Macrovascular invasion (MVI) in patients with hepatocellular carcinoma (HCC) is a very poor prognostic factor. Treatment in such cases is still a matter of debate. The goal of this study is to assess short- and long-term results of liver resection and thrombectomy in a series of patients with HCC and MVI.
MethodsRetrospective cohort study of patients who underwent liver resection for HCC in the period 2007–2015 (n = 120). Of all the patients, 108 did not have MVI, while 12 presented with MVI: 1 patient in the common portal vein (Vp4), 8 patients in first-order portal branches (Vp3), 1 patient in a sectorial branch (Vp2), 1 patient in a segmental branch (Vp1); another patient presented with tumor thrombus in a main hepatic venous branch in the confluence with the vena cava (Vv2).
ResultsPatients with MVI needed major hepatic resection more frequently than patients without MVI (83.3% vs 25.9%, P < .0001), with no differences in postoperative mortality or severe morbidity. Patients with MVI required a longer operative time and developed more frequently postoperative ascites (33.3% vs 9.3%, P = .034).
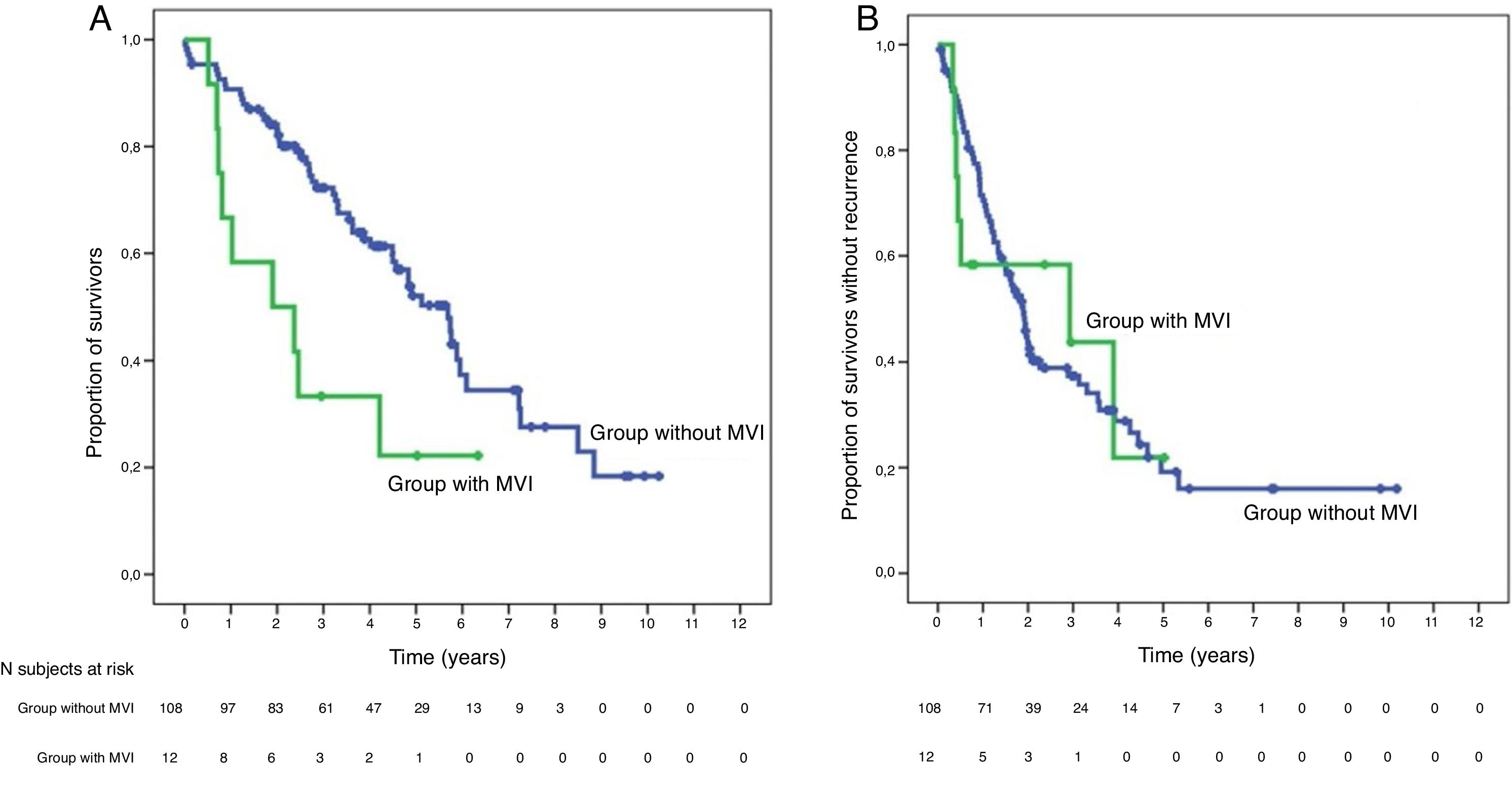
Global survival at 1, 3 and 5 years was 66.7%, 33.3% and 22.2% in patients with IMV, and 90.7%, 72.4% and 52.2% in patients without IMV (P = .009), respectively.
ConclusionsHepatectomy associated with thrombectomy might be justified in a selected group of patients with HCC and MVI, offering a potential benefit in survival with acceptable morbidity.
La invasión macrovascular (IMV) en los pacientes con carcinoma hepatocelular (CHC) es un factor de muy mal pronóstico. El tratamiento de estos casos es todavía controvertido. El objetivo de este estudio es valorar los resultados a corto y largo plazo de la resección hepática asociada a trombectomía en una serie de pacientes con CHC asociado a IMV.
MétodosEstudio retrospectivo de cohortes en los pacientes sometidos a resección hepática por CHC durante el período 2007–2015 (n = 120). Del total, 108 pacientes no presentaban IMV, mientras 12, presentaban al diagnóstico IMV: 1 paciente presentaba IMV en la porta común (Vp4), 8 pacientes en ramas portales de primer orden (Vp3), 1 paciente en ramas sectoriales (Vp2), 1 paciente en ramas segmentarias (Vp1); además, 1 paciente presentaba trombosis en una vena suprahepática principal hasta la entrada en vena cava (Vv2).
