Frailty degree can influence more than age or severity in the outcome of patients older than 70 years undergoing surgery of the digestive system that require immediate postoperative control in the ICU.
MethodsA prospective and observational study of patients over 70 years of age who were admitted to the surgical ICU of a third level hospital immediately after an elective or emergent surgical intervention on the digestive system from June 1, 2018 until June 1, 2019. The variables age, frailty Clinical Frailty Scale (CFS), and modified Frailty Index (mFI), severity (APACHE II), type of surgery, surgical pathology were recorded upon admission. A bivariate analysis was performed to assess the influence of frailty and severity on hospital morbidity and mortality and baseline situation of the patient (in terms of dependence) at 6 months.
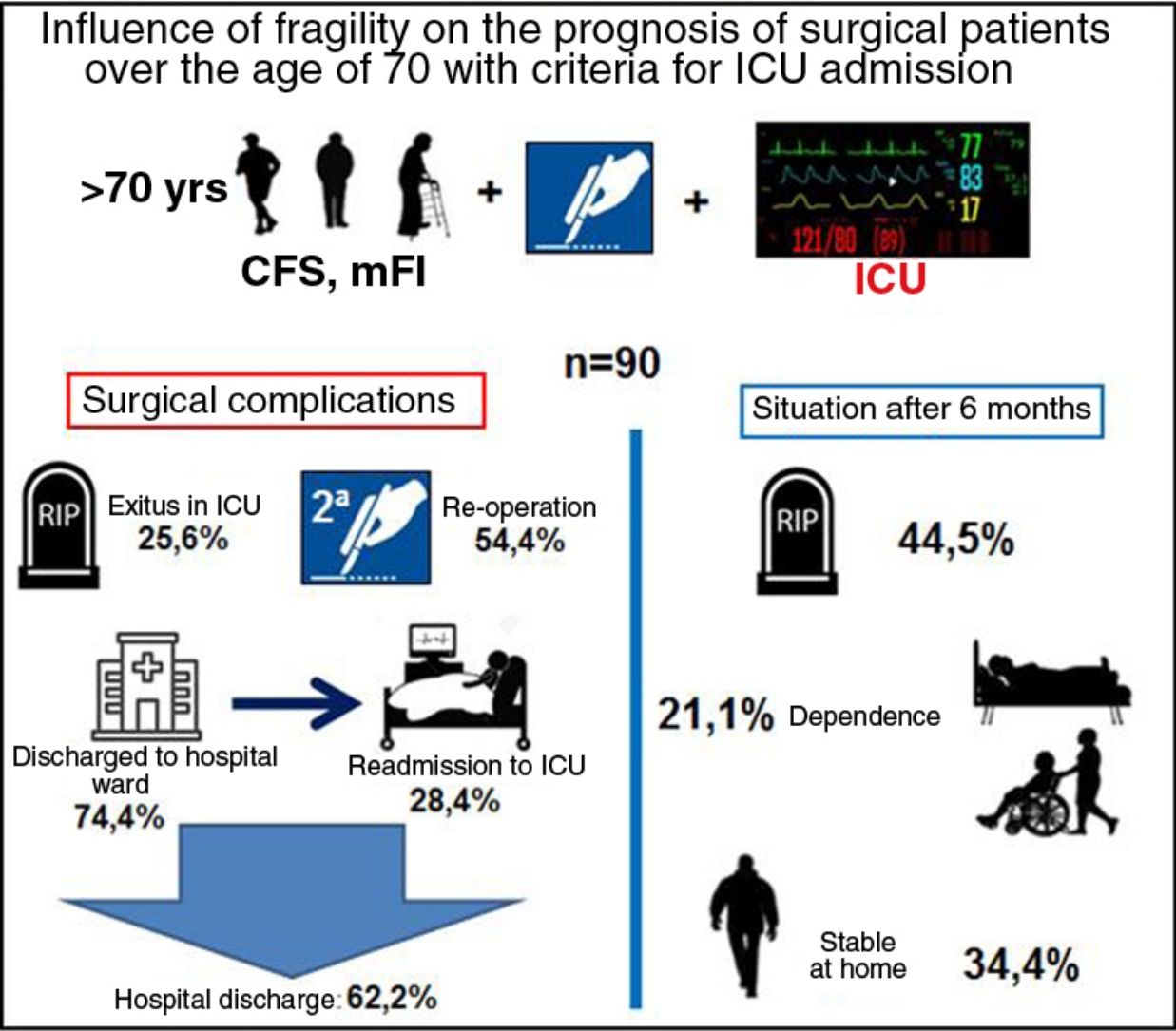
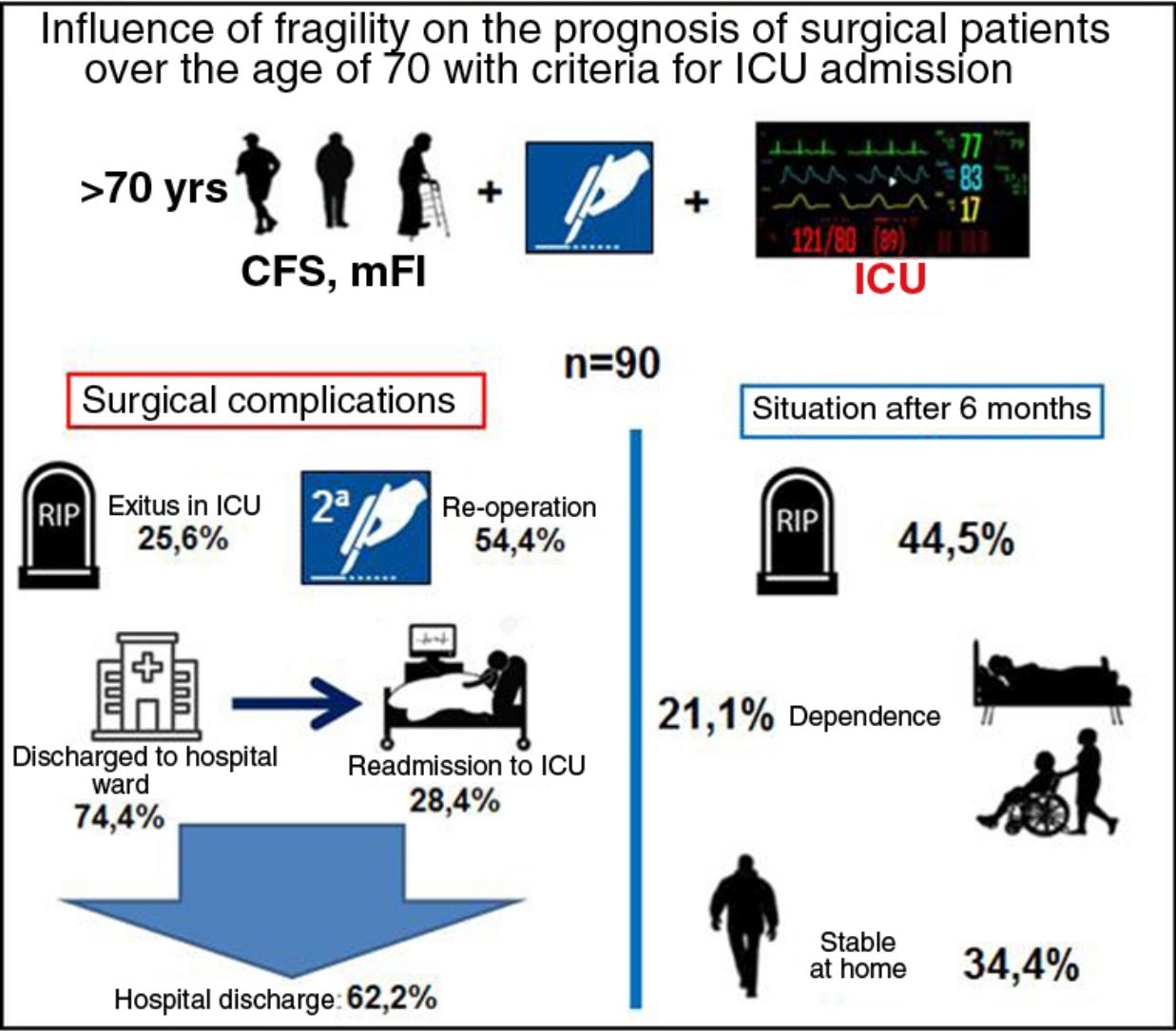
ResultsA total of 90 patients were recruited, 54.4% of whom were reoperated; 74.4% were initially discharged from the ICU, with 28.4% of readmission and directly associated to frailty (CFS and mFI: P<0.01). The overall mortality at 6 months was 44.5% being CFS (OR=64.3; P<0.05, 95% CI: 12.3–333.9) and APACHE II (OR=1.17; P<0.05; 95% CI: 1.04–1.32) the covariates that best related.
