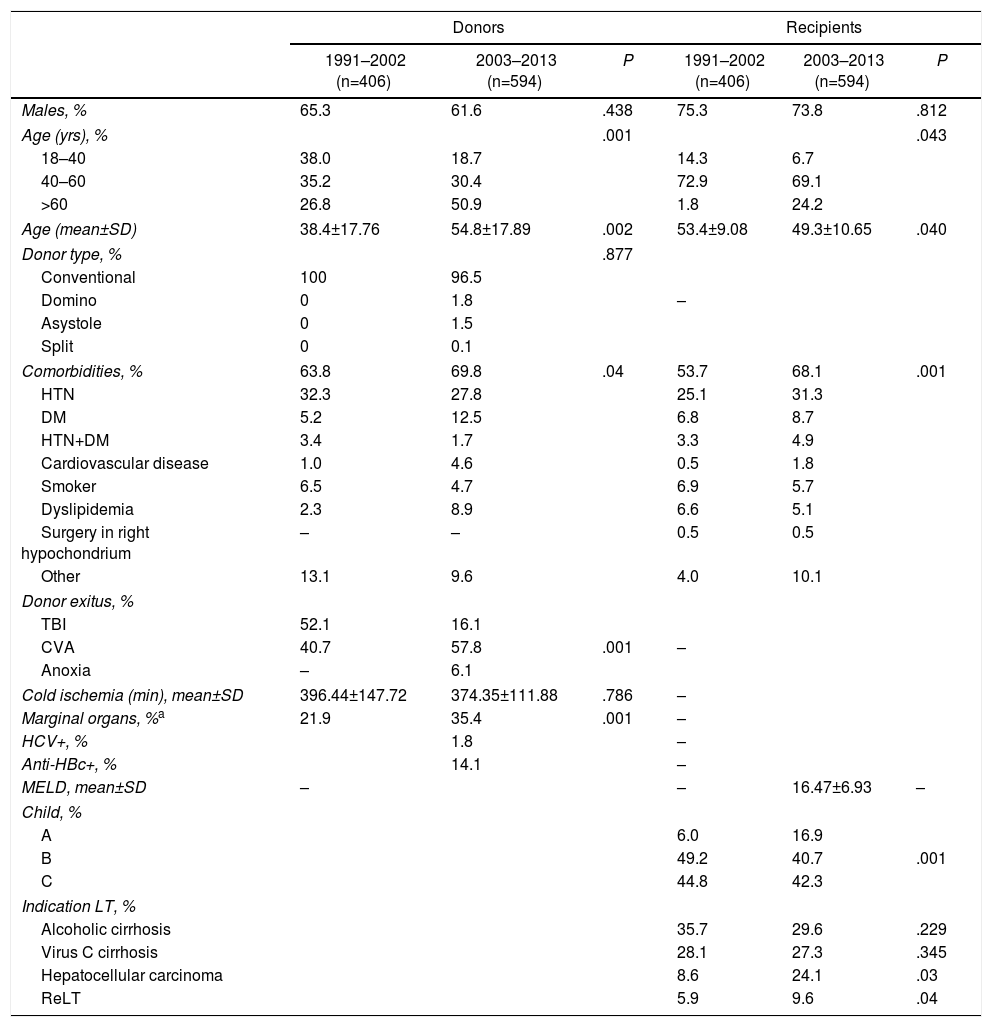
ResultsDonorsIn the first stage, donors came from all over Spain as a result of the national allocation of donors; after 2003, 88.2% of the grafts came from donors in Andalusia, 44.4% of which belonged to the Seville healthcare sector (Seville and Huelva), 43.8% from the other 3 Andalusian sectors, 11.6% from the rest of the country and 2 donors from other European medical centers. The type of donation was mainly complete grafts from brain dead donors (98.9%), although 11 were complete grafts from living donors affected by familial amyloid polyneuropathy (FAP). A change in donor age was observed during these 22 years, increasing from 27.7 years in 1990 to 62.9 years in 2012 (P=.001). The group of donors > 75 years was significantly higher in the second stage, 0.1% in the first stage (one donor) and 6.6% in the second stage (P=.0001), with the majority in the last 5 years (50 donors) (Table 1).