ResultadosLos pacientes con IMV necesitaron con mayor frecuencia una hepatectomía mayor frente a los sin IMV (83.3% vs 25.9%, p < 0,0001) sin diferencias en cuanto a mortalidad y morbilidad grave postoperatoria. Los casos con IMV requirieron un tiempo operatorio más largo y desarrollaron con más frecuencia ascitis postoperatoria (33.3% vs 9.3%, p = 0,034).
La supervivencia global a 1, 3 y 5 años fue del 66.7%, 33.3% y 22.2% respectivamente, en los pacientes con IMV y del 90.7%, 72.4% y 52.2% en el grupo sin IMV (p = 0,009).
ConclusiónLa hepatectomía asociada a trombectomía parece estar justificada en un grupo seleccionado de pacientes con CHC e IMV, pudiendo aportar un beneficio de supervivencia con una aceptable tasa de morbilidad.
Hepatocellular carcinoma (HCC) has a tendency to spread through the intrahepatic portal and venous system, and macrovascular invasion (MVI) is a criterion of advanced disease and a very poor prognosis.1 MVI in the portal trunk can contribute to increased portal pressure, with the risk of bleeding from varicose veins, ascites and hepatic decompensation. Tumor thrombosis in the hepatic veins or their extension to the vena cava can cause pulmonary embolism or intrapulmonary dissemination after embolization of tumor thrombi into the right atrium. Without treatment, HCC with MVI is associated with a median survival of less than 3 months.2
Treatment options in these advanced cases are partially directed in Western countries by the current guidelines of the American Association for the Study of Liver Diseases (AASLD)3 and the European Association for the Study of the Liver (EASL),4 based on the therapeutic algorithm of the Barcelona Clinic for Liver Cancer (BCLC),5 which recommends systemic therapy in cases of MVI (stage C).
However, a select group of patients with HCC and portal vein thrombosis may also benefit from surgical treatment or intra-arterial therapies, which is why both the American Hepato-Pancreato-Biliary Association6 and the Japanese Hepatology Society7 have included these treatments as therapeutic options.
In this study, we have analyzed the surgical technique, postoperative complications and long-term survival of a series of patients with HCC associated with MVI, comparing them with those of patients treated surgically in the same period without MVI. In addition, univariate and multivariate studies have been used to try to detect the risk factors associated with a worse prognosis.
MethodsWe present a cohort study in patients undergoing liver resection for HCC with and without macroscopic thrombosis between 2007 and 2015. The study was approved by the Clinical Research Ethics Committee of the Hospital General Universitario Gregorio Marañón.
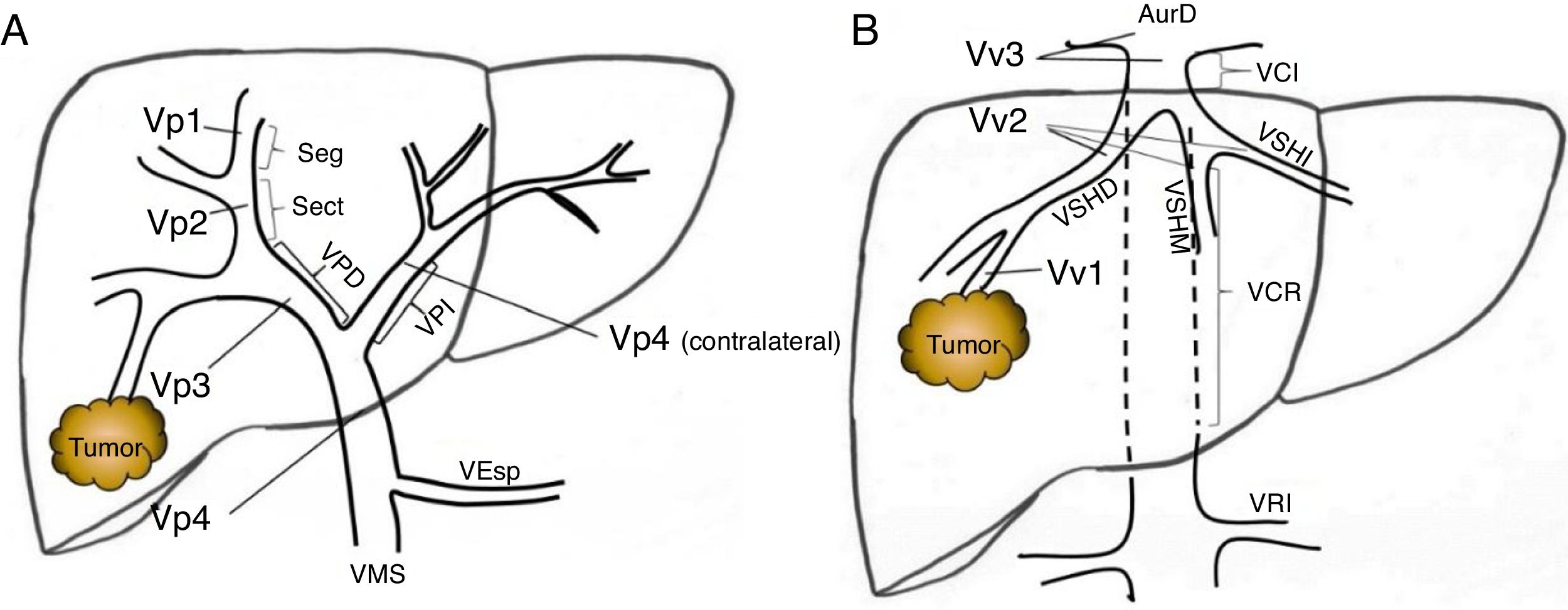
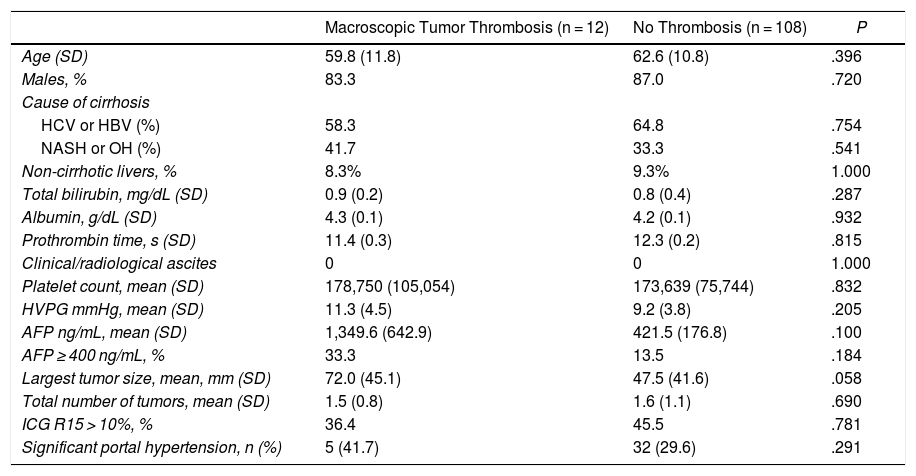
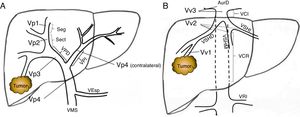
Preoperative StagingExtrahepatic disease was ruled out by thoracic-abdominal-pelvic computed tomography (CT) scan and a scintigraphy. Liver tumor burden was studied with CT and/or magnetic resonance imaging (MRI), at least. The differential diagnosis between benign thrombus and tumor thrombus was made preoperatively using radiological criteria (arterial phase hypervascularization with portal phase washout), by CT or with contrast ultrasound (Sonovue®, Bracco, Italy). In uncertain cases, an ultrasound-guided needle biopsy (18 G needle) was performed for histological study of the thrombus. The level of portal and venous tumor involvement was classified according to the nomenclature of the Japanese staging system8 (Fig. 1).
