Weight loss depends directly on the adhesion to the postoperative diet in patients undergoing a sleeve gastrectomy. The aim of this study is to evaluate the effect of different preoperative feeding patterns and the adhesion to a preoperative diet on short and mid-term postoperative weight loss.
Materials and methodsA prospective study of all morbidly obese patients undergoing a laparoscopic sleeve gastrectomy as a bariatric procedure between 2008 and 2012 was performed. Preoperative feeding patterns and weight loss, preoperatively and postoperatively at 12 and 24 months, were evaluated.
ResultsA total of 50 patients were included, with a mean preoperative BMI of 51.2+7.9kg/m2. All the patients presented a feeding pattern of big eaters, 44% of snackers, 40% of sweet eaters and 48% reported regular ingestion of “light” soft drinks. Mean preoperative excess weight loss (EWL) was 13.4% (range 10–31.4%). At 12 months mean EWL was 83.7% and at 24 months 82.4%. Pre and postoperative EWL showed a direct correlation at 12 and 24 months. Mean EWL was significantly lower in snackers, sweet eaters and those drinking “light” soft drinks regularly.
ConclusionPreoperative weight loss correlates directly with postoperative weight loss at 1 and 2 years. Snackers, sweet eaters and “light” soft drink consumers, associated with a big eater pattern, achieve a significantly lower postoperative weight loss.
En la gastrectomía vertical, la pérdida de peso depende directamente de la adherencia a la dieta postoperatoria. El objetivo de este estudio es evaluar el efecto de los patrones alimentarios preoperatorios y de la adherencia a la dieta pautada antes de la cirugía, sobre la pérdida de peso a corto y medio plazo.
Material y métodosSe realizó un estudio prospectivo de todos los pacientes obesos mórbidos intervenidos mediante una gastrectomía vertical laparoscópica como procedimiento bariátrico entre 2008 y 2012. Se evaluaron los hábitos alimentarios preoperatorios de los pacientes y se registró la pérdida de peso pre- y postoperatoria a los 12 y 24 meses de la cirugía.
ResultadosSe incluyó a 50 pacientes en el estudio con un IMC preoperatorio medio de 51,2+7,9 kg/m.2 Todos los pacientes presentaban un patrón alimentario de grandes comedores. Además, el 44% de los pacientes presentaba un patrón de picoteador, el 40% reconocían ingesta abundante de dulces y el 48% tomaban refrescos «light» como bebida en las comidas. El porcentaje de exceso de peso perdido (PEP) medio preoperatorio fue de 13,4% (rango 10-31,4%). Al año el PEP era de 83,7% y a los 2 años, de 82,4%. La pérdida de peso y el PEP preoperatorios mostraron una correlación directa con la pérdida obtenida a los 12 y 24 meses. El PEP medio a los 12 y 24 meses fue significativamente peor en pacientes picoteadores, tomadores de dulces y bebedores de refrescos «light».
ConclusiónLa pérdida de peso preoperatoria se correlaciona con la pérdida de peso al año y a los 2 años. Aquellos pacientes, que además de grandes comedores, también son picoteadores, tomadores de dulces o de bebidas «light», logran una menor pérdida de peso.
Obesity is one of the most prevalent diseases in developed countries. In Spain, its prevalence in both sexes is 15.5%, although a progressive increase has been observed in recent years.1,2 The initial treatment of obesity should be based on a low-calorie diet and physical exercise. Nonetheless, in patients with morbid obesity, bariatric surgery has been shown to be the most effective method to achieve long-lasting substantial weight loss, while improving the comorbidities associated with obesity.3 The laparoscopic approach is currently the method of choice for most bariatric procedures; however, it is a complex surgery performed in patients with multiple pathologies and can therefore involve important morbidity and mortality rates.4,5 Thus, given the diversity of surgical techniques, it is necessary to define preoperative indicators for good post-surgical results in order to choose the best procedure for each patient.
Laparoscopic vertical sleeve gastrectomy (LVSG) is a restrictive procedure that involves resection of the fundus and body of the stomach, converting the stomach into a tubular duct relying on the lesser curvature.5 As there is no malabsorptive component, weight loss depends directly on the type of foods consumed after surgery and compliance with the postoperative diet.6
The objective of this study is to evaluate the effect of preoperative eating habits and compliance with the recommended diet before surgery on short- and mid-term weight loss.
Patients and MethodsWe performed a prospective study including all morbidly obese patients treated with LVSG as a bariatric procedure between February 2008 and January 2012.
