The persistence of obesity favors the failure of the Fundoplication (FP) in the treatment of Gastroesophageal Reflux (GER). However, the weight loss obtained with the performance of a Gastric Bypass (GBP) allows a good resolution of symptoms, without increasing the incidence of postoperative complications. All of this leads us to consider that while FP is the indication in patients with BMI < 30, in those patients with BMI > 35, GBP appears to be the procedure of choice. But there is still no position in the case of patients with a BMI between 30 and 35, although we must take into account that an increase in GER recurrence has been described after FP in patients with a BMI > 30. Although Sleeve Gastrectomy (SG) is one of the most frequently used bariatric procedures in recent years, its association with a high rate of postoperative GER has led several authors to propose its performance associated with an anti-reflux procedure in patients with GER symptoms. Likewise, if the existence of an Hiatal Hernia is verified, it must be treated by hiatoplasty, both during the performance of a GBP and a SG. This simultaneous treatment is not associated with an increase in complications.
La persistencia de obesidad favorece el fracaso de la Funduplicatura (FP) en el tratamiento del Reflujo Gastro-esofágico (RGE). Sin embargo, la pérdida de peso obtenida con la realización de un Bypass Gástrico (BPG) permite una buena resolución de síntomas, sin incrementar la incidencia de complicaciones postoperatorias. Todo ello lleva a considerar que mientras la FP es la indicación en el paciente con IMC < 30, en aquellos pacientes con IMC > 35 el BPG se muestra como el procedimiento de elección. Pero todavía no existe un posicionamiento en el caso de pacientes con IMC entre 30 y 35, si bien deberemos tener en consideración que se ha descrito un aumento de recurrencia del RGE tras FP en pacientes con un IMC > 30. Aunque la Gastrectomía Vertical (GV) es uno de los procedimientos bariátricos más frecuentemente utilizado en los últimos años, su asociación a una tasa elevada de RGE postoperatorio ha llevado a varios autores a proponer su realización asociada a un procedimiento anti-reflujo en aquellos pacientes con síntomas de RGE.