ConclusionsThe estimation of frailty by CSF and mFI is directly associated to the surgical morbidity and readmission of elderly and severe patients admitted to the ICU. In addition, CFS and mFI has been efficient as a predictive of mortality at 6 months.
El grado de fragilidad puede influir más que la edad o la gravedad en el pronóstico de pacientes mayores de 70 años intervenidos de cirugía del aparato digestivo que precisan control postoperatorio inmediato en UCI.
MétodosEstudio prospectivo y observacional de pacientes mayores de 70 años que ingresaron en UCI quirúrgica de un hospital de tercer nivel inmediatamente después de una intervención quirúrgica electiva o urgente sobre el aparato digestivo desde el 1 de junio de 2018 hasta el 1 de junio de 2019. Se registraron al ingreso las variables edad, fragilidad (Clinical Frailty Scale, CFS, y Modified Frailty Index, mFI), gravedad (APACHE II), tipo de cirugía y entidad quirúrgica. Se realizó un análisis bivariante para evaluar la influencia de la fragilidad y gravedad en la morbimortalidad hospitalaria y situación basal del paciente (en cuanto a dependencia) a 6 meses.
ResultadosFueron seleccionados 90 pacientes, de los que el 54,4% fueron reintervenidos; el 74,4% fueron dados de alta inicialmente en UCI, con un reingreso del 28,4% y con relación directa con la fragilidad (CFS y mFI: p<0,01). La mortalidad global a los 6 meses fue 44,5%, con CFS (OR=64,3; p<0,05; IC 95%: 12,3–333,9) y APACHE II (OR=1,17; p<0,05; IC 95%: 1,04–1,32) fueron las covariables que mejor se relacionaron.
