Portal hypertension (PHT) is a clinical syndrome characterized by an increased pressure gradient between the portal vein (PV) and the suprahepatic veins. Symptoms appear when the gradient exceeds 10−12 mmHg. One of the causes of prehepatic PHT is PV thrombosis, secondary to liver cirrhosis or thrombophilic diseases, although it can also be idiopathic in origin. Thrombosis can be asymptomatic or manifest as upper gastrointestinal hemorrhage (UGH), ascites, or produce portal hypertension colopathy whose most frequent clinical manifestation is lower gastrointestinal bleeding.1 Management of portal vein thrombosis is fundamentally medical and complemented with interventional radiology and digestive endoscopy. Surgical options include portosystemic shunt surgeries, liver transplantation (LT), and multivisceral transplantation (MVT).
We present the case of a 26-year-old male with no medical or surgical history. As a result of an episode of UGH, he was diagnosed with complete idiopathic thrombosis of the portal venous axis causing a PHT syndrome. As part of the diagnostic process, studies were performed to rule out liver disease and thrombophilia, with no pathological findings; then, a gastroscopy was conducted, which revealed esophageal varices and mild PHT gastropathy. Initially, the patient was treated with non-selective beta-blockers, providing good control of the esophageal varices. Six years later, he began to present episodes of rectal bleeding, anemia, and the need for periodic transfusions. He did not present hydropic decompensation or hepatic encephalopathy. This patient’s case was presented at a joint session to assess whether to perform MVT versus portosystemic shunt surgery. Given his normal liver function, no thrombophilic diseases, no surgical history, good control of the esophageal varices, and the predominant symptoms of colopathy due to PHT, we decided to perform a mesocaval shunt.
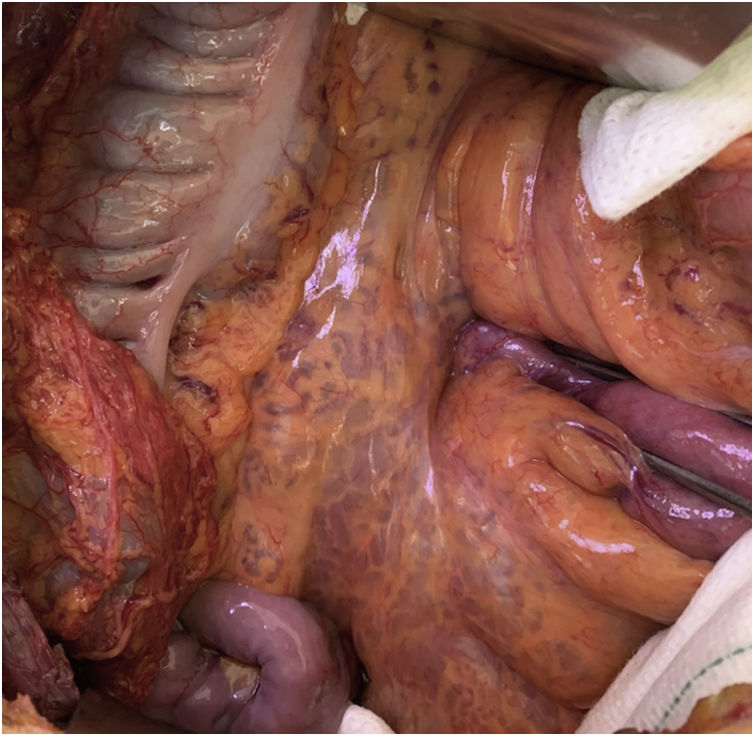
During surgery, an extensive cavernous network of lymphatic vessels was observed at the root of the mesentery (Fig. 1). The superior mesenteric artery was located at the root of the mesentery and, immediately to the right, the anterior and lateral side of the superior mesenteric vein (SMV) was dissected over a length of 5−6 cm. By extending the peritoneal incision, the inferior vena cava (IVC) was observed below the second duodenal flexure. It was necessary to dissect 6−8 cm from the anterior side of the IVC to perform the anastomosis with superior and inferior vascular control. A mesocaval anastomosis requires the placement of a shunt, which can be either a heterologous graft from the organ bank or, as in our case, a 12-mm ringed Teflon prosthesis.2 The first side-to-end anastomosis between the SMV and the shunt was performed with two continuous 6/0 Dacron sutures. The second anastomosis was end-to-side between the shunt and the IVC (Fig. 2). This was carried out with the same technique and under lateral clamping of the IVC to minimize hemodynamic repercussions. The postoperative period was uneventful, and treatment was started with enoxaparin at prophylactic doses. Three months later, when the platelet count exceeded 75,000/μL, treatment was changed to clopidogrel. In the following 12 months, the patient presented no hemorrhages, hepatic encephalopathy, or altered liver function.3 The portal pressure prior to the intervention was 22 mmHg, which dropped to 11 in the postoperative period. Hepatic arterial flow also decreased, which had increased as a compensatory mechanism against the portal hypoflow4 (arterial resistance index [RI] before surgery = 0.85; postoperative = 0.65).
Portosystemic shunt surgeries can completely divert the flow from the PV to the IVC, or they can be partial, such as Warren’s selective distal splenorenal shunt or the mesocaval shunt, whose objective is to reduce the pressures of the venous return system of the colon to avoid bleeding episodes, while preserving residual portal perfusion2 to ensure adequate hepatic flow. After the surge in LT and radioguided interventions, these surgeries have taken a backseat and are reserved for patients who are not transplant candidates, or for certain life-threatening emergency situations.3,5–7 The objective is to reduce portal pressure below 12 mmHg, thereby reducing the risk of hemorrhage.3 These are interventions with high perioperative morbidity and mortality, partly due to the difficult surgical technique, but also due to the risk of liver dysfunction secondary to decreased portal flow.3 In this context, MVT is considered a surgical option for: patients with extensive thrombosis of the portomesenteric axis (Yerdel grade IV thrombosis)8; patients with associated liver dysfunction or dysfunction of another abdominal organ (eg, desmoid tumor in Gardner syndrome); patients with protein C deficiency due to the high risk of thrombosis of the venous bypass and IVC, as well as other coagulation disorders that favor the failure of portosystemic shunts; and finally patients with multiple previous abdominal surgeries or after the failure of more conservative techniques, such as portosystemic shunts.5 In general, these are technically complex surgeries with very limited indications that should be concentrated at specialized hospitals, and cases should be previously discussed by a multidisciplinary committee.