Preoperative EvaluationAll patients were evaluated by a multidisciplinary team made up of surgeons, endocrinologists, anesthetists, endoscopists, psychiatrists, psychologists and nursing staff specialized in nutrition. The diagnostic tests included abdominal ultrasound, upper gastrointestinal endoscopy, respiratory function tests and complete blood workup with nutritional profile. The psychiatrists and psychologists had individual interviews with the candidates for bariatric surgery in order to evaluate their commitment to compliance with the postoperative diet. Trained nurses explained to the patients the recommended 1200kcal/day Mediterranean diet to be followed after surgery. A weight loss of at least 10% of excess weight was considered an essential requirement to be selected as a candidate for LVSG. Patients with evidence of gastroesophageal reflux or those who did not reach the required weight loss (a sign of poor compliance with the diet) were excluded from the study and selected for gastric bypass.
Surgical TechniqueA laparoscopic approach was used in all patients. Five ports were inserted: subxiphoid, supraumbilical, right hypochondrium, left hypochondrium and left flank. The short vessels of the greater curvature of the stomach were dissected with a harmonic scalpel (Ultracision, Ethicon Endosurgery, NY, USA). Longitudinal gastric resection was performed from the cardiac notch up to approximately 3–4cm proximal to the pylorus, using an endostapler (Echelon Flex, Ethicon Endosurgery, NY, USA). The gastric tube was previously calibrated with a 50 French catheter (Foucher), placed along the lesser curvature of the stomach. Methylene blue was used to check for any leaks. A Jackson-Pratt drain tube was left along the staple line of the gastric tube.
Postoperative Course24h after the procedure, the patient was administered a vial of methylene blue diluted in 200ml of water, taken orally. After confirming that the contrast did not leak through the drain, patients began ingesting water or infusions. On the 2nd day post-op, they were allowed to consume up to 600ml of high-protein liquid supplements. On the 3rd day post-op, patients were discharged if there had been no postoperative incidences.
Follow-upThe follow-up rate was 100%. All the patients were monitored by the surgeon and endocrinologist at 1, 3, 6, 12, 18 and 24 months after the operation. The patients were also followed by the nursing staff to reinforce compliance with the diet.
Weight loss was registered along with the evolution of comorbidities. Pharmacological treatments were adjusted to the needs of each patient. Patients were prescribed daily multivitamin supplements and proton pump inhibitors.
Patients were also recommended to do at least 1h of physical exercise each day (swimming, walking, etc.).
VariablesWe recorded weight loss and the percentage of excess weight loss (EWL), both preoperative and postoperative at 12 and 24 months after surgery. We evaluated the preoperative eating patterns of the patients (binge eater, snacker, sweet eater and “diet” beverage drinker). Binge eaters were defined as patients who reported consuming large quantities of food at a time. Snackers were characterized by continuously eating small quantities of food between meals. Sweet eaters ate sweet foods containing refined sugars on a daily basis. Last of all, “diet” soda drinkers drank sweetened carbonated drinks with and between meals. The different eating patterns were not mutually exclusive, so one single patient could present several or even all the eating patterns described.
Statistical AnalysisAll statistical analyses were done with the SPSS 17.0 program (SPSS Inc., Chicago, IL, USA). The quantitative variables that followed normal distribution were defined as mean and standard deviation; for the non-Gaussian variables, the median and range were used. The qualitative variables were defined by number of cases and percentage. The comparison between variables was done with the Student's t and Pearson's correlation tests for quantitative variables with Gaussian distribution, and the Mann–Whitney and Spearman's tests for non-Gaussian variables. P values <.005 were considered significant.
ResultsA total of 50 patients were included in the study: 44 women (88%) and 6 men (12%), with a mean age of 43.2±10.2 and a range between 20 and 62. Patient comorbidities included type 2 diabetes mellitus in 26% of the cases, dyslipidemia in 50% (40% hypercholesterolemia and 10% hypertriglyceridemia), arterial hypertension in 30%, osteoarthritis in 18% and obstructive sleep apnea syndrome in 14%. All the diabetic, hypertensive or dyslipidemic patients were receiving pharmacological treatment with an acceptable control of comorbidities. Mean preoperative BMI was 51.2±7.9kg/m2.
All the patients included presented binge eating patterns. Furthermore, 44% of the patients presented snacking patterns, 40% reported an abundant consumption of sweets and 48% drank “diet” soft drinks with meals.
Preoperative weight loss was 10.2±3.8kg, with a mean EWL of 13.4% (range 10–31.4%).