ConclusionesLa estimación de la fragilidad mediante CSF y mFI tiene relación directa con la morbilidad quirúrgica y el reingreso de pacientes graves de edad avanzada ingresados en UCI. Además, CFS y mFI han resultado eficientes como predictores de mortalidad a los 6 meses.
Currently, frailty is considered an important medical syndrome that is strongly associated with age. Its characteristics include loss of strength, resistance and physiological function, which increase individual vulnerability and reduce the patient’s ability to face different situations that cause stress.1,2
The significant increase in life expectancy3 has resulted in a greater number of elderly patients being admitted to surgical intensive care units,4–7 frequently as a result of digestive surgery.8 This patient group presents significant heterogeneity in terms of comorbidities, level of dependence, cognitive status, socioeconomic circumstances and general physical condition.9
These differences, implicit in the concept of frailty, could be a determining factor in the postoperative prognosis of these patients, regardless of the disease treated. There is evidence that frailty is associated with an increase in postoperative mortality, irrespective of age.10,11
One of the main models used in the calculation of frailty is the ‘accumulation of deficits’ or Frailty Index (FI) proposed by Rockwood.12 It defines deficits as the presence of a clinical or analytical alteration, loss of functional capacity and the deterioration in the social and cognitive sphere, and it is obtained by dividing the number of deficits present between the total number of deficits contemplated. Given the high number of deficits contemplated (70) in the original index, other indices have been proposed, such as the Modified Frailty Index (mFI)13 (Fig. 1), which uses a smaller number of deficits.
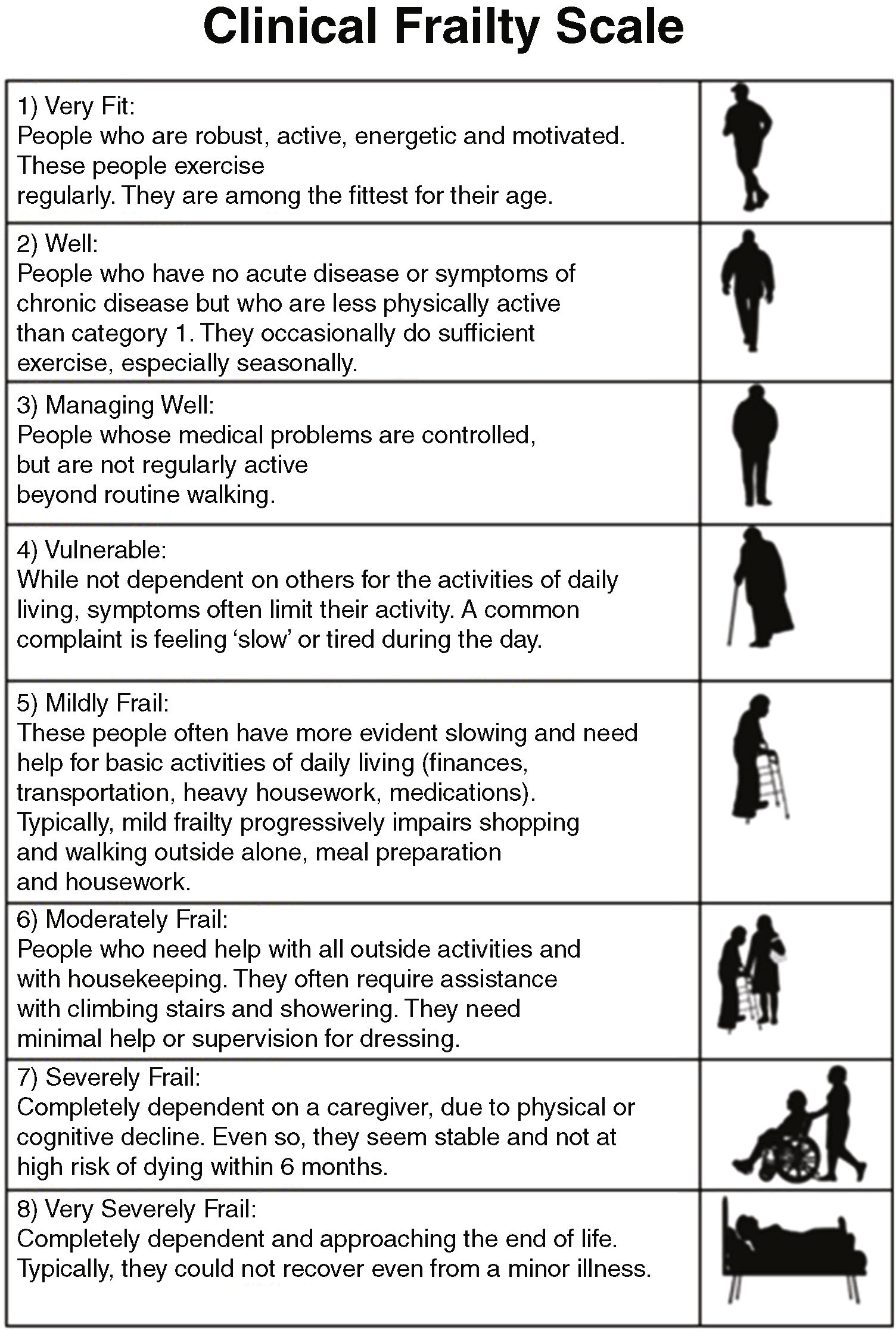
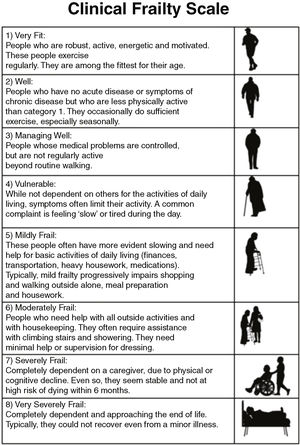
Based on this model of deficit accumulation, Rockwood proposed the Clinical Frailty Scale (CFS)14 (Fig. 2) in 2005. According to this scale, it is possible to classify patients into one of the 8 levels of frailty proposed using data from the patient interview, clinical history and examination, and by means of reasoning and clinical judgment.