Así mismo, en caso de constatarse la existencia de una HH, ésta debe ser tratada mediante hiatoplastia, tanto durante la realización de un BPG como de una GV. Este tratamiento simultáneo no se asocia con un incremento de complicaciones.
As mentioned in previous editions, there are several pathophysiological mechanisms that play a role in the development of gastro-oesophageal reflux (GOR) in obese patients, including a hypotonic lower oesophageal sphincter, motor alterations of the oesophagus or anatomical or physiological disruption of the oesophagogastric junction secondary to increased intra-abdominal pressure.1 Regardless of this, different studies have shown the existence of a correlation between the increase in body mass index (BMI) and the level of GOR.2 The prevalence of GOR symptoms is 23%, 27% and 50% of patients depending on whether the BMI is <25, 25–30, or >30 kg/m2, and up to 55% in patients who are candidates for bariatric surgery.3
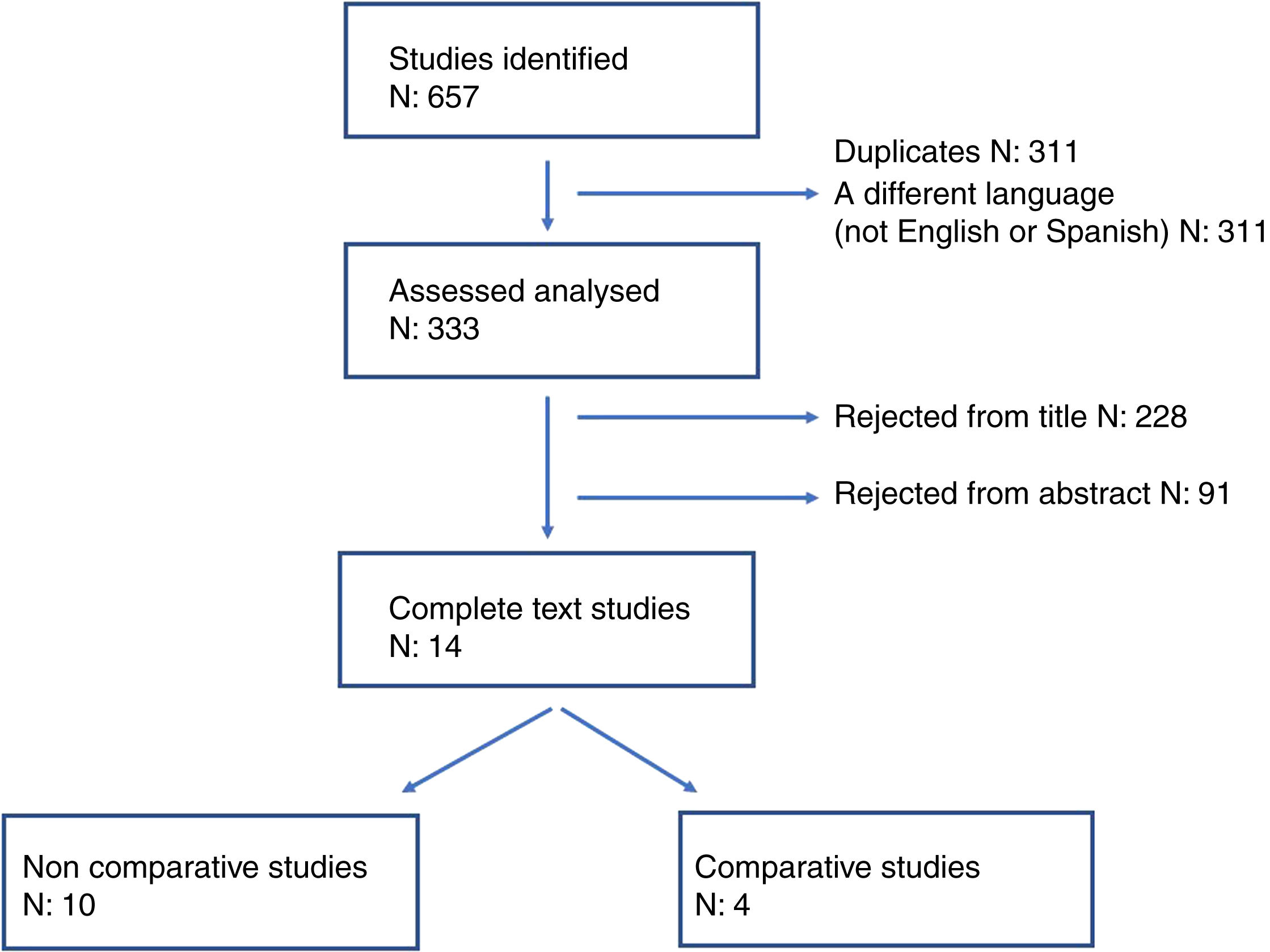
Anti-reflux surgery vs. bariatric surgery as anti-GOR treatmentTo respond to this question through evidence-based surgery, we carried out a systematic review of the literature until September 2022 and selected those studies that report the clinical outcomes obtained comparing gastric bypass (GBP) and fundoplication (FP) in obese patients with GOR. To identify the studies, we used the following terms: “obesity”, “obese patient”, “antireflux”, “bariatric surgery”, “gastric bypass”, “fundoplication”, “Nissen”, “Toupet”, “Dor”. The references of the selected articles were used to identify other potentially relevant articles. Of the 657 articles obtained, 324 were discarded, including duplicates and those written in a language other than Spanish or English. Based on the title and abstract of the publications, another 319 articles were excluded, leaving us with 14 clinical studies that analyse the results of performing FP or GBP in obese patients with GOR (see flow chart in Fig. 1). Of these 14 studies, only 4 compare the performance of antireflux surgery with GBP (Table 1) and their non-uniformity and characteristics impede any quantitative analysis.
