The glomus body is a specialized arteriovenous anastomosis that controls thermoregulation through blood flow. These bodies are located in the skin, subungual regions and, less frequently, in the subcutaneous tissue.1 Glomus tumors are rare benign vascular neoplasms derived from these bodies. They belong to a family of tumors that are characterized by their composition: glomus cells, blood vessels and smooth muscle in different proportions.2 Classically, they are located under the nails, but they have also been reported in the respiratory tract, mediastinum and lungs, gastrointestinal tract and genitourinary system. Most glomus tumors of the digestive system are located in the stomach, and their location in the hepatobiliary tract is extremely rare.3
Glomus tumors occur most frequently in young adults (20–40 years of age). Their incidence is similar in both sexes, except for digital lesions, which are more prevalent in women,3 and gastrointestinal tract tumors, which are more frequent in older women. These tumors are usually solitary and manifest as painful nodules.1
We present the case of a 53-year-old male in good general condition, whose only medical history was an open appendectomy and resection of a basal cell carcinoma from the nose. He reported non-specific pain in the right hypochondrium, which was not accompanied by abdominal discomfort or changes in bowel habit. Upon examination, mild hepatomegaly was palpable with pain upon deep palpation in the epigastrium.
Laboratory studies for tumor markers (CEA, CA 19.9, CA 15.3, PSA and alpha-fetoprotein) were negative.
A CT scan demonstrated a mass in the left liver lobe measuring 65×80mm that was heterogeneous, composed of multiple hypointense lesions and had a clustered appearance. An ultrasound-guided biopsy provided the diagnosis of a poorly differentiated mesenchymal neoplasm. The study was completed with positron emission tomography (PET) and somatostatin receptor SPECT/CT (OctreoScan®); no other tumor foci were observed.
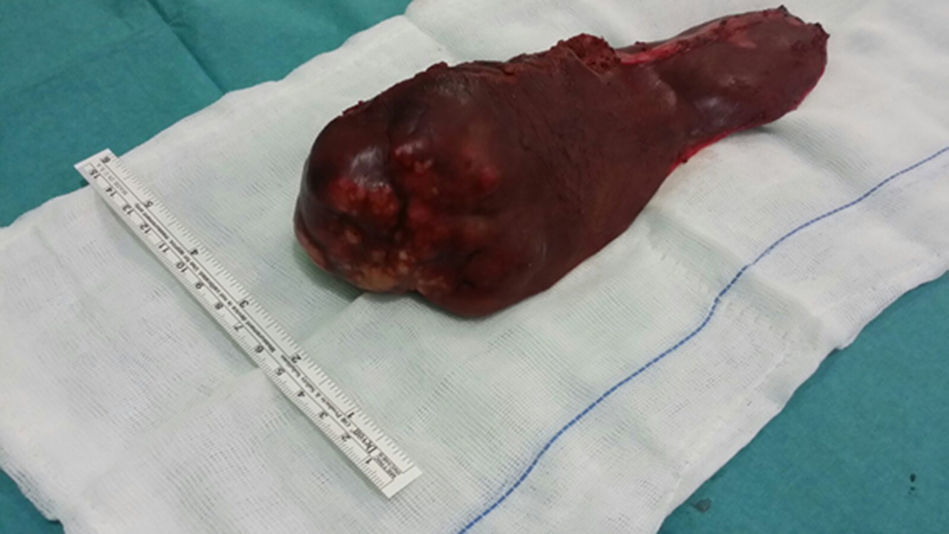
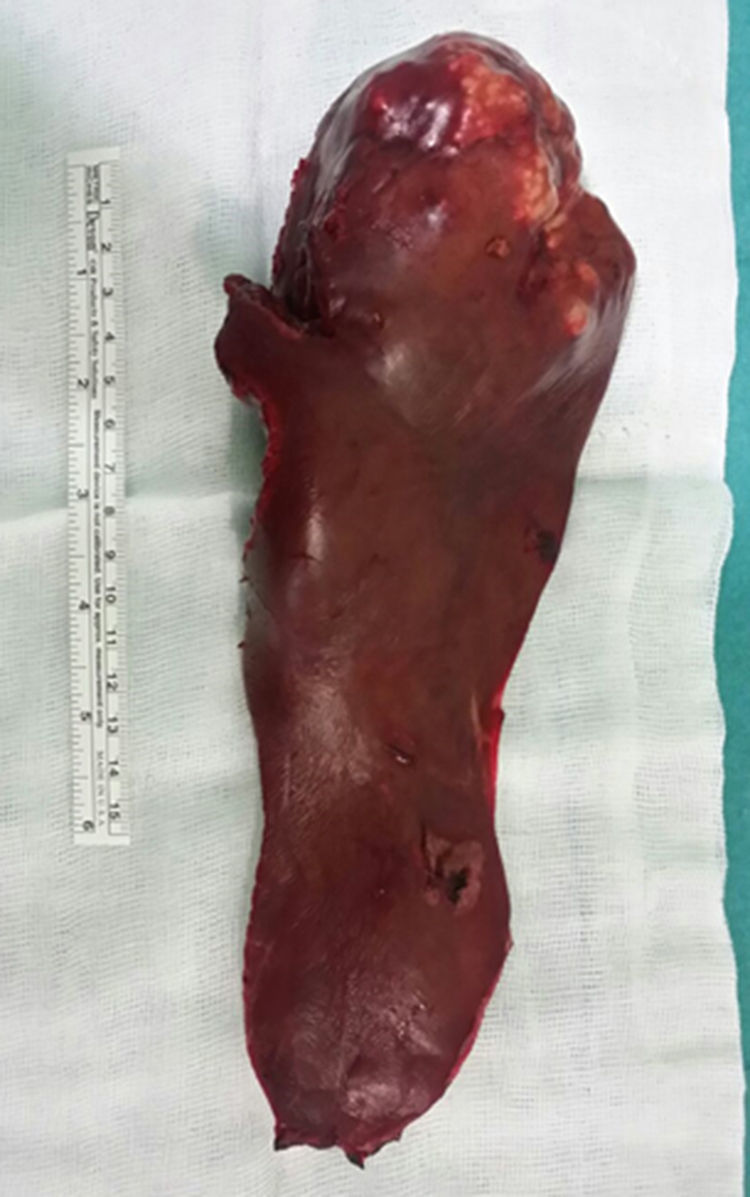
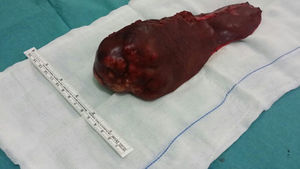
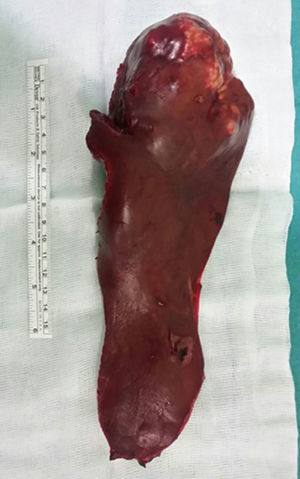
Given these findings, we decided to conduct a left hepatic lobectomy (Figs. 1 and 2), without incident, and the patient was discharged on the sixth postoperative day.
Macroscopically, a mass was observed with well-defined lobed edges and a surface that was medium in consistency, fibrous in appearance and whitish in color.
Microscopically, the hepatic parenchyma was observed to be infiltrated by a solid-pattern neoplasm, comprised of monomorphic appearing cells with rounded nuclei and eosinophilic cytoplasms, alternating with cells showing a variable degree of nuclear atypia and fusiform morphology. The neoplastic cells showed a tendency toward perivascular disposition, with no vascular or perineural invasion. The neoplasm presented an infiltrative growth pattern, forming multiple nodules and showing areas of sclerosis. There were focal signs of necrosis and frequent figures of mitosis (20–25/50 high-power fields).