The aim of the study is to analyze the influence of frailty on the prognosis of patients over the age of 70 who underwent digestive system surgery and who require, a priori, immediate postoperative control in the ICU, quantifying its severity upon admission using the Acute Physiology and Chronic Health disease Classification System II (APACHE II).
MethodsWe designed a prospective, observational study with a 6-month follow-up, conducted in a surgical intensive care unit (ICU) at a tertiary hospital from June 1, 2018 to June 1, 2019. The main objective was to analyze the influence of frailty, quantified by applying 2 validated scales on the postoperative complications and prognosis of patients 6 months after hospital discharge.
The cohort was made up of a series of successive patients >70 years of age who were admitted to the ICU immediately after digestive system surgery, either on a scheduled or urgent basis. The aim of the study was to evaluate the influence of frailty in the prognosis of surgical patients >70 years with criteria for admission to the ICU, regardless of whether it was urgent or scheduled surgery, focusing on only severity criteria. Surgical patients who were admitted from the hospital ward during the postoperative period due to a complication (including surgical reoperation) were excluded. The level of severity and prognosis at admission was estimated using the APACHE II. The study was approved by the Research Commission and by the Center’s Ethics Committee.
Evaluation of frailty: as one of the objectives of the study, patient selection was defined by age (>70 years) and the indication for postoperative admission to the ICU by the anesthesiologist. For this reason, the evaluation was made during the first hours of admission to the ICU, after informed consent for participation in the study was given by the patient or family member (depending on the level of consciousness). For the frailty estimates, the mFI and CFS were used:
- The mFI is based on the frailty model of deficit accumulation and contemplates 11 pathological antecedents (Fig. 1). Furthermore, it has a continuous quantitative nature, with values that vary between 0 and 1. It was proposed and developed by the National Surgical Quality Improvement Program of the American College of Surgeons. The 11 items of the mFI were selected from among the 70 deficits of the original FI, and the index has been validated by other studies.15,16
- The CFS assesses the level of frailty based on clinical judgment. It grades frailty on a scale from 1 to 8, with values 1 and 2 representing ‘robust’ patients, 3 and 4 ‘pre-frail’ patients and 5 or more ‘frail’ patients.4,17 It was calculated by interviewing the patient (if the level of consciousness so allowed) or their family. This scale has been shown to have a good correlation with the FI.11
The outcome variables were the following:
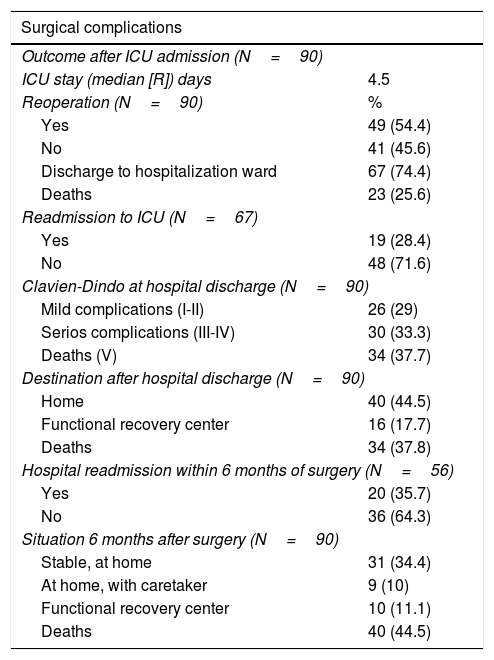
Postoperative complications during hospital admission: days of ICU stay, ICU mortality, need for reoperation (Clavien-Dindo IIIa-IIIb) and need for ICU readmission (Clavien-Dindo IVa-IVb) (Table 1).
Result variables.
| Surgical complications | |
|---|---|
| Outcome after ICU admission (N=90) | |
| ICU stay (median [R]) days | 4.5 |
| Reoperation (N=90) | % |
| Yes | 49 (54.4) |
| No | 41 (45.6) |
| Discharge to hospitalization ward | 67 (74.4) |
| Deaths | 23 (25.6) |
| Readmission to ICU (N=67) | |
| Yes | 19 (28.4) |
| No | 48 (71.6) |
| Clavien-Dindo at hospital discharge (N=90) | |
| Mild complications (I-II) | 26 (29) |
| Serios complications (III-IV) | 30 (33.3) |
| Deaths (V) | 34 (37.7) |
| Destination after hospital discharge (N=90) | |
| Home | 40 (44.5) |
| Functional recovery center | 16 (17.7) |
| Deaths | 34 (37.8) |
| Hospital readmission within 6 months of surgery (N=56) | |
| Yes | 20 (35.7) |
| No | 36 (64.3) |
| Situation 6 months after surgery (N=90) | |
| Stable, at home | 31 (34.4) |
| At home, with caretaker | 9 (10) |
| Functional recovery center | 10 (11.1) |
| Deaths | 40 (44.5) |
R: interquartile range; ICU: intensive care unit.