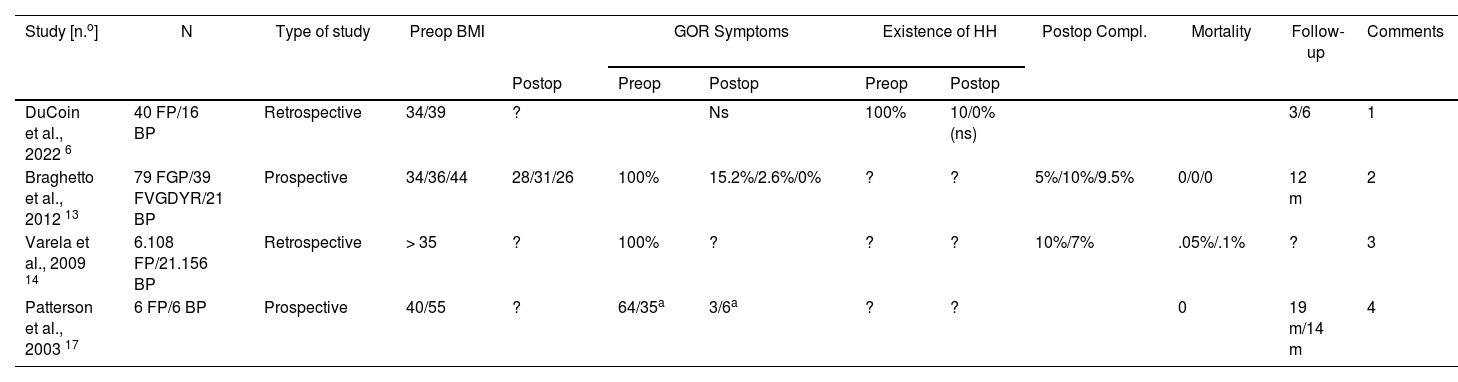
Results of comparative bypass vs fundoplication studies.
| Study [n.o] | N | Type of study | Preop BMI | GOR Symptoms | Existence of HH | Postop Compl. | Mortality | Follow-up | Comments | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postop | Preop | Postop | Preop | Postop | ||||||||
| DuCoin et al., 2022 6 | 40 FP/16 BP | Retrospective | 34/39 | ? | Ns | 100% | 10/0% (ns) | 3/6 | 1 | |||
| Braghetto et al., 2012 13 | 79 FGP/39 FVGDYR/21 BP | Prospective | 34/36/44 | 28/31/26 | 100% | 15.2%/2.6%/0% | ? | ? | 5%/10%/9.5% | 0/0/0 | 12 m | 2 |
| Varela et al., 2009 14 | 6.108 FP/21.156 BP | Retrospective | > 35 | ? | 100% | ? | ? | ? | 10%/7% | .05%/.1% | ? | 3 |
| Patterson et al., 2003 17 | 6 FP/6 BP | Prospective | 40/55 | ? | 64/35a | 3/6a | ? | ? | 0 | 19 m/14 m | 4 | |
GBP: Gastric bypass; FGP: fFndoplication + gastropexia posterior; FP: Fundoplication; FVGDYR: Fundoplication + vagotomy + gastrectomy with Y-Roux.
Comments:
1. Greater reduction of HH recurrence after gastric bypass without statistical significance.
2. Similar regression of intestinal metaplasia (favouring GBP) but fundoplication only in short Barrett and the other two techniques in long Barrett.