Characteristics of Donors and Recipients.
| Donors | Recipients | |||||
|---|---|---|---|---|---|---|
| 1991–2002 (n=406) | 2003–2013 (n=594) | P | 1991–2002 (n=406) | 2003–2013 (n=594) | P | |
| Males, % | 65.3 | 61.6 | .438 | 75.3 | 73.8 | .812 |
| Age (yrs), % | .001 | .043 | ||||
| 18–40 | 38.0 | 18.7 | 14.3 | 6.7 | ||
| 40–60 | 35.2 | 30.4 | 72.9 | 69.1 | ||
| >60 | 26.8 | 50.9 | 1.8 | 24.2 | ||
| Age (mean±SD) | 38.4±17.76 | 54.8±17.89 | .002 | 53.4±9.08 | 49.3±10.65 | .040 |
| Donor type, % | .877 | |||||
| Conventional | 100 | 96.5 | ||||
| Domino | 0 | 1.8 | – | |||
| Asystole | 0 | 1.5 | ||||
| Split | 0 | 0.1 | ||||
| Comorbidities, % | 63.8 | 69.8 | .04 | 53.7 | 68.1 | .001 |
| HTN | 32.3 | 27.8 | 25.1 | 31.3 | ||
| DM | 5.2 | 12.5 | 6.8 | 8.7 | ||
| HTN+DM | 3.4 | 1.7 | 3.3 | 4.9 | ||
| Cardiovascular disease | 1.0 | 4.6 | 0.5 | 1.8 | ||
| Smoker | 6.5 | 4.7 | 6.9 | 5.7 | ||
| Dyslipidemia | 2.3 | 8.9 | 6.6 | 5.1 | ||
| Surgery in right hypochondrium | – | – | 0.5 | 0.5 | ||
| Other | 13.1 | 9.6 | 4.0 | 10.1 | ||
| Donor exitus, % | ||||||
| TBI | 52.1 | 16.1 | ||||
| CVA | 40.7 | 57.8 | .001 | – | ||
| Anoxia | – | 6.1 | ||||
| Cold ischemia (min), mean±SD | 396.44±147.72 | 374.35±111.88 | .786 | – | ||
| Marginal organs, %a | 21.9 | 35.4 | .001 | – | ||
| HCV+, % | 1.8 | – | ||||
| Anti-HBc+, % | 14.1 | – | ||||
| MELD, mean±SD | – | – | 16.47±6.93 | – | ||
| Child, % | ||||||
| A | 6.0 | 16.9 | ||||
| B | 49.2 | 40.7 | .001 | |||
| C | 44.8 | 42.3 | ||||
| Indication LT, % | ||||||
| Alcoholic cirrhosis | 35.7 | 29.6 | .229 | |||
| Virus C cirrhosis | 28.1 | 27.3 | .345 | |||
| Hepatocellular carcinoma | 8.6 | 24.1 | .03 | |||
| ReLT | 5.9 | 9.6 | .04 | |||
CVA: cerebrovascular accident; SD: standard deviation; DM: diabetes mellitus; HTN: hypertension; ReLH: liver re-retransplantation; IQR: interquartile range; TBI: traumatic brain injury; LT: liver transplantation; HCV: hepatitis C virus.
The classic Starzl extraction technique was used in 61.6% of the donors, the Nakazato rapid technique in 37.2% and in the remaining 1.2% different techniques were used (domino, split).
RecipientsThe first 1000 LT were carried out in 916 patients: 17 received multiple-organ transplants (15 liver-kidney, one liver-pancreas and one liver-heart), 11 received complete grafts from living donors with FAP, and one patient received a reduced graft from an adult donor. The remaining 84 LT were reLT.
The median age of the LT recipients during the period of analysis was 53.5 years (Q1: 46, Q3: 49), with an observed increase in the age of the LT recipients in the post-2003 phase (49 vs 53 years, P=.043). Ages ranged between 14 and 69 years, and 71.3% of the patients belonged to the 40–59 age group. The age limit of our group is 70 years for patients without comorbidities (Table 1).
The most frequent indications for LT in the series were hepatopathies of hepatocellular origin (alcoholic cirrhosis, hepatitis B and C virus cirrhosis) in 488 cases (48.8%), followed by hepatocellular carcinoma (HCC) in 178 (17.8%), reLT in its different acute and elective modalities in 84 cases (8.4%) and cholestatic diseases in 36 patients (3.6%). There were no differences between the stages, except in the case of reLT and HCC.
95.5% of the LT were performed electively, and 4.5% were urgent for different indications: fulminant hepatic failure (2.2%), liver trauma (0.2%) and acute reLT (1.9%), with no differences between the two phases.
The prevalence of C virus was 27.6% in the total of the series, without no differences observed between the 2 stages analyzed (114 [28.08%] until 2002 and 162 [27.3%] after 2003, P=.23). There were no differences in survival by stages in transplants for C virus in patients (P=.096) or in grafts (P=.170). On the other hand, there was a significant increase in LT indications for HCC in the second stage (8.6% and 24.1%, P=.03). There was also an observed increase in the prevalence of reLT in the second stage (5.9% vs 9.6%, P=.04) (Table 1).