Classification of the portal tumor thrombosis level (A) and the suprahepatic/cava venous system (B) according to the Japanese nomenclature.
A: Vp1, presence of the tumor thrombus in segmental (Seg) or subsegmental branches; Vp2, tumor thrombus in the secondary portal branches (sectorial, Sect); Vp3, tumor thrombus in the first-order branches (right vena porta or left vena porta); Vp4, tumor thrombus in the main portal trunk and/or in the contralateral portal system to the affected lobe.
B: Vv1, presence of tumor thrombus in the peripheral hepatic venous branches, including microvascular invasion; Vv2, tumor thrombus in the main suprahepatic venous branches; Vv3, tumor thrombus in the inferior vena cava.
AurR: right auricle; IVC: inferior vena cava; RVC: retrohepatic vena cava; VSp: splenic vein; SMV: superior mesenteric vein; RVP: right vena porta; LVP: left vena porta; LRV: left renal vein; RSHV: right suprahepatic vein; LSHV: left suprahepatic vein; MSHV: medial suprahepatic vein.
Preoperative planning included liver volumetry and transjugular measurement of hepatic venous pressure gradient (HVPG) to determine the degree of portal hypertension (considered clinically significant if ≥10 mmHg)9 and a clearance study of indocyanine green (ICG) to determine the hepatic functional reserve.10
In 47 patients (39.2%), since there was no direct measurement of HVPG, platelet counts of less than 100/nL associated with hypersplenism greater than 12 cm were considered criteria for clinically significant portal hypertension.11 Of the patients classified with significant portal hypertension (a total of 37 in the entire series), only for 3 (8.1%) was the indirect criterion used. Only 1 patient (8.3%) of the cohort with MVI (no significant portal hypertension data) was classified based on indirect criteria.
Criteria for Surgical EesectionAll patients were evaluated by the Multidisciplinary Committee for Hepatocellular Carcinoma, which, despite using the BCLC algorithm,5 extended the criteria for surgical resection supported by the results of several international centers.12–14
Surgery was indicated in patients with macroscopic thrombosis if they met the following criteria: 1) preserved liver function (Child-Pugh A) without the presence of ascites or previous decompensation; 2) possibility to perform a complete tumor resection (R0), including the tumor thrombus and leaving a liver remnant volume greater than 40% of the total volume in case of cirrhotic liver; 3) absence of contralateral portal and vena cava invasion; and 4) absence of extrahepatic disease.
The presence of significant portal hypertension was not considered a strict exclusion criterion for resection.15,16
Surgical ProcedureIn cases with MVI, the following was performed: 1) intraoperative re-staging with ultrasound in B-mode and with contrast to determine the size and extension of the MVI, in addition to checking for possible secondary modifications of the portal or venous flow; 2) first the portal and parenchymal resection with minimal manipulation, using an anterior approach; and then, 3) anatomical liver resection.