3. Patients in the bypass group had more comorbidities.
4. Postoperative pHmetries were standardised in all cases. Both groups were not sufficiently comparable.
One of these 4 studies4 analyses the immediate postoperative period of a series of 27,264 patients with morbid obesity who underwent laparoscopic FP (n = 6108) or laparoscopic GBP (n = 21,156), observing a significantly lower incidence of postoperative complications in the GBP group, with no differences in terms of hospital stay, mortality or hospital costs. But, as the authors acknowledge, postoperative information is very limited with regards to time and no information is provided regarding the effectiveness in the treatment of GOR and the evolution of BMI. For its part, the study by Braghetto et al.5 focuses on the analysis of obese patients who, in addition to GOR, present with Barrett's oesophagus (BO) and fundamentally compares the results of three surgical procedures on the evolution of intestinal metaplasia: group I: FP with posterior gastropexy (n = 79), group II: FP with vagotomy + distal gastrectomy and Roux-en-Y reconstruction (n = 39) and group III: GBP (n = 21). In this study, the length of the BO segment determined the decision to perform one or another intervention, indicating FP with gastropexy in patients with short BO segment, while the other two techniques were performed in patients with long BO segment. The three procedures obtained good results in terms of postoperative reduction of GOR symptoms, as well as regression of intestinal metaplasia (group I: 51.9%, group II: 51.3%, group III: 61. 9%). The other two studies2,6 compare the antireflux effectiveness of FP and GBP, confirming an evident improvement in GOR symptoms in the postoperative period, with no significant difference between both procedures. However, there are important biases, such as differences in BMI or GOR scores between patients in both groups, which make it difficult to draw definitive conclusions.
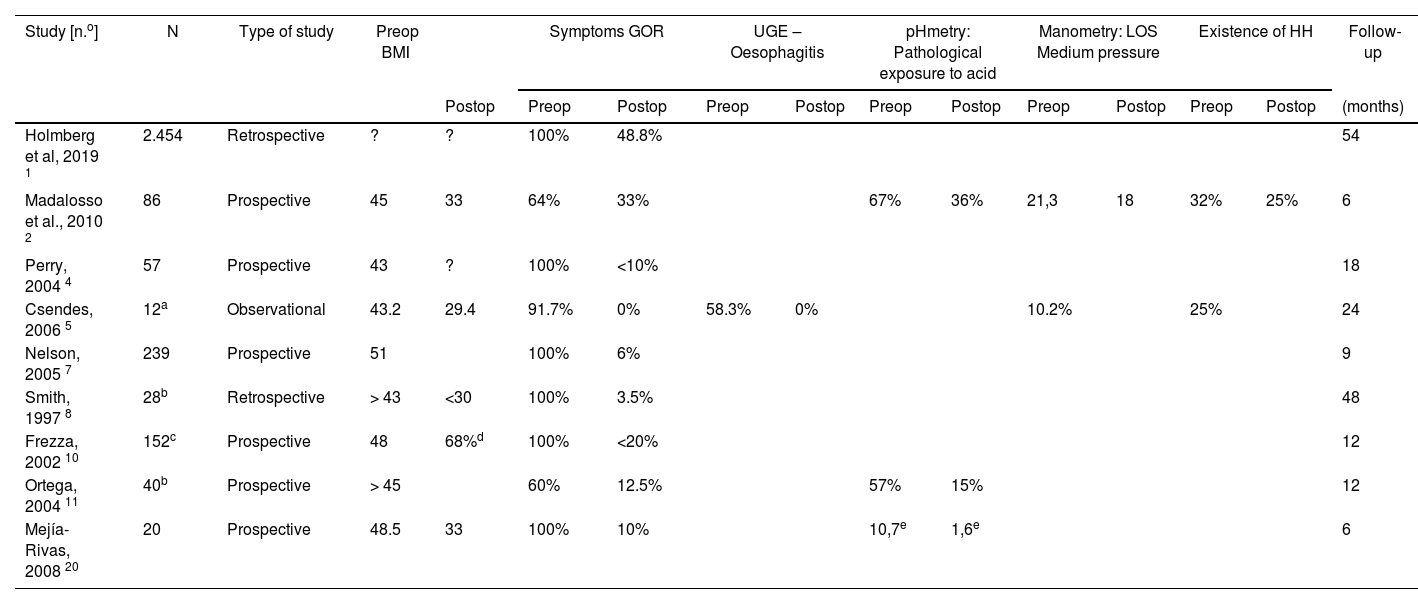
Of the remaining 10 studies, 9 analyse the application of GBP in obese patients with GOR (Table 2) and the last is a meta-analysis of the results of antireflux surgery in obese patients.7 The 9 publications that analyse the application of GBP concur in the detection of a disappearance of symptoms in more than 50% of patients (in some up to 90%) after performing GBP. Although follow-up is scarce in most studies, good results are maintained in those with follow-up of more than 4 years.8,9 Regarding the functional study of these patients, the limited existing data confirm a significant reduction in the percentage of patients with pathological pH monitoring after surgery. This data coincides with those observed by the comparative study by Patterson et al.2 where both groups (FP and GBP), in the postoperative period, presented pHmetry and manometry with results within normality, although the preoperative data were more altered. in patients undergoing FP, giving rise to more significant changes than those observed in patients undergoing GBP. For this reason, the authors acknowledge that they cannot conclude that the two procedures achieve the same results, although both are effective, especially in symptom control.