Prognostic variables at hospital discharge were: discharge destination (home, functional recovery center, or death), need for hospital readmission within 6 months after discharge, baseline situation 6 months after surgery (stable at home, at home with need for caregiver, institutionalized, death).
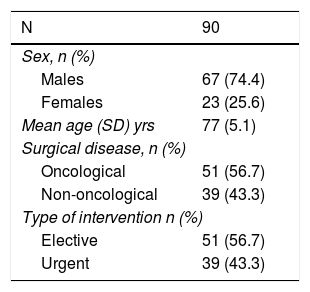
In addition to the above, control and adjustment variables were also collected, including sociodemographic data (such as age and sex), type of surgical disease (oncological or benign), and elective or urgent nature of the surgery (Table 2).
The statistical analysis was carried out using the SPSS version 21 statistical program, with a descriptive study of the variables. The mean was calculated for the adjusted variables, and the median for those that were not. A bivariate analysis was used to study the relationship of the epidemiological, surgical, clinical and frailty covariates with the outcome variables. For the qualitative variables, the chi-square test was applied, while the non-parametric tests of the Mann–Whitney and Kruskal–Wallis U were used for the quantitative variables (according to the number of categories of the variable under study). In cases with a significant Kruskal–Wallis test, multiple comparisons were made between the groups to see which of them had significant differences. When both variables were quantitative, the Spearman correlation coefficient was used. In the multivariate analysis, logistic regression models were used to evaluate the influence of the 5 variables that were significant (age, surgical disease, APACHE II, mFI and CFS) in the bivariate analysis of 6-month mortality after hospital discharge. Results with a P<.05 were considered statistically significant.
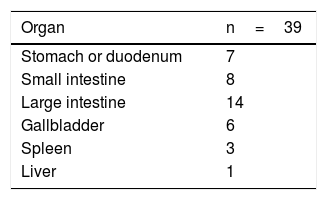
ResultsThe study included 92 patients, but 2 were lost to follow-up within 6 months and therefore excluded. Out of the 90 patients, 67 were men (74.4%). The mean age was 77 years (range 70–92). There were 51 (56.6%) elective procedures, all of which were hepatobiliary-pancreatic surgeries (hepatic resection or pancreaticoduodenectomy), tabulated as complex major surgery according to the British United Provident Association and scored as II or III according to the American Society of Anesthesiologists. This surgical type is usually the main cause for admission to the ICU of General Surgery patients treated with elective procedures versus other types of surgeries. The remaining surgical procedures were urgent. This surgery was the first during hospitalization (Table 3). Regarding the surgical disease of the whole group, 51 patients (56.6%) were oncological.
The mean stay in the ICU was 7.5 days, and the median was 4.5. Regarding frailty, 28 patients were considered ‘robust’ and 62 had different degrees of frailty (pre-frail and frail).
In terms of prognostic variables, 23 patients died in the ICU and 49 (54.4%) were re-operated. Out of the 67 patients (74.4%) who were discharged to the hospital ward, 19 (28.4%) had to be readmitted to the ICU. Fifty-six patients (57.2%) were discharged from hospital: 40 (44.5%) to home, and 16 (17.7%) to a functional recovery center. In the following 6 months, 20 patients (35.7%) were readmitted to the hospital. Finally, the overall 6-month mortality of the series (90 patients) was 44.5% (40 patients).
With regards to the frailty and functional status of the remainder, 31 (34.4%) patients remained stable, with baseline situations similar to their previous situations (possibly because they were robust patients and some were pre-frail). The status of the other patients had worsened: 9 (10%) were at home, but needed a caregiver; and 10 (11.1%) were admitted to a functional recovery center (Table 1), showing no correlation with the group of origin (oncological vs non-oncological).