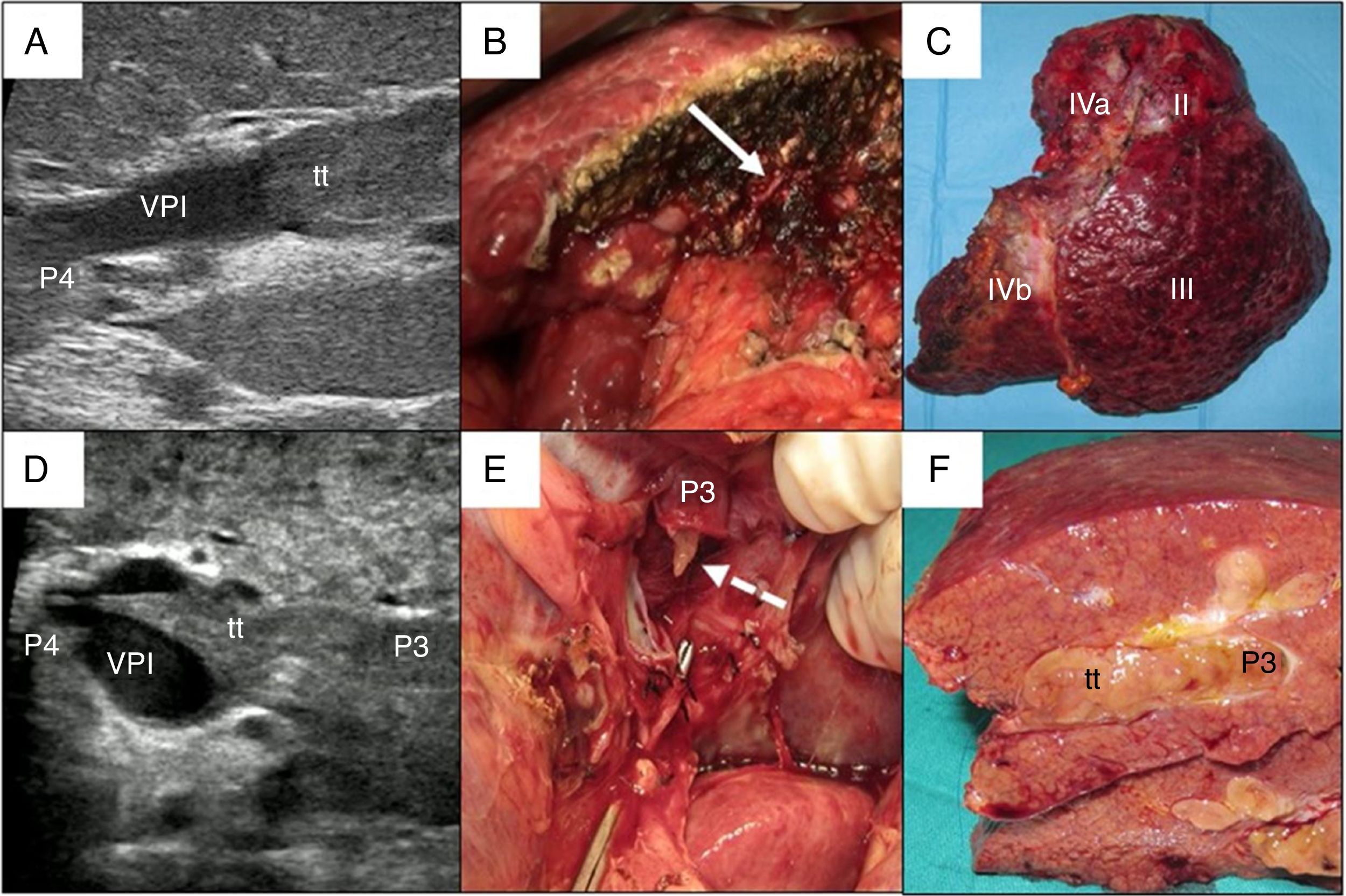
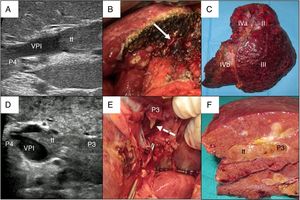
In cases with malignant portal thrombus, two hepatectomy modalities were used: 1) en bloc resection17 (Fig. 2A–C), when there was sufficient remaining volume and a cuff between the portal branch to be divided and the proximal end of the thrombus; and 2) the ‘peeling off’ technique18 (Fig. 2D–F) in cases with reduced ICG clearance and/or insufficient remaining liver volume in the case of performing an en bloc resection. Peeling off saves parenchyma through a portal thrombectomy when the front of the thrombus extends beyond the portal branch to be ligated. This technique includes: 1) vascular control and proximal portal clamping distal-lateral to the area of origin of the thrombus; 2) portal venotomy and thrombus extraction; 3) lavage of the portal branches with heparinized saline under pressure and drag after proximal partial detachment; and 4) suture of the venotomy (remnant liver). In the patient with tumor thrombus located in the suprahepatic vein, the procedure was reversed, first performing parenchymal resection with the anterior approach and then lateral clamping of the retrohepatic vena cava to verify the complete removal of the thrombus.
Examples of en bloc left hepatectomy (case 1, A–C) and lateral sectorectomy with the ‘peeling off’ technique (case 2, D–F).
Case 1. A) Intraoperative ultrasound of the lateral sector showing the front of the tumor thrombus (tt) originating in the portal branch of segment II, penetrating partially towards the lumen of the left portal vein (LPV), leaving free the portal branches of segment IV (P4); B) with an adequate liver remnant, en bloc resection is executed (left portal vein stump indicated with arrow); C) Left hepatectomy (segments II-III-IV).
Case 2. D) In this other case, the intraoperative ultrasound of the lateral sector shows the tumor thrombus (tt) that, from segment III, completely crosses the lumen of the left portal vein (LPV) and enters the portal vein of segment IV (P4); E) Given the insufficient remnant volume to perform a left hepatectomy, the peeling off technique allows us to peel off the front of the tumor thrombus through a venotomy in the umbilical sector of the LPV. The dashed arrow indicates the tt appearing through the P3 (portal vein of segment III); F) Lateral sectorectomy piece, with tumor thrombus (tt) completely occupying the portal lumen.
A total of 120 patients with HCC resected surgically were included in the study. The study cohort (n = 12) included patients with HCC and malignant thrombosis (11 patients with MVI in the portal system and 1 patient with MVI located in the hepatic venous system); the cohort without MVI included 108 patients, 15 of which were stage 0 (13.9%), 69 in stage A (63.9%) and 24 in stage B (22.2%).
We have retrospectively analyzed: demographic variables, etiology of the hepatopathy, preoperative liver function parameters, liver tumor burden (including preoperative alpha-fetoprotein [AFP] values), surgical technique (type of hepatectomy, transient ischemia time, associated procedures such as radiofrequency ablation (RFA), alcoholization or atypical limited liver resections (tumorectomies), operative time and need for transfusion of packed red blood cells (pRBC). General postoperative complications (classified according to the Dindo-Clavien scale) were studied19 along with other specific complications of postoperative hepatic dysfunction (liver failure according to the “50-50 criteria”,20 development of ascites). The AFP values have been studied as a continuous and categorical variable, using 400 ng/mL as a cut-off point, in accordance with the CLIP staging system.21 In addition, data were also collected for the postoperative treatments of possible recurrences.