Gastric bypass patients. Results of published studies.
| Study [n.o] | N | Type of study | Preop BMI | Symptoms GOR | UGE – Oesophagitis | pHmetry: Pathological exposure to acid | Manometry: LOS Medium pressure | Existence of HH | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postop | Preop | Postop | Preop | Postop | Preop | Postop | Preop | Postop | Preop | Postop | (months) | ||||
| Holmberg et al, 2019 1 | 2.454 | Retrospective | ? | ? | 100% | 48.8% | 54 | ||||||||
| Madalosso et al., 2010 2 | 86 | Prospective | 45 | 33 | 64% | 33% | 67% | 36% | 21,3 | 18 | 32% | 25% | 6 | ||
| Perry, 2004 4 | 57 | Prospective | 43 | ? | 100% | <10% | 18 | ||||||||
| Csendes, 2006 5 | 12a | Observational | 43.2 | 29.4 | 91.7% | 0% | 58.3% | 0% | 10.2% | 25% | 24 | ||||
| Nelson, 2005 7 | 239 | Prospective | 51 | 100% | 6% | 9 | |||||||||
| Smith, 1997 8 | 28b | Retrospective | > 43 | <30 | 100% | 3.5% | 48 | ||||||||
| Frezza, 2002 10 | 152c | Prospective | 48 | 68%d | 100% | <20% | 12 | ||||||||
| Ortega, 2004 11 | 40b | Prospective | > 45 | 60% | 12.5% | 57% | 15% | 12 | |||||||
| Mejía-Rivas, 2008 20 | 20 | Prospective | 48.5 | 33 | 100% | 10% | 10,7e | 1,6e | 6 | ||||||
UGE: Upper gastrointestinal endoscopy; LOS: Lower oesophageal sphincter; HH: Hiatus hernia; BMI: Body Mass Index; GOR: Gastroesophageal reflux.
Weight loss, which can reduce the thoracoabdominal pressure gradient, is considered to be one of the factors that influence the resolution of GOR symptoms after performing a GBP. Other factors considered are the reduction of acid production from the reservoir or the reduction of possible bile reflux through a food loop of sufficient length.
Different studies have shown a correlation between increased BMI and the level of GOR.2 This correlation is also observed after antireflux surgery, as observed in the meta-analysis by Abdelrahman et al.,7 where it is confirmed that after performing FP there is a greater recurrence of GOR symptoms in those patients with BMI > 30 compared to those who have a BMI < 30. In this regard, preoperative BMI > 35 was a predictor of recurrence after performing FP in a series of 174 patients with a follow-up of 10 years.10 Some series including obese patients undergoing revision antireflux surgery for previous FP failure report that the most frequent cause of recurrence was related to a disruption of FP, suggesting obesity as a possible causal factor due to the greater intra-abdominal pressure exerted.11
In contrast, after performing a GBP, Nelson et al.12 did not observe that the improvement in GOR symptoms was related to weight loss, probably because in this case the other factors mentioned play a sufficiently important role (lower production of acid and control of possible bile reflux) that allow the effect of weight loss to be largely ignored.