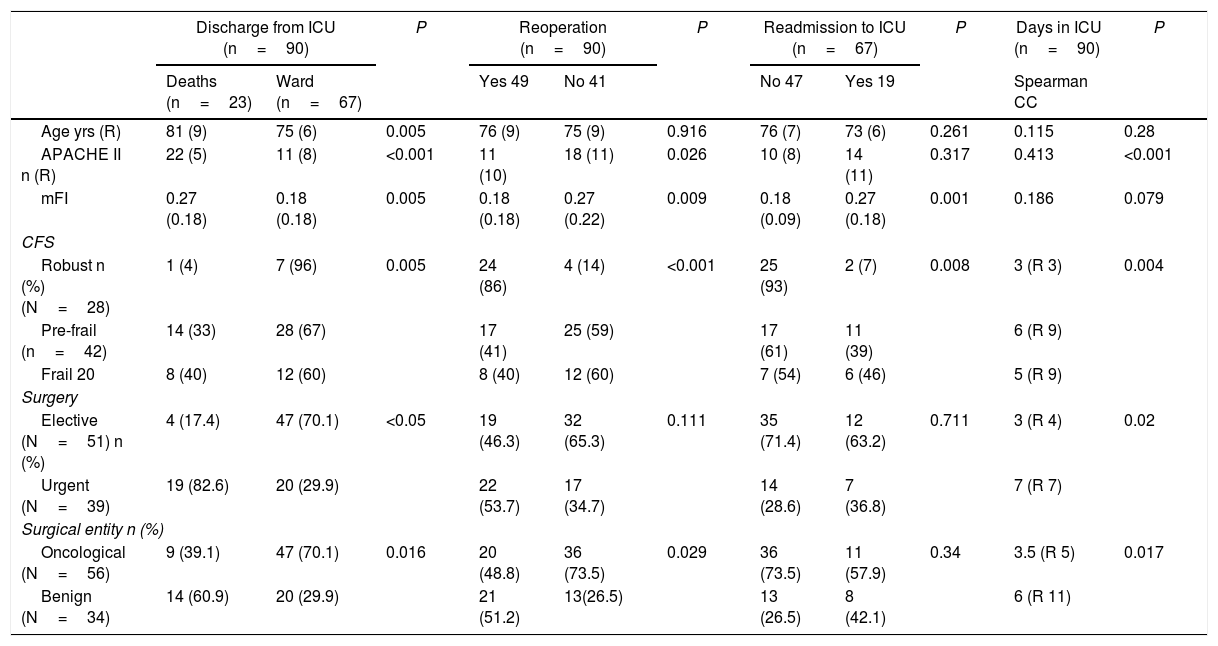
In the bivariate analysis of the surgical complications during hospitalization (Table 4), both the urgent nature of the intervention and the higher APACHE II, age and mFI were associated with greater ICU mortality (P<.05). Furthermore, patients classified as frail and pre-frail according to CFS showed higher mortality compared to robust patients (P<.01). However, of all the cited variables, those with the highest grades on the frailty scales (mFI and CFS) were the only ones that were also related with the need for surgical reintervention or readmission to the ICU (P<.01).
Surgical complications during hospitalization.
| Discharge from ICU (n=90) | P | Reoperation (n=90) | P | Readmission to ICU (n=67) | P | Days in ICU (n=90) | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (n=23) | Ward (n=67) | Yes 49 | No 41 | No 47 | Yes 19 | Spearman CC | |||||
| Age yrs (R) | 81 (9) | 75 (6) | 0.005 | 76 (9) | 75 (9) | 0.916 | 76 (7) | 73 (6) | 0.261 | 0.115 | 0.28 |
| APACHE II n (R) | 22 (5) | 11 (8) | <0.001 | 11 (10) | 18 (11) | 0.026 | 10 (8) | 14 (11) | 0.317 | 0.413 | <0.001 |
| mFI | 0.27 (0.18) | 0.18 (0.18) | 0.005 | 0.18 (0.18) | 0.27 (0.22) | 0.009 | 0.18 (0.09) | 0.27 (0.18) | 0.001 | 0.186 | 0.079 |
| CFS | |||||||||||
| Robust n (%) (N=28) | 1 (4) | 7 (96) | 0.005 | 24 (86) | 4 (14) | <0.001 | 25 (93) | 2 (7) | 0.008 | 3 (R 3) | 0.004 |
| Pre-frail (n=42) | 14 (33) | 28 (67) | 17 (41) | 25 (59) | 17 (61) | 11 (39) | 6 (R 9) | ||||
| Frail 20 | 8 (40) | 12 (60) | 8 (40) | 12 (60) | 7 (54) | 6 (46) | 5 (R 9) | ||||
| Surgery | |||||||||||
| Elective (N=51) n (%) | 4 (17.4) | 47 (70.1) | <0.05 | 19 (46.3) | 32 (65.3) | 0.111 | 35 (71.4) | 12 (63.2) | 0.711 | 3 (R 4) | 0.02 |
| Urgent (N=39) | 19 (82.6) | 20 (29.9) | 22 (53.7) | 17 (34.7) | 14 (28.6) | 7 (36.8) | 7 (R 7) | ||||
| Surgical entity n (%) | |||||||||||
| Oncological (N=56) | 9 (39.1) | 47 (70.1) | 0.016 | 20 (48.8) | 36 (73.5) | 0.029 | 36 (73.5) | 11 (57.9) | 0.34 | 3.5 (R 5) | 0.017 |
| Benign (N=34) | 14 (60.9) | 20 (29.9) | 21 (51.2) | 13(26.5) | 13 (26.5) | 8 (42.1) | 6 (R 11) | ||||
CC: Spearman’s correlation coefficient; R: interquartile range.