Postoperative Follow-upThe follow-up of the surgical patients has been conducted routinely with thoracoabdominal-pelvic CT and AFP one month after surgery and then (except for intercurrent clinical findings) with CT and AFP every 3 months. In case of doubt of intrahepatic recurrence, MRI was performed instead of CT. All the corresponding diagnostic and therapeutic decisions were agreed upon in the HCC Multidisciplinary Committee of our institution.
Statistical AnalysisThe categorical variables between the groups have been compared using the chi-squared or Fisher’s exact test, while continuous variables have been compared with the Student’s t or the Mann–Whitney test. The survival analysis was calculated with Kaplan–Meier curves. Survival curves have been compared with the log-rank test. A multivariate Cox analysis was used to identify variables that were independently associated with a worse survival among the significant variables in the univariate analysis. The data have been analyzed using SPSS® statistical software (IBM®, version 20).
ResultsThe two cohorts did not present significant preoperative differences, although it should be noted that there was a tendency in patients with MVI to present a moderately larger tumor size (Table 1).
Comparison of Demographic and Preoperative Data Between the Two Groups.
| Macroscopic Tumor Thrombosis (n = 12) | No Thrombosis (n = 108) | P | |
|---|---|---|---|
| Age (SD) | 59.8 (11.8) | 62.6 (10.8) | .396 |
| Males, % | 83.3 | 87.0 | .720 |
| Cause of cirrhosis | |||
| HCV or HBV (%) | 58.3 | 64.8 | .754 |
| NASH or OH (%) | 41.7 | 33.3 | .541 |
| Non-cirrhotic livers, % | 8.3% | 9.3% | 1.000 |
| Total bilirubin, mg/dL (SD) | 0.9 (0.2) | 0.8 (0.4) | .287 |
| Albumin, g/dL (SD) | 4.3 (0.1) | 4.2 (0.1) | .932 |
| Prothrombin time, s (SD) | 11.4 (0.3) | 12.3 (0.2) | .815 |
| Clinical/radiological ascites | 0 | 0 | 1.000 |
| Platelet count, mean (SD) | 178,750 (105,054) | 173,639 (75,744) | .832 |
| HVPG mmHg, mean (SD) | 11.3 (4.5) | 9.2 (3.8) | .205 |
| AFP ng/mL, mean (SD) | 1,349.6 (642.9) | 421.5 (176.8) | .100 |
| AFP ≥ 400 ng/mL, % | 33.3 | 13.5 | .184 |
| Largest tumor size, mean, mm (SD) | 72.0 (45.1) | 47.5 (41.6) | .058 |
| Total number of tumors, mean (SD) | 1.5 (0.8) | 1.6 (1.1) | .690 |
| ICG R15 > 10%, % | 36.4 | 45.5 | .781 |
| Significant portal hypertension, n (%) | 5 (41.7) | 32 (29.6) | .291 |
AFP: alpha-fetoprotein; ICG R15: indocyanine green retention test (15 min); NASH: non-alcoholic steatohepatitis; HBV: hepatitis B virus; HCV: hepatitis C virus.
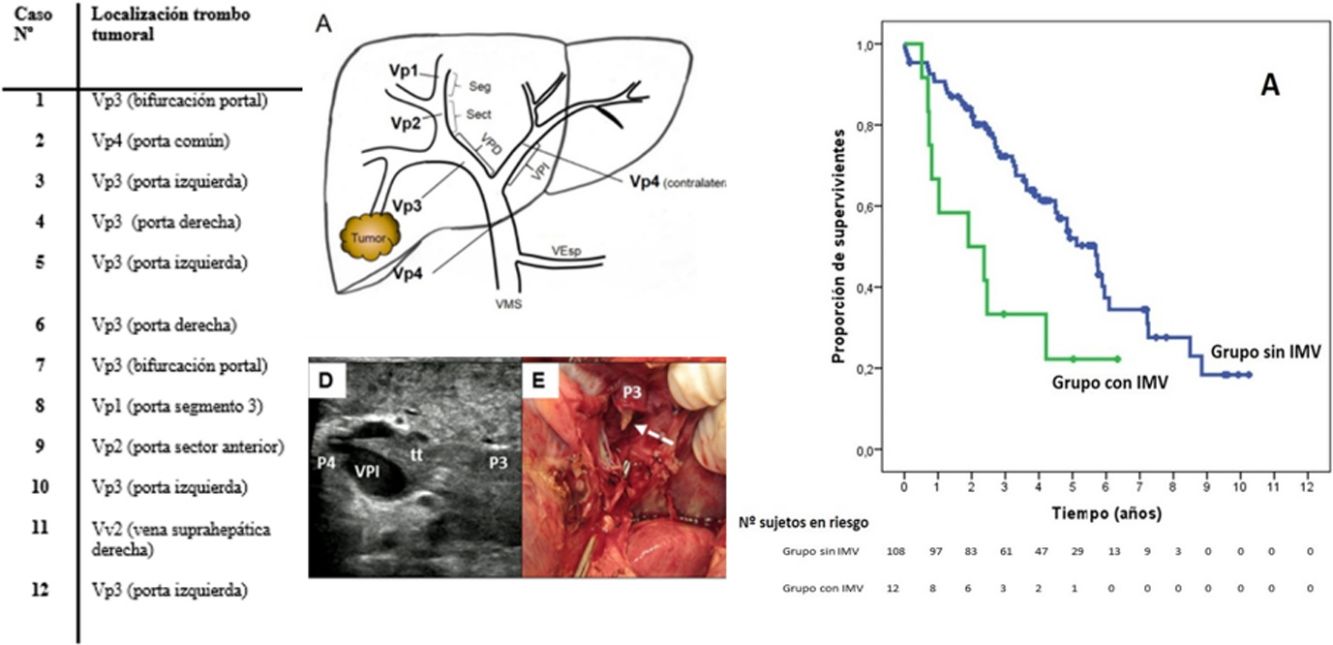
The 12 patients with MVI were treated with 10 major resections (3 or more liver segments according to the Couinaud classification) and 2 minor resections (up to 2 segments) (Table 2). In proportion, in the group with MVI, major resections were performed in 83.3% of the cases compared to 25.9% in the group without MVI (P < .0001). The operative time was on average longer in the patients with MVI than in the cohort without MVI (348.3 ± 71.8 vs. 283.4 ± 108.1 min, P = .045).