Complications appeared in 4 patients (8%): 2 patients with organ/space SSI (intraabdominal abscess); one staple-line leak; and one iatrogenic perforation of the middle third of the esophagus when the calibration catheter was inserted. Mortality rate was 2% (the patient with the iatrogenic esophageal perforation presented massive pulmonary embolism). The 2 patients with organ/space SSI presented no incidences in the immediate postoperative period and were discharged on the 3rd day post-op. On the 5th and 6th days after discharge, respectively, these 2 patients came to our Emergency Department with fevers above 38°C. Abdominal CT scan with oral and intravenous contrast showed intraabdominal collections in both cases measuring 3 and 5.5cm in maximum diameter, respectively, located in the left hypochondrium and gastrohepatic ligament. No extravasation of oral contrast was observed. In the patient with the 5.5cm collection, percutaneous drainage was used and antibiotic treatment was initiated (piperacillin/tazobactam 4.5g/8h for 5 days). In the other patient, antibiotic treatment was used alone for 7 days. Both patients recovered satisfactorily and were discharged 8 days after hospital admittance, with no later incidences. The patient who presented a staple-line leak was diagnosed 24h after surgery, when methylene blue was observed through the drain. A covered stent was placed endoscopically and left in for 4 weeks. Afterwards, the stent was withdrawn, with no further complications. The patient with iatrogenic esophageal perforation presented with tachycardia the day after the procedure. There was no evidence of methylene blue through the drain. Thoracoabdominal CT scan showed evidence of minimal contrast extravasation at the esophageal area. Because it was impossible to perform an emergency upper gastrointestinal endoscopy, exploratory laparoscopy was performed, where we observed a minimal contrast leak through the esophageal hiatus, originating in the mediastinum. A drain was inserted in the mediastinum. The following day, endoscopy demonstrated an esophageal microperforation that may possibly have been caused by trauma (it had been difficult to insert the catheter during surgery). A covered stent was inserted, covering the esophagus and gastric tube. The patient was admitted to the ICU, where he began to have sudden dyspnea 6 days later. Thoracic CT showed a massive left pulmonary embolism, which led to his death.
Mean weight loss one year after surgery was 45.8±11.7kg, with a mean BMI of 27.7±2.8kg/m2 and a mean EWL of 83.7%. Two years later, mean weight loss was 45.5±10.2kg, obtaining a mean BMI of 27.9±2.8kg/m2 and a mean EWL of 82.4%.