In view of the data provided by the literature, FP remains the preferred treatment in patients with GOR and a BMI under 30 kg/m2. Furthermore, the persistence of obesity will enhance the failure of surgery in the case of FP. At the same time, GBP has been shown to be very effective in remission of GOR in patients with BMI > 35 kg/m2 with low recurrence rates. Furthermore, it achieves this without increasing the incidence of postoperative complications and adding the health benefits of the weight loss obtained with this procedure.4,13 All of the above leads to the consideration of GBP as the procedure of choice in the morbidly obese patient with GOR.2
What must however be taken into consideration are the technical aspects in the realisation of GBP, such as the size of the reservoir. If we perform a longer reservoir, we may encourage greater acid production to persist, and this would negatively influence the outcomes, since the pressure of the lower oesophageal sphincter (LOS) after performing a GBP does not present significant changes with respect to the preoperative values,14 unlike that which occurs after FP. Madalosso et al.14 observed, through pHmetry that the acidic gastric reservoir in the postoperative period correlated with greater exposure of the oesophagus to acid compared to patients without acidity in the gastric reservoir (47% vs 17%), and that they suffered more frequently from oesophagitis (26% vs 0%).
Few studies analyse the resolution of GOR and provide sufficient information on the existence and characteristics of a possible associated hiatus hernia (HH). If the existence of HH is confirmed, it must be treated by hiatoplasty during the GBP. This simultaneous treatment is not associated with an increase in complications, as Lewis et al.15 have recently published.
Another aspect to consider is the attitude to take towards obese patients with BO, whose incidence is between 1.3% and 3.8%, more than doubles the prevalence in the general population.16 Furthermore, approximately 50% of these patients do not have symptoms. A recently carried out systematic review17 shows that GBP is associated with a regression of BO and dysplasia in 36%–62% of patients with preoperatively confirmed BO.
In 2014, Pagé et al.18 published the results of a survey conducted among members of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) aimed at determining the position of surgeons in the surgical treatment of GOR in obese patients, in keeping with their BMI. While in patients with BMI > 30, 94% would perform FP, this percentage drops to 57.8% when the BMI is >35, and with BMI > 40, GBP was the technique of choice for 80% of surgeons. Currently, in view of the published data, it is not surprising that scientific societies such as the American College of Gastroenterology (ACG) or SAGES recommend GBP as the gold standard in the population with GOR and BMI > 35; It is likely that other scientific societies, in future updates of their guidelines, will also adopt this position.
Notwithstanding, there remains no positioning in the case of patients with a BMI between 30 and 35, although we must take into consideration that the meta-analysis by Abdelrahman et al.7 refers to the increase in recurrence after FP in obese patients, placing the cut-off point at a BMI > 30.
New surgical proposalsAlthough GBP seems to be the most recommended bariatric surgery in obese patients with GOR, in recent years new procedures have been proposed that open the range of therapeutic possibilities.