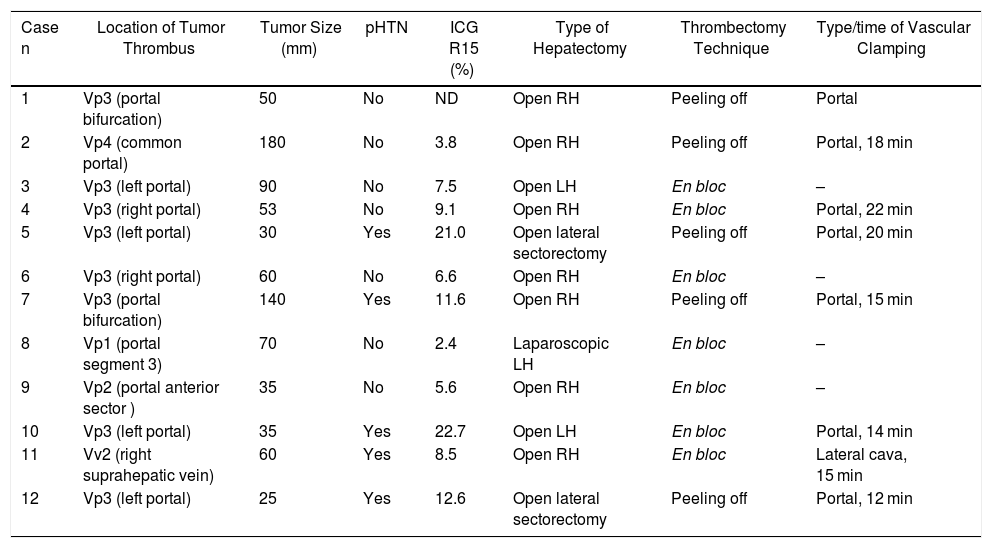
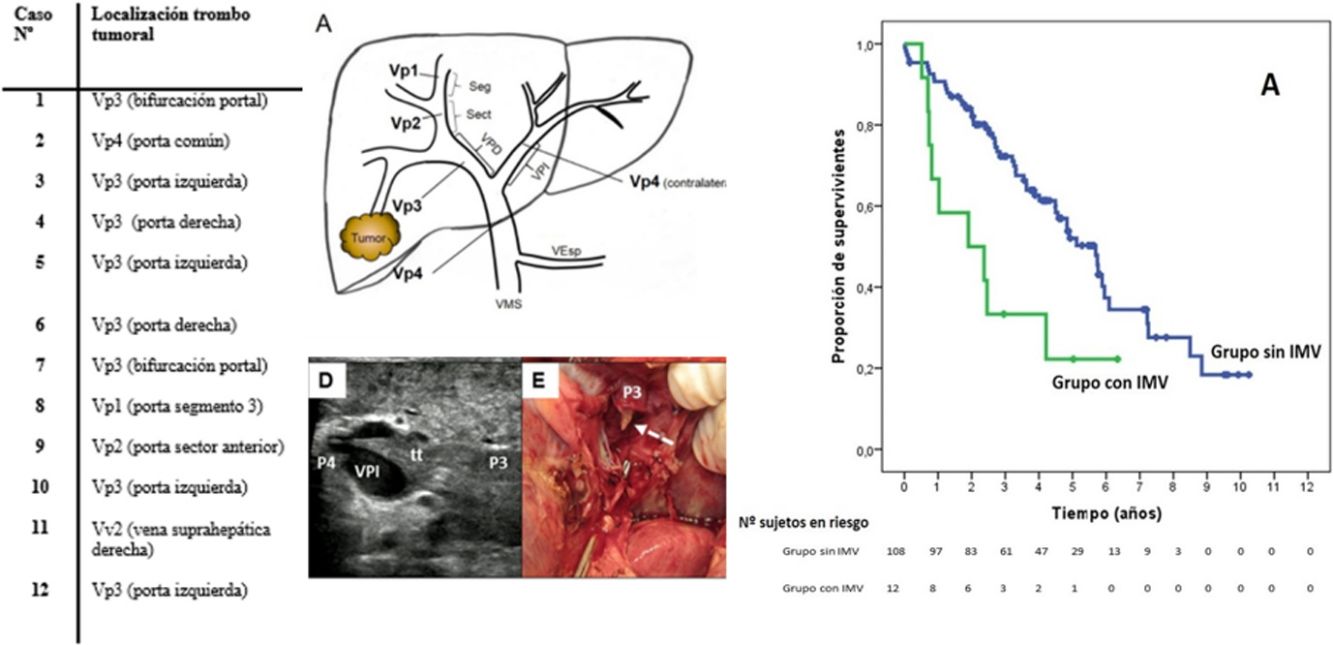
Extension of the Macrovascular Invasion (MVI) in the 12 Cases and Technique Used.