The immunohistochemistry study demonstrated positivity in the tumor cells for smooth muscle actin (SMA), H-caldesmon, calponin, vimentin, collagen IV, Bcl-2, TLE-1, INI1, WT1, CD99, EMA, nestin and cathepsin K. The study was negative for pan cytokeratin, keratin AE1/AE3, keratin 5.6, desmin, smoothelin, CD117, CD34, DOG1, chromogranin, synaptophysin, CD56, CD31, D240, S-100 protein, HMB45, Melan-A, estrogen receptors, progesterone receptors, mesothelin, calretinin, HBME1, FLI1, MITF, MyoD1, myogenin, sarcomeric actin, CD1a and CD68.
Occasionally, this type of neoplasm shows unusual characteristics (large size, deep location, mitotic activity, nuclear atypia and necrosis). In 2001, Folpe et al.4 proposed histological criteria for malignancy in this type of neoplasms, classifying as malignant those tumors that meet one or more of the following criteria:
- -
Deep location and size greater than 2cm;
- -
Presence of atypical mitoses;
- -
Moderate-intense nuclear atypia, and a number of mitosis equal to or greater than 5 mitoses/50 high-power fields (HPF).
In the case of this neoplasm, at least 2 criteria were met (>2cm and number of mitoses), so it was therefore considered malignant.
Due to the rarity of glomus tumors in this location, several types of tumors were included in the differential diagnosis.
It is necessary to differentiate between glomus tumors and other types of tumors: hemangioendotheliomas, gastrointestinal stromal tumors (GIST), paragangliomas, perivascular epithelioid cell tumors (PEComas), neuroendocrine tumors, etc.
Negativity for CD34 and positivity for SMA rule out hemangioendothelioma. Negativity for S-100, chromogranin, CD117 and synaptophysin rule out paraganglioma and GIST. Neuroendocrine tumors are typically positive for chromogranin A, synaptophysin, neuron specific enolase, CD56 and cytokeratin.3,5 The absence of HMB45 and desmin rules out PEComa.
Histologically, it is easy to make the distinction between glomus tumors and hemangiomas or angiosarcomas.3 Hepatocellular carcinoma and hepatoblastoma are ruled out due to the low levels of alpha-fetoprotein.5 The negativity of desmin in conjunction with the histologic distribution rules out a vascular leiomioma.1
Glomus tumors from any part of the body can metastasize to the liver. Nonetheless, in our patient, no other primary tumor was found.
Despite having completely resected the tumor, and due to its uncertain malignant potential, the patient is currently in appropriate clinical follow-up with periodical studies.
We conclude that glomus tumors should be considered in the differential diagnosis of liver tumors with positive immunohistochemistry for SMA, which has been shown to be a useful diagnostic method.
Authorship- -
Study design: FNF and JAJR;
- -
Data collection: ECA and NSV;
- -
Analysis and interpretation of the results: FNF, ECA and NSV;
- -
Article composition: ECA and NSV;
- -
Critical review and approval of the final version: FNF and JAJR.
Please cite this article as: Calcerrada Alises E, Sarabia Valverde N, Navarro Freire F, Jiménez Ríos JA. Localización atípica de tumor glómico en el hígado. Cir Esp. 2017;95:234–235.