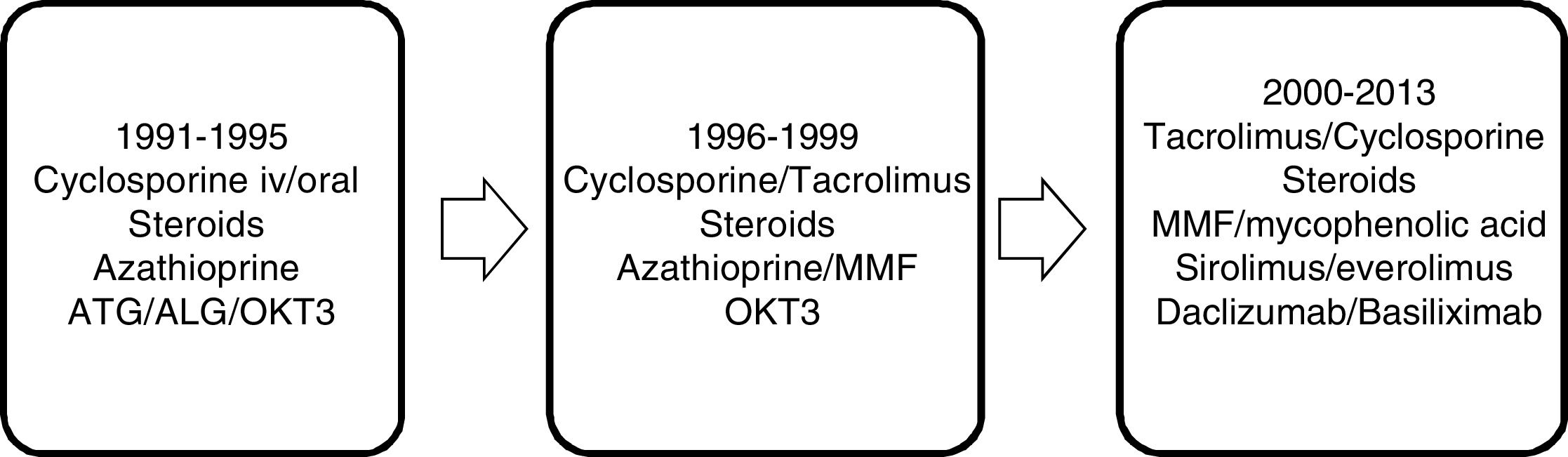
Immunosuppressive therapy has changed over the years. From the beginning of the program until 1995, the immunosuppression protocol was based on cyclosporine and steroids, modulated by azathioprine, with an acute rejection rate (clinical-analytical) of up to 65%. From 1995 to 1999, with the introduction of tacrolimus, this progressively replaced cyclosporine as the main immunosuppressant; the same was true with azathioprine in favor of mycophenolate mofetil, registering an acute rejection rate (clinical-analytical) of 43%. In the period from 2000 to 2013, the main immunosuppressants were tacrolimus and mycophenolic acid, and in recent years m-Tor inhibitors and monoclonal antibodies have been introduced, with an acute rejection rate confirmed by biopsy of 19.9% (Fig. 1).
The surgical procedure used during hepatectomy in the recipient was the classic technique with retrohepatic vena cava excision1 in the first 130 LT. Subsequently, this technique was used sporadically in 11 LT for different reasons (neoplasms proximal to the vena cava or technical problems). After the first piggyback in December 1992, this procedure was performed in 85.9% of LT, even in 10 FAP recipients whose grafts were used to perform domino transplants (all of which belonged to the second stage).
In 9.1% of the LT in the series, portacaval shunt was performed, representing 6.4% of the LT in the first stage and 10.9% in the second stage (P=.014), indicated in cases of poor collateral circulation (FAP or acute liver failure, for example). The drainage of the suprahepatic veins has evolved throughout the series from an anastomosis of 2 (middle-left) in 28.3% of the cases in the beginning to performing it later with a plasty between the 3 suprahepatic veins in 57.4% of LT, using exceptionally a side-to-side cavo-caval anastomosis in 11 cases.
The next vascular anastomosis performed in 99% of the LT in the series was between the portal vein of the donor and the recipient. Portal thrombosis (partial or complete) was present in 13% (125) of the recipients at the time of implantation (12.0% [48] vs 12.9% [77] between the first and second stage, respectively; P=.804), in different degrees, and thrombectomy was performed in most cases. In 11 cases, it was not possible to perform the thrombectomy or the portal flow was not adequate, requiring other portal revascularization techniques, such as anastomosis with choledochal varices in 3 cases, cavo-portal transposition in 2 cases, reno-portal anastomosis in one case, a 10-mm Gore-Tex® ringed prosthesis in one case and in 4 cases the interposition of a vascular graft from the donor was necessary, while portal arterialization of the portal vein was not necessary in any case.