| Case n | Location of Tumor Thrombus | Tumor Size (mm) | pHTN | ICG R15 (%) | Type of Hepatectomy | Thrombectomy Technique | Type/time of Vascular Clamping |
|---|---|---|---|---|---|---|---|
| 1 | Vp3 (portal bifurcation) | 50 | No | ND | Open RH | Peeling off | Portal |
| 2 | Vp4 (common portal) | 180 | No | 3.8 | Open RH | Peeling off | Portal, 18 min |
| 3 | Vp3 (left portal) | 90 | No | 7.5 | Open LH | En bloc | – |
| 4 | Vp3 (right portal) | 53 | No | 9.1 | Open RH | En bloc | Portal, 22 min |
| 5 | Vp3 (left portal) | 30 | Yes | 21.0 | Open lateral sectorectomy | Peeling off | Portal, 20 min |
| 6 | Vp3 (right portal) | 60 | No | 6.6 | Open RH | En bloc | – |
| 7 | Vp3 (portal bifurcation) | 140 | Yes | 11.6 | Open RH | Peeling off | Portal, 15 min |
| 8 | Vp1 (portal segment 3) | 70 | No | 2.4 | Laparoscopic LH | En bloc | – |
| 9 | Vp2 (portal anterior sector ) | 35 | No | 5.6 | Open RH | En bloc | – |
| 10 | Vp3 (left portal) | 35 | Yes | 22.7 | Open LH | En bloc | Portal, 14 min |
| 11 | Vv2 (right suprahepatic vein) | 60 | Yes | 8.5 | Open RH | En bloc | Lateral cava, 15 min |
| 12 | Vp3 (left portal) | 25 | Yes | 12.6 | Open lateral sectorectomy | Peeling off | Portal, 12 min |
RH: right hepatectomy; LH: left hepatectomy; pHTN: clinically significant portal hypertension; ICG R15: indocyanine green 15-min clearance retention rate; Vp1: tumor thrombus in segmental portal branch; Vp2: tumor thrombus in sector; Vp3: tumor thrombus in first-order portal branch; Vp4: tumor thrombus in common or contralateral portal vein; Vv2: tumor invasion of main hepatic vein branch.
There was no difference in the two groups regarding the need for transfusion of perioperative packed red blood cell units (Table 3).
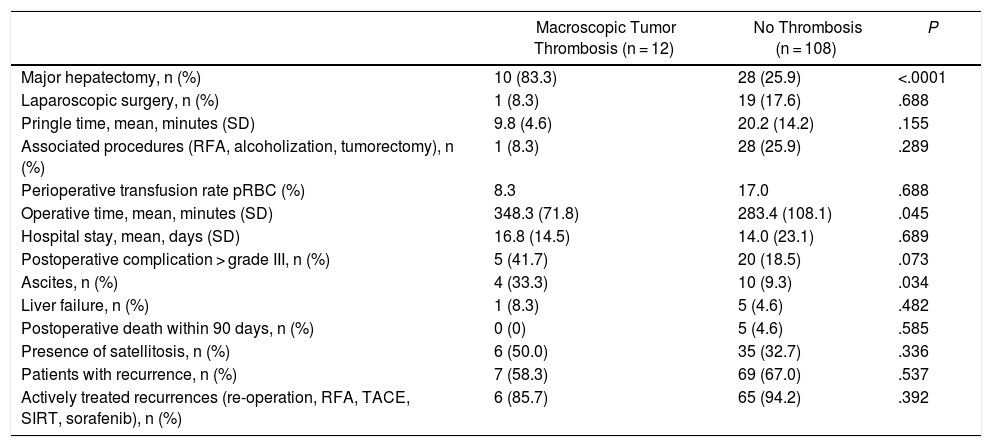
Comparison of Intraoperative and Postoperative Variables Between the Two Cohorts.
| Macroscopic Tumor Thrombosis (n = 12) | No Thrombosis (n = 108) | P | |
|---|---|---|---|
| Major hepatectomy, n (%) | 10 (83.3) | 28 (25.9) | <.0001 |
| Laparoscopic surgery, n (%) | 1 (8.3) | 19 (17.6) | .688 |
| Pringle time, mean, minutes (SD) | 9.8 (4.6) | 20.2 (14.2) | .155 |
| Associated procedures (RFA, alcoholization, tumorectomy), n (%) | 1 (8.3) | 28 (25.9) | .289 |
| Perioperative transfusion rate pRBC (%) | 8.3 | 17.0 | .688 |
| Operative time, mean, minutes (SD) | 348.3 (71.8) | 283.4 (108.1) | .045 |
| Hospital stay, mean, days (SD) | 16.8 (14.5) | 14.0 (23.1) | .689 |
| Postoperative complication > grade III, n (%) | 5 (41.7) | 20 (18.5) | .073 |
| Ascites, n (%) | 4 (33.3) | 10 (9.3) | .034 |
| Liver failure, n (%) | 1 (8.3) | 5 (4.6) | .482 |
| Postoperative death within 90 days, n (%) | 0 (0) | 5 (4.6) | .585 |
| Presence of satellitosis, n (%) | 6 (50.0) | 35 (32.7) | .336 |
| Patients with recurrence, n (%) | 7 (58.3) | 69 (67.0) | .537 |
| Actively treated recurrences (re-operation, RFA, TACE, SIRT, sorafenib), n (%) | 6 (85.7) | 65 (94.2) | .392 |
pRBC: packed red blood cells; RFA: radiofrequency ablation.
The morbidity rates of grade III or higher on the Dindo-Clavien scale19 were comparable in the two cohorts (Table 3). However, the incidence of postoperative ascites was 3 times higher in patients with MVI (33.3% vs. 9.3%, P = .034). The incidence of postoperative liver failure and hospitalization time were similar in both groups. There was no 90-day mortality in patients with MVI, while 5 patients (4.6%) in the group without MVI died in the postoperative period (P = .585) due to liver failure in 3 cases and refractory multiple organ shock after polytransfusion in the other 2.
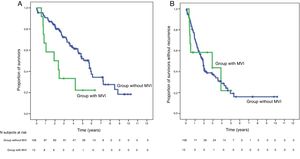
SurvivalWith a median follow-up time of 81.3 months, the overall 1, 3 and 5-year survival rates in patients with MVI were 66.7, 33.3 and 22.2%, respectively, with a median study time of 22.8 months, and 90.7, 72.4 and 52.2% in the group without MVI (P = .009), with a median of 65.7 months (Fig. 3A). There were no differences in recurrence-free survival in the two groups, which was 58.3, 43.7 and 21.9% in cases with MVI, and 70.5, 37.4 and 19.2% in the group without MVI after 1, 3 and 5 years, respectively (P = .988) (Fig. 3B). Following the criteria of the Hepatocellular Carcinoma Committee, we actively treated six out of the 7 patients (85.7%) who relapsed in the cohort with MVI (with sorafenib, transarterial chemoembolization [TACE], RFA) and 65 of the 69 patients (94.2%) without MVI who relapsed (with re-surgery, RFA, TACE, sorafenib) (P = .392) (Table 3).