The resolution rates for diabetes mellitus and arterial hypertension were 84.6% and 86.7%, respectively. Hypertriglyceridemia, osteoarthritis and obstructive sleep apnea syndrome improved in all cases, and medication and CPAP were suspended in 100% of the patients. Hypercholesterolemia showed limited improvement, although hypolipidemic medication was not able to be suspended in any of the cases.
Correlation Between Preoperative Weight Loss and Postoperative Weight Loss at 12 and 24 MonthsPreoperative weight loss showed a direct correlation with weight loss reached at 12 months (Pearson 0.597; P=.003) and 24 months (Pearson 0.573; P=.001). Preoperative EWL also significantly correlated with EWL at 12 months (Pearson 0.848; P=.043) and 24 months (Pearson 0.822; P=.012).
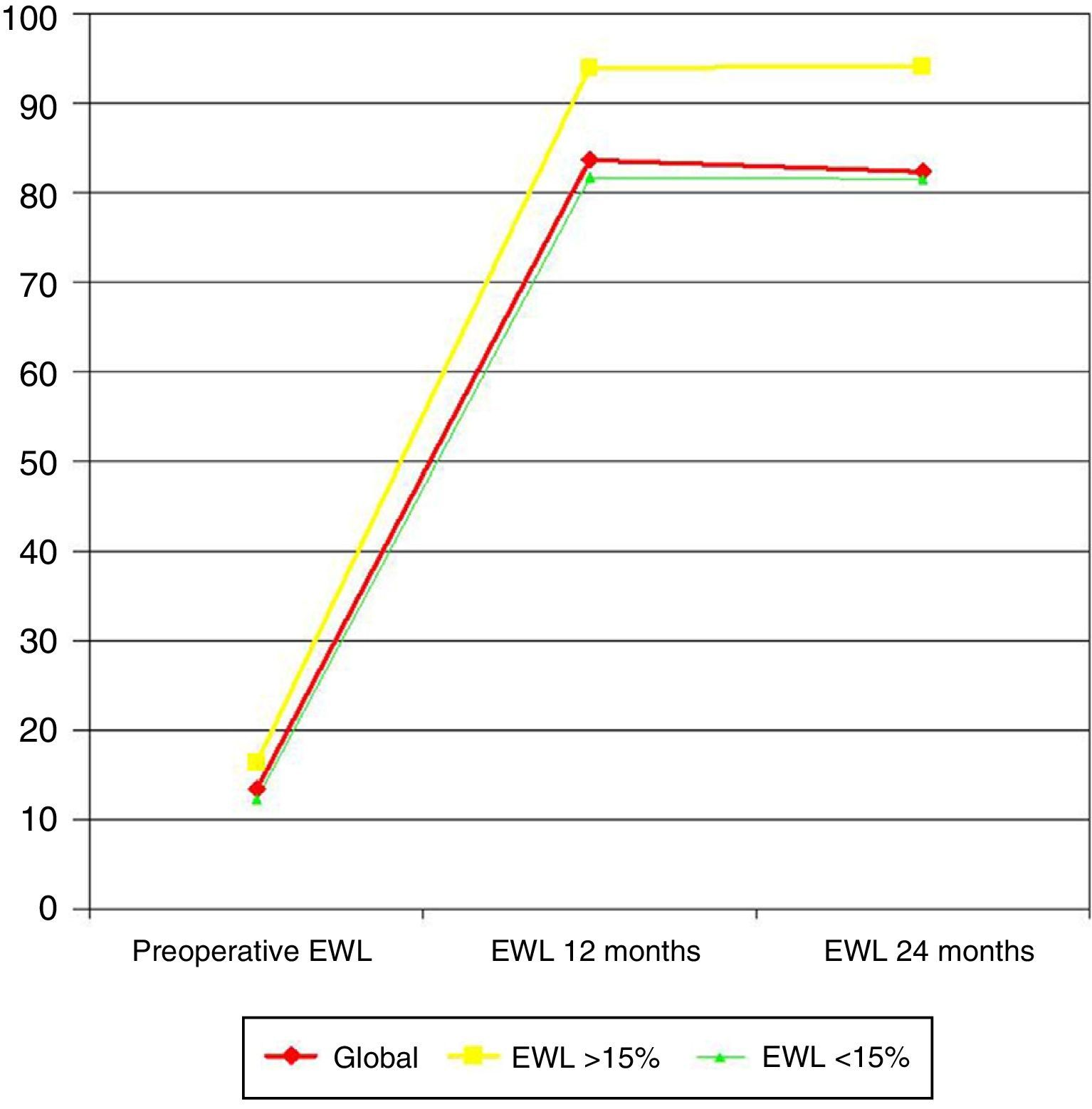
When preoperative weight loss surpassed 15% EWL (10 patients [20%]), EWL at 12 months was significantly greater (93.9 versus 81.6%; P=.014), as well as at 24 months (94.1 versus 81.5%; P=.002) (Fig. 1).
Association Between Eating Patterns and Weight LossTwelve months after surgery, mean EWL in snacker patients was 84.6 versus 89.8% in non-snackers (P=.037). After 24 months, the EWL in snackers was 83.1 versus 90.2% in non-snackers (P=.008) (Fig. 2).
In patients who often ate sweets, EWL at 12 months was 80.8 versus 92.3% in patients without this eating habit (P=.013). At 24 months, EWL in sweet eaters was 78.3 versus 93.9% in non-sweet eaters (P<.001) (Fig. 3).
The patients who drank “diet” sodas showed an EWL at 12 months of 85.7% versus 90.6% in non-soda drinkers (P=.041). After 2 years, the EWL in the former was 83.2 versus 92.8% in the latter (P=.022) (Fig. 4).
DiscussionLVSG is a safe and effective bariatric technique that achieves significant weight loss and a high percentage of resolution of comorbidities. In recent years, it has become more relevant and is now the second most frequent bariatric technique performed in our setting after gastric bypass. In terms of weight loss, the EWL that is reached is estimated at 70%.7 In our series, it is striking that the EWL after one year was 83.7% and 82.4% after 2 years. In an earlier study by our group,8 these excellent results were explained by patient selection. All our patients who had been selected for LVSG surgery presented a binge eater eating pattern, so they would theoretically benefit the most from gastric restriction. Furthermore, an essential condition was good compliance with the preoperative diet, with an EWL of at least 10%.