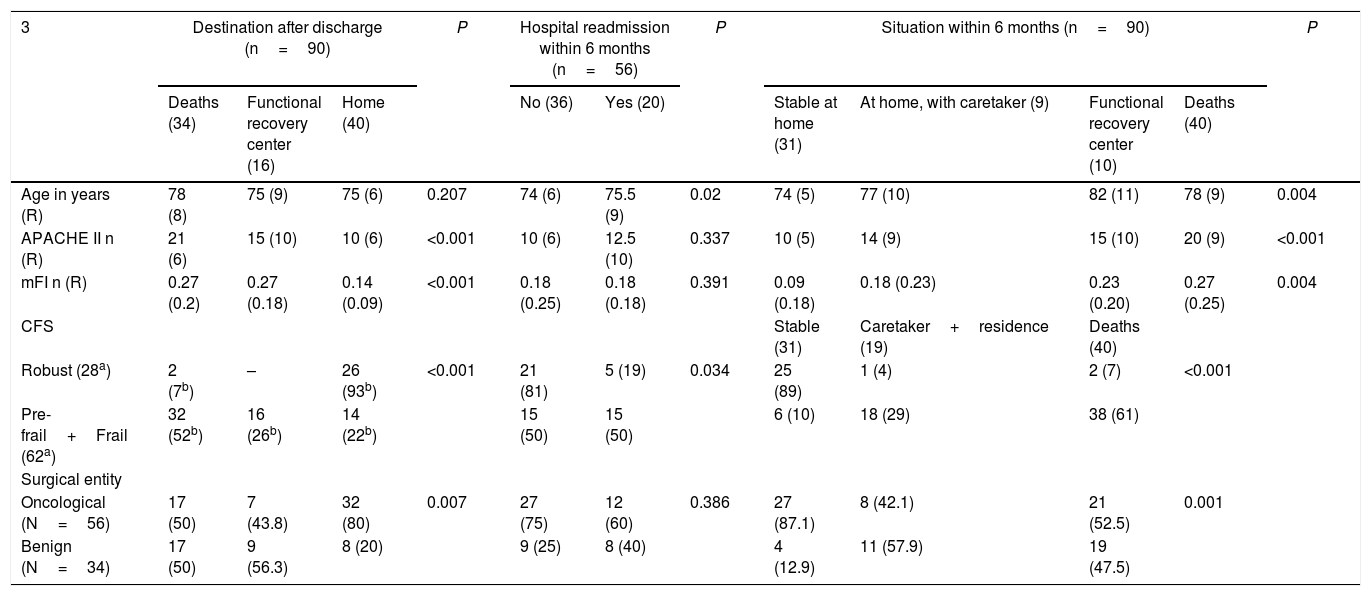
Neither age nor mFI showed a correlation with the number of days spent in the ICU, although APACHE II (P=.01) and CFS did, as it was found that patients classified as robust according to CFS had shorter ICU stays than frail and pre-frail patients (P<.01). As for the rest of the prognostic variables (Table 5), age was the only one that did not correlate with the destination after hospital discharge. In contrast, we observed that oncological disease (P=.007), robust patients (P<0.001), lower mFI scores (P<.001) and APACHE II (P<.001) were related with hospital discharge to home.
Prognostic variables at hospital discharge.
| 3 | Destination after discharge (n=90) | P | Hospital readmission within 6 months (n=56) | P | Situation within 6 months (n=90) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (34) | Functional recovery center (16) | Home (40) | No (36) | Yes (20) | Stable at home (31) | At home, with caretaker (9) | Functional recovery center (10) | Deaths (40) | ||||
| Age in years (R) | 78 (8) | 75 (9) | 75 (6) | 0.207 | 74 (6) | 75.5 (9) | 0.02 | 74 (5) | 77 (10) | 82 (11) | 78 (9) | 0.004 |
| APACHE II n (R) | 21 (6) | 15 (10) | 10 (6) | <0.001 | 10 (6) | 12.5 (10) | 0.337 | 10 (5) | 14 (9) | 15 (10) | 20 (9) | <0.001 |
| mFI n (R) | 0.27 (0.2) | 0.27 (0.18) | 0.14 (0.09) | <0.001 | 0.18 (0.25) | 0.18 (0.18) | 0.391 | 0.09 (0.18) | 0.18 (0.23) | 0.23 (0.20) | 0.27 (0.25) | 0.004 |
| CFS | Stable (31) | Caretaker+residence (19) | Deaths (40) | |||||||||
| Robust (28a) | 2 (7b) | – | 26 (93b) | <0.001 | 21 (81) | 5 (19) | 0.034 | 25 (89) | 1 (4) | 2 (7) | <0.001 | |
| Pre-frail+Frail (62a) | 32 (52b) | 16 (26b) | 14 (22b) | 15 (50) | 15 (50) | 6 (10) | 18 (29) | 38 (61) | ||||
| Surgical entity | ||||||||||||
| Oncological (N=56) | 17 (50) | 7 (43.8) | 32 (80) | 0.007 | 27 (75) | 12 (60) | 0.386 | 27 (87.1) | 8 (42.1) | 21 (52.5) | 0.001 | |
| Benign (N=34) | 17 (50) | 9 (56.3) | 8 (20) | 9 (25) | 8 (40) | 4 (12.9) | 11 (57.9) | 19 (47.5) | ||||
R: interquartile range.
Compared to the baseline situation, after 6 months both age and frailty showed significant differences and, using multiple comparisons, we observed that the youngest patients, cancer patients, patients with lower mFI scores and robust patients according to CFS were associated with being ‘stable at home’ (P<.01). The patients who were readmitted to hospital within 6 months after discharge were older (P=.02), frail and pre-frail according to the CFS classification (P=.034).
In the multivariate analysis, after analyzing several models, we found that the one that more closely predicted 6-month mortality included CFS as covariates (OR=64.3; P<.05; 95% CI: 12.3–333.9) and APACHE II (OR=1.17; P<.05; 95% CI: 1.04–1.32).
DiscussionThe main contribution of this study is that frailty assessment in senior surgical patients can help predict postoperative morbidity and mortality as well as age or other classic tools, such as severity at admission quantified by APACHE II.