The univariate analysis identified variables for a poor prognosis, including the presence of MVI, tumor size greater than 10 cm, the need for transfusion of pRBC and the presence of satellitosis in the pathology results. The multivariate model confirmed as poor prognostic factors the presence of MVI (hazard ratio [HR]: 2.248; 95% confidence interval [CI]: 1.067–4.734; P = .033) and satellitosis (HR: 2.015; 95% CI: 1.140–3.561; P = .016).
DiscussionIn the current American (AASLD)3 and European (EASL)4 guidelines based on the BCLC therapeutic algorithm,5 the recommended treatment in the presence of MVI (stage C) is systemic therapy with sorafenib, with a median reported survival of 10.7 months.22 It should be noted that the latest European guidelines contemplate the possibility of surgical resection in selected cases with portal segmental or sector invasion, but not as a standard treatment.
The HCC Multidisciplinary Committee at our institution made the exceptional decision to indicate surgery for these patients based on the extensive experience of several Eastern and Western groups, which, in clinical practice, exceed the indications of the AASLD-EASL with acceptable survival benefits for the patients.12,17,23–25 Therefore, our series is one of the pioneers in Spain to collect the results of liver resection in stage C of BCLC in a select group of patients.
Liver transplantation is contraindicated in the presence of macroscopic tumor thrombosis because it is a factor of early tumor recurrence (within 1 year) and because it affects overall survival.26–29
Among the locoregional treatments, TACE (despite being historically contraindicated due to the supposed risk of triggering atrophy or hepatic necrosis in an area that is already compromised from a vascular standpoint30) has demonstrated a good safety profile even in patients with MVI in the common portal thanks to more selective arterial catheterization techniques31–33 and results similar to those obtained with sorafenib, although prospective comparative studies are still needed.
Selective internal radiotherapy (SIRT) with yttrium microspheres90 does not seem to have better oncological outcomes for intermediate and advanced stages in terms of overall survival than systemic treatment with sorafenib, as demonstrated by the recent SARAH34 and SIRveNIB35 randomized clinical trials.
Another promising therapeutic option is stereotactic body radiation therapy (SBRT), which can offer local disease control also in the presence of MVI.36,37
Accidentally, Kumada et al.38 used thrombectomy at the beginning of the 1990s as an urgent portal decompression method to prevent the risk of variceal hemorrhage: the patients had a longer-than-expected survival of up to 16 months on average. Next, Minagawa et al.39 presented a first series (n = 18) of patients undergoing surgical resection, after having responded to TACE, as a treatment for hepatocellular carcinoma with portal MVI and without distant metastasis, demonstrating a 5-year survival of 42%. Since then, the Japanese national registry has been making an inventory of the long-term results of the indication for surgical resection in cases of portal MVI (both Child A and B). Recently published data by Kokudo et al.25 indicate that the median survival in patients after surgical resection (n = 2,093) was 1.77 years longer than that of the non-surgical group (n = 4,381), who had received locoregional treatment (TACE, ablation), systemic chemotherapy (except sorafenib) or supportive treatment.
This experience from Asian countries has also been extended to other Western tertiary hospitals. A multicenter study published in 201323 that collected data from 10 hepatobiliary-pancreatic surgery departments (only 3 Asian, 4 European and 3 North American) shows that in ‘real life’, and not only in Asia, many teams are looking for alternatives to the rigid indications of the BCLC algorithm, offering surgery with radical intention also to patients with HCC in stages B and C of the BCLC.
Our study has certain limitations: 1) its retrospective design; 2) a selection bias, as only patients with relatively preserved liver function, without invasion of the contralateral portal or vena cava, were selected for surgical resection; 3) a limited sample size (n = 12) and belonging to a single center; and 4) other postoperative treatments (sorafenib, RFA, re-operation, TACE, SIRT) have been applied in a dispersed and individualized manner (dose, time of indication, tolerance), so they are difficult to interpret and regroup for statistical analysis in a large part of the patients in both cohorts, which may have influenced the overall survival of the entire series.
It should be noted that, according to the recommendations of the AASLD-EASL, stage C patients only receive systemic treatment for purely palliative purposes. However, the surgical treatment of selected patients in our series has made it possible to prolong survival (with a median of almost 2 years), leaving the door open to treatments for possible recurrences.
We can report that no differences in recurrence-free survival were found in the two groups. In our opinion, this can be explained by two recognized risk factors for recurrence of HCC: the high prevalence (>90%) in both groups of cirrhotic livers, and the presence of 24 stage B patients (22.2%) in the group without MVI.
Despite the limitations listed above, this series demonstrates that, in patients with HCC associated with MVI without extrahepatic dissemination and presenting an acceptable hepatic functional reserve, liver resection with thrombectomy is a valid therapeutic option except in cases with contralateral portal and vena cava involvement. It offers a median survival of up to 22.8 months at the expense of an elevated risk of postoperative ascites and a longer operative time.
Conflict of InterestsThe authors have no conflict of interests to declare regarding the publication of this manuscript.
The authors would like to thank María del Carmen de la Cruz, from the Instituto de Investigación Sanitaria del Gregorio Marañón (IiSGM), for her help editing the protocol for the Ethics Committee. Thanks also go to José María Bellón, from the Unidad de Apoyo al Diseño de Proyectos y Análisis estadístico (IiSGM), for his help with the statistical analysis.
Please cite this article as: Resección hepática con trombectomía en el tratamiento del carcinoma hepatocelular con invasión vascular macroscópica Cortese S, Morales J, Martín L, Kayser S, Colón A, Ramón E, et al. Resección hepática con trombectomía en el tratamiento del carcinoma hepatocelular con invasión vascular macroscópica. Cir Esp. 2020;98:9–17.