Several authors have mentioned preoperative weight loss as a predictive factor for the success of bariatric surgery in terms of weight loss. It has been widely demonstrated that lifestyle changes and behavioral modification are a clear indicator of the long-term success of the weight loss achieved. In recent years, more and more groups are focusing on preoperative weight loss, not as a mid- to long-term predictive factor, but instead as a means to reduce operative risk and improve the surgical field, decreasing intraabdominal fat and fatty liver symptoms. With the objective to correlate preoperative with postoperative weight loss, Alvarado et al.9 developed a study in which they observed that for every 1% of preoperative weight loss, 1.8% of weight was lost post-op. They concluded that preoperative weight loss was a barometer of the patient's motivation to maintain physical exercise and diet in the postoperative period. Along the same lines, Alger-Meyer et al.10 showed that patients who had been able to lose 10% of the weight before surgery presented better results for maintaining the weight loss at 3–4 years, while the Alami group11 observed that preoperative weight loss of 10% was related with greater weight loss in the early post-op. Nonetheless, others authors did not find significant differences in the correlation between short- and long-term weight loss in those patients with preoperative weight loss.12–14 In a systematic review of this topic that included 14 articles, half of the studies indicated a positive correlation between pre- and postoperative weight loss, while the other half did not observe this association.15
To date, most published studies about this topic refer to gastric bypass and none specifically deal with LVSG, where preoperative weight loss, as an indicator of compliance with the diet, should be much more relevant than in mixed or malabsorptive techniques. This is confirmed in our series, since the preoperative weight loss directly correlated with weight loss and EWL at 1 and 2 years after the procedure. In fact, patients who reached 15% EWL had even better results.
Another factor to take into consideration is the manner in which preoperative weight loss is achieved. Some authors propose maximum weight loss in two weeks by administering a diet that is very low in calories or even a liquid diet based on high-protein, low-calorie nutritional supplements with a maximum intake of 800kcal/day. Meanwhile, other groups like ours prefer slower weight loss for at least 8 weeks prior to surgery with low-calorie diets of 1200kcal/day.16,17 In our opinion, the latter method is a true reflection of patient compliance with the diet, as long as the preoperative diet is similar to postoperative recommendations. If the patient does not show good compliance before surgery, it would be risky to assume that he/she would follow the diet after the operation. It would be very difficult for rapid weight loss methods with very low-calorie diets to be able to predict compliance with another diet type after the intervention.
The other parameter analyzed in our study is the association of eating habits with weight loss at 12 and 24 months. As we have mentioned previously, all the patients met the binge eater criteria, which was a requisite to be selected for this bariatric technique. Nonetheless, 44% of patients were also snackers, 40% were sweet eaters, and 48% drank “diet” soft drinks with the pretense that they do not have calories. The snacker eating pattern is considered a predictive factor for poor results after any type of bariatric technique, but even more so in restrictive procedures.18 Snacker patients continually consume small quantities of food, so there is no onset of gastric restriction and, therefore, intake is not curbed. This explains the lower weight loss in the snacker patients of our series.
Likewise, patients who frequently eat sweets also achieved less weight loss. Sweets are foods with simple carbohydrates, which have high caloric values and little satiating effect. In these patients, the restrictive component is also not effective. Sugerman et al. reported that, in patients who ate sweets and had undergone vertical gastroplasty, weight loss was significantly lower than in non-sweet eaters. Nevertheless, in patients who had undergone gastric bypass, a technique with an important malabsorptive component, there were no significant differences in weight loss between patients who ate sweets and those who did not.19
Similar to the situation observed in snackers and sweet eaters, patients who drank “diet” sodas either with or without meals also achieved less weight loss. There is abundant evidence in the literature that the consumption of sweetened drinks is a risk factor for developing obesity. This is based on the fact that the extra caloric content of sugar-sweetened drinks is not compensated with greater physical activity.20,21 In theory, this deleterious effect would disappear with “diet” drinks, which are sweetened with artificial sweeteners and not with the addition of sugar, resulting in 0-calorie drinks. However, some authors have observed that the consumption of these drinks not only does not reduce the consumption of other foods, but, in fact, it leads to increased intake. Some authors have attributed this phenomenon to the gas in carbonated drinks, which distends the lumen of the stomach while increasing the release of ghrelin, resulting in increased appetite.22
Patients with a binge eating pattern who achieve an excess weight loss of at least 10% before surgery are able to achieve excellent results with LVSG. Preoperative weight loss correlates with weight loss at 1 and 2 years. Patients who, in addition to being binge eaters, are also snackers, sweet-eaters or “diet” soda drinkers lose less weight.
Conflict of InterestsThe authors declare having no conflict of interests.
Members of the OBELCHE Group: M. Remedios Alpera, Antonio Aroyo, Elena Miranda, José Luis Muñoz, Ana Murcia, José María Rico, Javier Sola-Vera and Luis Fernando Vences.
Please cite this article as: Ruiz-Tovar J, Boix E, Bonete JM, Martínez R, Zubiaga L, Díez M, et al. Efecto de los patrones de conducta alimentaria y de la pérdida de peso preoperatoria sobre los resultados a corto y medio plazo en pérdida de peso tras gastrectomía vertical laparoscópica. Cir Esp. 2015;93:241–247.