It is worth noting that the number of oncology and elective surgery patients (mainly hepatobiliary-pancreatic, which is the elective surgery most frequently associated with ICU admission at our hospital) is the same in this study. This is merely a coincidence. However, most of the scheduled procedures were oncological, while a small percentage of urgent procedures were due to digestive oncological complications.
Many studies agree on the importance of quantifying the frailty of patients with surgical procedures (urgent or scheduled) for prognostic purposes. Most have been designed retrospectively9 and used cohorts of frail or elderly patients who have undergone surgery, demonstrating the significant association that frailty has with poor postoperative outcomes.18 Our study is prospective, however, and one more variable has been added: the quantification of severity using the APACHE II scale, which, measured in critically ill surgical patients in the first 24h of admission to the ICU, is able to provide a prognosis of morbidity and mortality by itself.19,20
The 3 frailty scales most commonly used in surgery21,22 are the CFS, mFI and Fried scale. The first 2 are easy to apply. But in light of the results of this study, the CFS is perhaps the most appropriate frailty scale for elderly and critically ill patients because it is based on clinical judgment, which is the gold standard for the European Society of Intensive Medicine and is the reference in European ICUs.23,24 The Fried scale is widely validated in elderly surgical25 and medical patients,7 and it has even been shown to better predict morbidity and mortality than the mFI in elderly surgical patients;22 however, its degree of complexity (requiring patient collaboration and involvement) means that it is not functional in urgent surgery or in critical patients.
Like similar studies, our study has confirmed that higher mFI levels15,26 and the condition of ‘frail elderly’ determined by the CFS27 are associated with worse postoperative results (mortality, hospital stay), as well as an increase in surgical complications: reoperation and ICU readmission. However, age, higher APACHE II values, the nature of the surgery, and the surgical disease have not been related with these complications. This fact could be justified because perhaps the baseline situation of the patient and her/his physiological reserve (evaluated by the frailty scales) could be decisive in the appearance of surgical complications causing reoperation or ICU readmission.
Regarding the prognosis and situation 6 months after hospital discharge, the patients who were identified as ‘robust’ by the CFS were the only group that was associated with better results for all variables, and specifically in terms of their frailty and functional status. This result contrasts with a similar study,28 in which CFS was not associated with hospital readmission but was instead associated with the rest of the variables. Likewise, it was observed that the oncological condition was related with a better prognosis, which may be justified by a selection bias: the oncology patients mostly underwent elective hepatobiliary-pancreatic surgery and had been previously evaluated by a tumor committee that took their baseline situation and life expectancy into consideration, thereby most likely selecting robust patients. Furthermore, the analysis of the subgroup of cancer patients showed that they presented lower scores in mFI and APACHE II compared to non-cancer patients (P=.006 and P<.05, respectively), as well as a greater association between the condition of oncological (according to the CFS) and robust (P=.004). Cancer patients were also older than non-cancer patients (P=.05), a fact that reinforces the hypothesis of a greater influence of frailty than age on the results.
It should be noted that, as in other studies,29,30 the frailty and severity of elderly surgical patients have been related with stays in functional recovery centers and specialized nursing care after hospital discharge.
Lastly, the overall mortality of the series 6 months after hospital discharge was 44.5%, a figure that differs from other series with lower mortality.26 This could be explained by the mean age (77 years) of the patients in our series and by their high severity rates (mean APACHE II in urgent surgery was 21.4, and all scheduled interventions were tabulated as ‘complex major’), which justified admission to the ICU in the immediate postoperative period. Like age, APACHE II, mFI, CFS and surgical disease were statistically related to overall mortality. A logistic regression model was designed, which showed that the 2 most influential variables were the CFS classification and the APACHE II (Table 5). Therefore, in our opinion, age would not be a single and sufficient objective criterion for decision-making in surgical patients.
Perhaps frailty explains the differences in postoperative recovery observed in elderly patients of a similar age who have undergone the same surgical procedure. Therefore, and in light of the results obtained, we propose that the routine use of preoperative frailty estimations in these patients can improve the predictive capacity of other scales like APACHE II. Thus, by combining the information from these scores, it will be possible to have a more solid forecast of postoperative complications and prognoses. This could help rationalize the subsequent treatment of these patients and promote the establishment of multimodal prehabilitation protocols to improve their degree of frailty, morbidity, mortality and postoperative functional status.
The main limitation of this study is its relatively small sample size, which prevents making comparisons between groups with sufficient power. An independent analysis of each of the treatment groups could not be done due to the limited sample size of the study.
In our opinion, the results obtained are indeed objective and ‘realistic’, given the type of patients and their severity, so it will be necessary to continue developing more studies with these parameters. We conclude that the baseline situation of patients and their physiological reserve (assessed by frailty according the CSF and mFI) may be deciding factors for the appearance of surgical complications that require reoperation or readmission in senior surgical patients admitted to the ICU. Furthermore, CFS and mFI have also been efficient as predictors of 6-month mortality.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ruiz de Gopegui Miguelena P, Martínez Lamazares MT, Miguelena Hycka J, Claraco Vega LM, Gurpegui Puente M. Influencia de la fragilidad en el pronóstico de pacientes quirúrgicos mayores de 70 años con criterios de ingreso en UCI. Cir Esp. 2021;99:41–48.