One of the most frequently used bariatric procedures is vertical gastrectomy (VG), and different studies have evaluated its feasibility, safety and reproducibility in terms of weight loss and reduction of comorbidities, compared to GBP. Despite the advantages, VG is associated with a high rate of postoperative GOR, regardless of the preoperative diagnosis.19 For this reason in recent years performing VG with an associated GOR preventive procedure has been proposed. One of the mechanisms involved in the aetiology of GOR is the presence of HH, and different authors have described a greater resolution of the symptoms if VG is associated with concurrent repair of HH (VG + HHR). Soricelli et al.20 performed a retrospective analysis of 97 patients who underwent VG + HHR. Forty-one of these patients presented preoperative symptoms of GOR, and after a postoperative follow-up of 18 months, 33 of them (80.4%) presented remission of symptoms, with no cases of de novo GOR. These results are also corroborated by Sucandy et al.21 in their retrospective series of 131 patients: of the 35 cases with HH and preoperative GOR symptoms who underwent VG + HHR, 32 cases (91%) presented complete resolution of the symptoms, and improvement thereof in the other 3 patients. But other studies do not present such optimal results, with a persistence of GOR symptoms in almost 50% of cases and the development of de novo GOR in more than 20% of patients, after performing VG + HHR.22
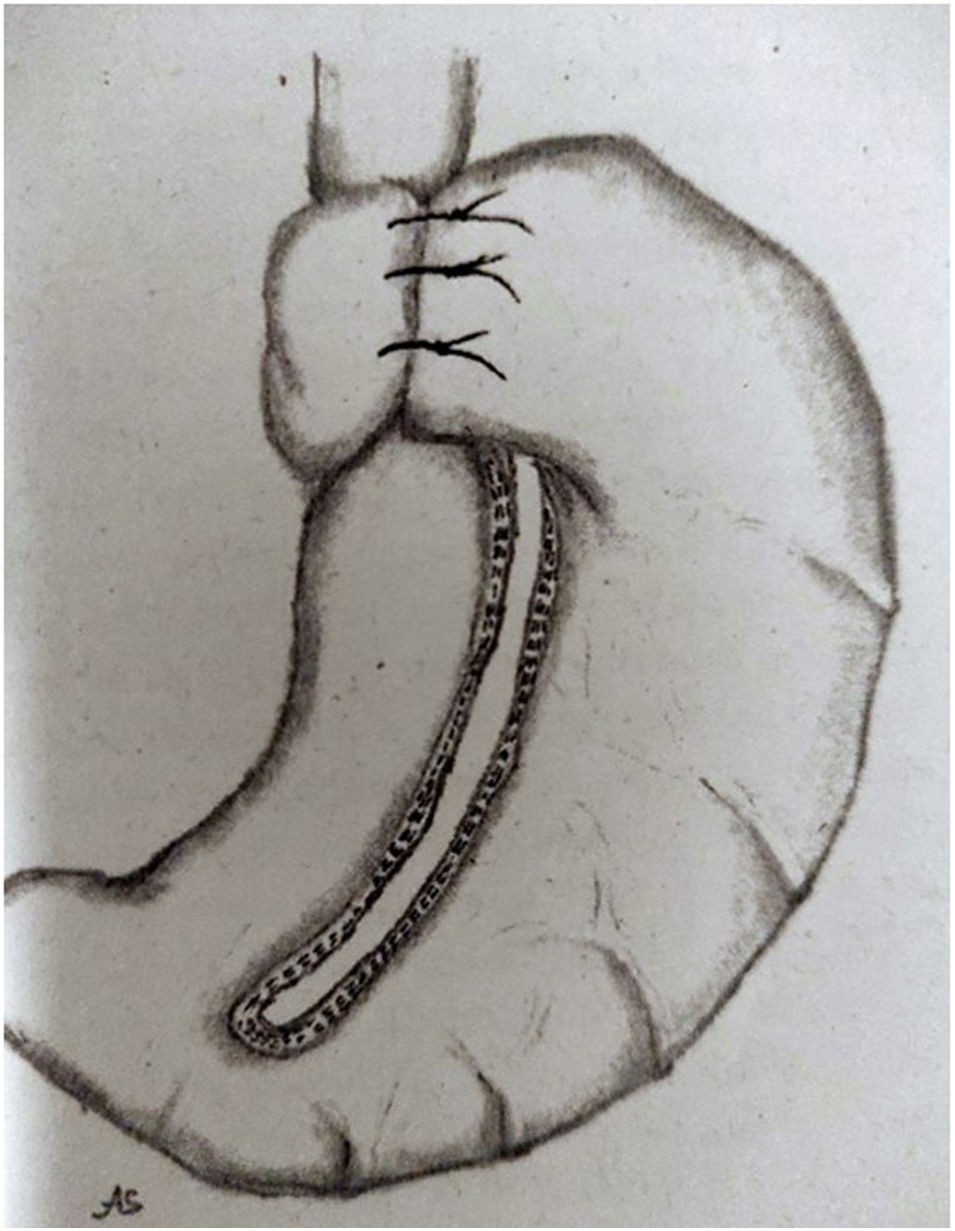
Since approximately 2015, several groups have begun to use the combination of VG with different antireflux systems, with promising preliminary outcomes. One of them is VG's association with the Magenstrasse-Mill operation and a Nissen FP. The Magenstrasse-Mill operation consists of performing a longitudinal section of the greater gastric curvature from 5 cm proximal to the pylorus to the angle of His and without resection of the sectioned stomach, since the fundus is used to perform a 360° FP (Fig. 2).23 For their part, Le Page y Martin24 proposed another type of FP associated with VG, consisting of a partial resection of the gastric fundus so that part of it remains for performing an anterior FP of 120° around the base of the oesophagus. Likewise, Sanchez-Pernaute et al.25 describe the possibility of the association of VG with Hill's gastropexy. However, these are proposals that report good outcomes in isolated cases, and do not currently offer outcome analyses in series large enough to allow them to be considered beyond mere possibilities.
Magenstrasse-Mill operation with FP Nissen.
Source: Fedenko y Evdoshenko.23