Arterial reconstruction was performed in 98.6% of arterial effluents of the hepatic artery of the recipient, and its most desirable location was in the bifurcation of the gastroduodenal artery or, less frequently, the bifurcation of the right-left hepatic artery. The use of other arterial reconstruction methods was exceptional: revascularization with the splenic artery in 6 cases, interposition of arterial grafts from the donor in 4 cases and anastomosis directly to the supraceliac aorta in 4 cases. Arterial thrombosis occurred in 41 cases (3.5% in the first stage and 4.4% in the second stage, P=.819).
The technique of biliary reconstruction most commonly used in both stages was the end-to-end choledocho-choledochal anastomosis in 92.5% of LT, with a bile duct T-tube (Kehr) in 55.4% (92.8% vs 29.8%, P<.001), being reserved in the second stage for those cases of differences in caliber or technical difficulties in the reconstruction. Bilioenteric shunts were performed in 6.5% of the LT in the series, grouped mostly in reLT and primary sclerosing cholangitis.
The median consumption of red cell concentrates between both stages changed from 7 (0–7) to 4 (0–10)units, plasma from 11 (6–19) to 0 (0–4)units and platelets from 5.5 (0–10) to 0 (0–2)units. The percentage of LT not requiring transfusion of blood products doubled in the second stage (6.16% vs 14.31%, P=.001).
In 91.7% of LT in the series, there were no vascular complications and in 8.3% there was a venous or arterial complication, most notably the presence of 4.1% (41) of arterial thrombosis, 1.2% (12) of portal thrombosis and one vena cava thrombosis; no significant differences were found between the 2 stages analyzed. In all cases of arterial thrombosis, the treatment was surgical, presenting graft dysfunction in 7.5%, segmental ischemia in 5% and hypertransaminasemia in 7.5%. The management of portal thrombosis was surgical when it was complete, while partial thrombosis was treated conservatively, with hepatic dysfunction appearing in 8.3% and hypertransaminasemia in 16.7%.
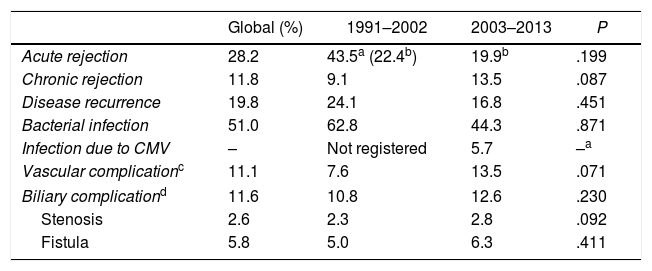
Biliary complications occurred in 11.6% of the LT in the analyzed series: 10.8% until 2002, and increasing to 12.6% in the second stage (P=.230). Major clinical events (Clavien–Dindo >3) are recorded in Table 2.
Major Clinical Events During Follow-up (25yrs).
| Global (%) | 1991–2002 | 2003–2013 | P | |
|---|---|---|---|---|
| Acute rejection | 28.2 | 43.5a (22.4b) | 19.9b | .199 |
| Chronic rejection | 11.8 | 9.1 | 13.5 | .087 |
| Disease recurrence | 19.8 | 24.1 | 16.8 | .451 |
| Bacterial infection | 51.0 | 62.8 | 44.3 | .871 |
| Infection due to CMV | – | Not registered | 5.7 | –a |
| Vascular complicationc | 11.1 | 7.6 | 13.5 | .071 |
| Biliary complicationd | 11.6 | 10.8 | 12.6 | .230 |
| Stenosis | 2.6 | 2.3 | 2.8 | .092 |
| Fistula | 5.8 | 5.0 | 6.3 | .411 |
CMV: cytomegalovirus.
Mean hospital stay in the first stage was 23.48±14.81 days, and in the second stage, from 21.99±18.77 (P=.112). The percentage of re-admissions was 18.3% in the first stage and 16.8% in the second stage (P=.776), and the most frequent cause of global re-entry was an unscheduled liver biopsy (18.4%), withdrawal of the T-tube (15.5%) and infectious causes (8.1%).
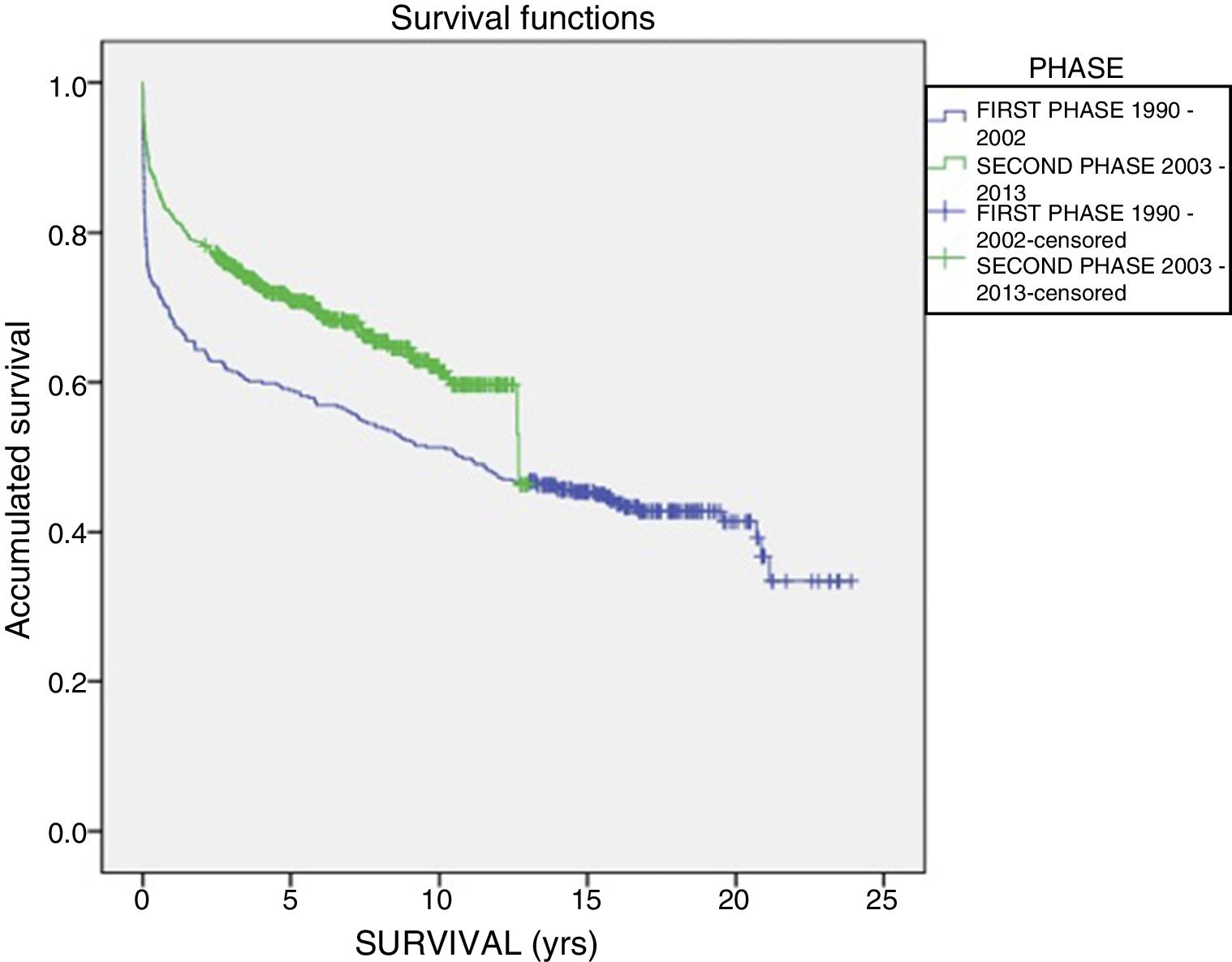
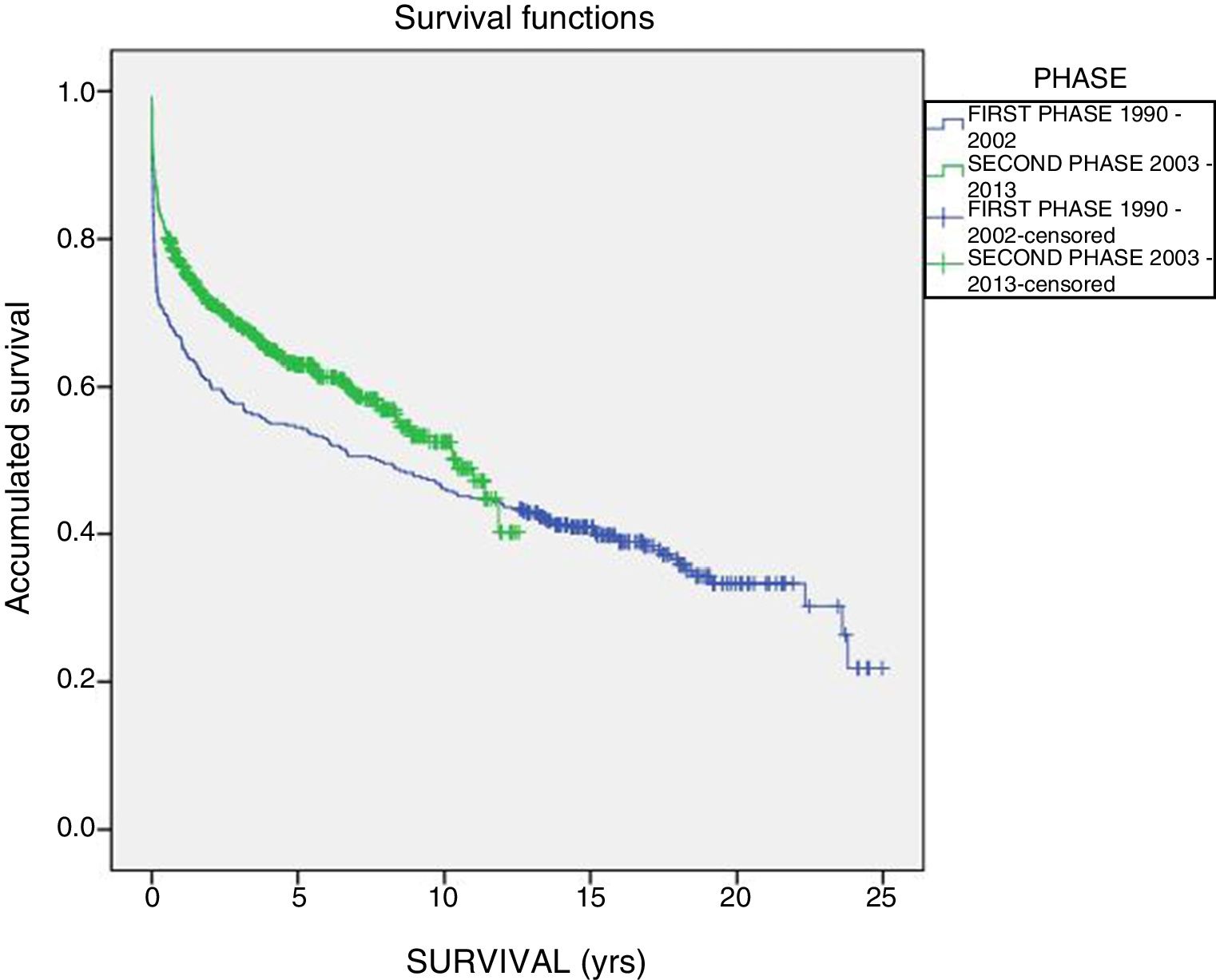
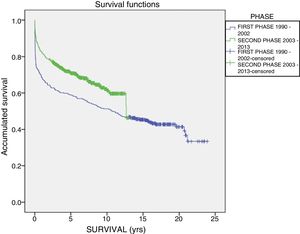
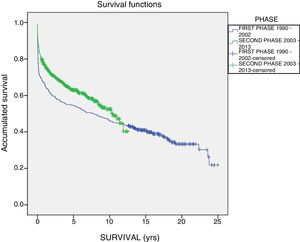
The one-year, 5-year and 10-year survival rates of all patients in the series (916 LT) were 77%, 63.5% and 51.3%, respectively; by stages, these rates were 68.5%, 58%, 6% and 51% in the first stage compared to 82%, 70.8% and 61.4% in the second stage (P<.001) (Fig. 2). The one-year, 5-year and 10-year survival rates of the grafts in the series (1000 LT) was 72%, 60.8% and 52.5%, respectively; by stages, these rates were 65.7%, 54.1% and 45.4% in the first stage compared to 76.4%, 65.5% and 57.1% in the second stage (P<.001) (Fig. 3). In the multivariate analysis, independent factors to predict mortality included: recipient age (OR 1.12, 95% CI 1.02–1.22, P=.015), the need for reLT (OR 456.33, 95% CI 57.49–3622, P<.001) and primary malfunction (OR 1.36, 95% CI 1.05–1.56, P=.015). Malfunction (hepatic functional alteration in the first 48h, requiring reLT or causing death) occurred in 44 cases, 33 of which progressed to exitus and 11 required urgent reLT.
The overall number of deaths was 426 (232 in the first stage and 194 in the second stage, P=.004). The most frequent causes of death were: bacterial sepsis (20.6% [48] vs 12.2% [24], P=.001), recurrence of the primary disease (9.9% [23] vs 10.2% [20], P=.553), de novo tumors (10.7% [25] vs 7.1% [14], P=.045), multiple-organ failure (5.6% [13] vs 9.2% [18], P=.091), cardiac complications (7.3% [17] vs 4.1% [8], P=.001) and hemorrhage (5.6% [13] vs 6.1% [12], P=.887).