The procedure about which we have the most information in recent years is the combination of VG with 360° FP (Rossetti, Nissen).26,27 This type of procedure is based on the initial performance of FP using the smallest possible part of the fundus in order to carry out the greatest resection of it during the next step, which consists of VG. The data presented offer favourable results for its use, with GOR symptoms disappearing in a high percentage of patients. Uccelli et al.28 present the 5 year results of a series of 127 patients who underwent VG associated with 360° Rossetti-type PF (R-VG). Of the 74.8% of patients with preoperative GOR symptoms undergoing R-VG, that percentage was reduced to 4.2% after 60 month follow-up, while describing weight loss and resolution of comorbidities comparable to those patients undergoing standard VG. They also observed the resolution of the two cases with BO prior to surgery.
We must not lose sight of the fact that in all cases with HH, its reduction must be associated with pillar closure, regardless of the antireflux procedure that will be applied associated with VG.
Other proposed procedures consist of the application of radiofrequency to the oesophagogastric junction endoscopically (Stretta®)29 or the placement at that level of a ring of titanium beads with a magnetic core that acts as a sphincter (LINX®),30 considered as therapeutic options for patients with GOR symptoms after VG. But more results and longer-term follow-up are still required to establish them as widely accepted procedures.
ConclusionsWhile FP is the preferred treatment in patients with GOR and a BMI less than 30 kg/m2, GBP is the procedure of choice in patients with BMI > 35 kg/m2. There are aspects to consider, such as the strategy in those patients with a BMI between 30 and 35 kg/m2 with severe GOR, or who, despite having an indication for bariatric surgery, are not interested in this surgical option.31
It has been observed that GBP is associated with a regression of BO and dysplasia in 36%–62% of patients with preoperatively confirmed BO.17
The majority of new surgical proposals for the treatment of these patients have been based on associating antireflux treatment with performing a VG, with preliminary results that seem to reduce the GOR but are not sufficiently conclusive at the moment.
The existence of a HH requires its resolution during the operation whether we are going to perform a GBP or a VG, without increasing the incidence of complications.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Balagué C, Nve E, Puértolas N, Rodriguez J. Cirugía antirreflujo vs cirugía bariátrica como tratamiento anti-RGE y de la hernia de hiato en el obeso. Nuevas propuestas quirúrgicas. Cir Esp. 2023. https://doi.org/10.1016/j.ciresp.2023.02.004