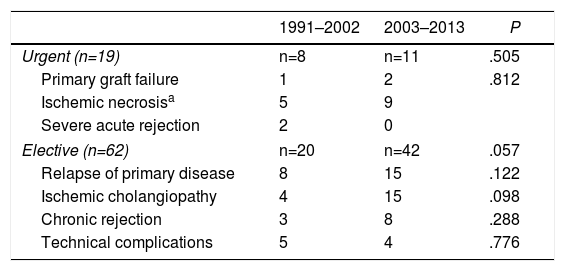
Urgent reLT was performed (<7 days after the primary LT) in 19 cases and elective in 62, with no statistical differences (P=.27). However, clinically, long-term survival (more than 10 years) was 77.9% for urgent cases and 64.5% for non-urgent cases. Recurrence of the primary disease was the main cause of elective reLT and constituted 37.11%, followed by ischemic cholangiopathy with 19 patients (30.64%), which surpassed chronic graft rejection with 11 cases (17.74%), and technical complications with 9 cases (14.51%). Table 3 shows the causes in the 2 phases.
Causes of Liver Retransplantation by Phases.
| 1991–2002 | 2003–2013 | P | |
|---|---|---|---|
| Urgent (n=19) | n=8 | n=11 | .505 |
| Primary graft failure | 1 | 2 | .812 |
| Ischemic necrosisa | 5 | 9 | |
| Severe acute rejection | 2 | 0 | |
| Elective (n=62) | n=20 | n=42 | .057 |
| Relapse of primary disease | 8 | 15 | .122 |
| Ischemic cholangiopathy | 4 | 15 | .098 |
| Chronic rejection | 3 | 8 | .288 |
| Technical complications | 5 | 4 | .776 |
The 3 liver retransplantations missing to reach 84 in total were second liver retransplantations.
Our progressively growing activity in LT, now surpassing 1000 transplantations, offers a broad and somewhat historical view of the evolution of this type of surgical programs. Without trying to perform an exhaustive analysis that could yield categorical conclusions, this general overview allows us to consider the improvements developed over time, as well as new issues to explore and improve.
DonorsDuring the initial years of the LT program at our hospital, advanced donor age was a contraindication for organ donation. That is why in the first stage the donor age was lower than after 2003, when we began to use grafts that had previously been considered too old; currently, donor age is no longer considered a limitation for liver donation.7,8 As a consequence of these events, in the series of the Spanish Hepatic Transplant Registry6 during the last 5 years, the number of elderly donors (>75 years old) grew considerably from a low 0.2% nation-wide in the beginning (1984–1995) to 19.1% in the years 2011–2013.
The surgical technique used in the donors, for the most part, was that described by Starzl et al. in 1984,9 which involves the careful dissection of the hepatic hilum and double aortic and splenic-portal cannulation. Only in cases of donor hemodynamic instability and in order to reduce surgical time to avoid graft loss was the rapid en bloc extraction technique by Nakazato et al.10 used, in which, without previous dissection of the hepatic hilum, rapid aortic and inferior mesenteric vein cannulation is carried out.
RecipientsIt seems evident that the consolidation of an LT team, together with an adequate volume, improved indication and exploration of grafts not contemplated in the past, has enabled us to increase the age for the indication of LT over the years. This fact is seen at the national level according to data issued by the Spanish Liver Transplant Registry,6 where, in the initial years of LT activity (1984–1995), 11.2% of these were performed in people over the age of 60, and in the 2002–2013 period, the percentage of people over 60 rose to approximately 30%.8
After performing 1000 LT, the indication of certain conditions, such as HCC (without a significant change in the indications of LT), has increased. This is probably influenced by the improvement in detection techniques during the follow-up of patients with chronic liver disease, as well as an optimization of percutaneous radiofrequency and chemoembolization techniques that enable us to rescue patients who were previously not candidates for LT, and patents affected by HCC have become included in the indications for LT. On the other hand, there has been an increase in the rate of reLT in the second phase, attributed to a greater indication of elective reLT due to recurrences of primary disease (HCV relapse and autoimmune diseases) and the appearance of chronic rejection of LT “accumulated” in the first stage.
Regarding the surgical technique for the implant, after the publication of the benefits of hepatectomy with preservation of the retrohepatic vena cava (piggyback),11 its use among LT surgeons became systematized and showed great benefits. In our series of 1000 LT, this technique was performed for the first time in LT number 9 and was consolidated as the standard surgical technique from LT number 25 on. In our series, it is difficult to analyze the benefits of this technique, since only a few LT were conducted with other techniques during a very early period. However, the improved hemodynamic stability, shorter surgical times and lower consumption of blood products have been widely documented.12
The use of the portacaval shunt during the anhepatic phase in order to minimize the adverse effects of splanchnic collapse was used in our series for the first time in 2002, reducing the dissection time of the retrohepatic vena cava and an improvement of the different hemodynamic patterns by preserving cardiac output.13 Subsequently, the indication was reserved for recipients who did not tolerate portal venous clamping or in the absence of evident portal hypertension.
During the first study phase, in practically all LT the cholecystic/bile duct anastomosis of choice was end-to-end choledocho-choledochal, and a Kehr bile duct tube was used. In the second phase, after some evidence warned that the systematic use of these devices did not provide any benefits,14 their use was reduced and reserved for cases with mismatched sizes, reLT or previous alterations of the bile duct. Biliodigestive diversions were indicated in patients with primary sclerosing cholangitis, reLT, or technical difficulties that prevented the primary anastomosis of the bile duct. Despite a decrease in the use of bile duct tubes, biliary complications remained stable, data that partially confirm the results described by Rolles et al.15 in 1994 in a series of 120 LT.
One of the great achievements comes from the decrease in the use of blood products in the last phase thanks to restrictive therapies, incorporation of the mobile laboratory in the surgical area in 2010, application of thromboelastometry, use of fibrinogen and improved patient selection. The use of the mobile laboratory has achieved intensive monitoring, with a reduction, in the second stage, to the minimum in terms of plasma and platelets, and also a lower use of packed red blood cells. As a result, the number of LT without any need for transfusions has doubled.
The appearance of acute rejection has remained stable throughout the entire period, although in the first stage the data were more difficult to evaluate due to less access at our hospital to early liver biopsy, with some cases of acute rejection after detecting clinical-analytical alterations. This situation was largely resolved in the second stage with diagnoses made exclusively by liver biopsy and the ability to correlate these clinical alterations with the histopathology result, which remains the gold standard.15,16 For chronic rejection, the slight percentage increase may be due to the transplants accumulated from the previous stage, as well as to a greater availability of liver biopsy.
The change in immunosuppression, led by the use of tacrolimus in daily doses, has possibly been another factor that has contributed to the results presented, taking into account that the clinical data (renal function, procarcinogenesis, adverse effects) were not the objective of this study.
Regarding the improved one-year survival, in the last stage this was attributed to LT program improvements, such as having a stable team of trained surgeons, better patient selection, improved anesthetic management and transfusion policy, as well as more individualized immunosuppression. In national6 and international series17,18 analyzing the survival results of patients and grafts, the evolution has been similar, especially in recent years. The accumulated experience and protocolization of the process have led to improved patient survival, in spite of an increasingly common use of older donors, use of partial grafts, some cases of more deteriorated recipients and the increasing indication of reLT of patients from previous stages because of deterioration of the grafts due to previous disease recurrences, among other causes.
As for reLT, the greatest decrease in survival occurs during the first year after reLT and it is in the first 3 months when most patients die. However, no significant differences in the overall survival of reLT have been observed between the two stages. There seems to be a better long-term survival of reLT performed urgently, although without statistical significance. This decrease in survival in the early post-reLT (3 months) is referenced in Berumen and Hemming's study from 2017.19
ConclusionsThe LT activity of the Virgen del Rocío Hospital has undergone an evolution similar to that experienced by the rest of the country's units. Its activity has become consolidated and now includes a majority of hepatocellular liver diseases among the indications for LT. There have been no changes in the prevalence of patients with HCV+ serology in the second stage; meanwhile, the indications for hepatocellular carcinoma and elective retransplantation have increased.
The donation criteria have evolved over time, so the donation age in the second phase has tripled. Vascular reconstruction, however, has remained unchanged throughout the program, and the use of biliary tutors decreased in the second phase. The consumption of blood products also dropped significantly in the second stage.
A greater loss of patients and grafts has been identified in the first month after the liver transplantation in the first phase, with significant improvements in survival rates in the last phase due to the decrease in mortality in the first month.
Conflict of InterestsThe authors have no conflicts of interest to declare for the publication of this study.
Juan Serrano, Ángel Bernardos, Inmaculada García, Felipe Pareja, Carmen Cepeda, Juan Manuel Pascasio, Teresa Ferrer, Jose Manuel Sousa, Manuel Sayago, Elisa Cordero, Álvaro Giráldez, Rafael Hinojosa and Francisco Porras.
Please cite this article as: Tinoco González J, Álamo Martínez JM, Bernal Bellido C, Suárez Artacho G, Marín Gómez LM, Barrera-Pulido L, et al. 1000 trasplantes hepáticos consecutivos. Análisis descriptivo y evolución de un centro. Cir Esp. 2018;96:268–275.