Iatrogenesis due to extravasation of chemotherapeutic drugs through subcutaneous reservoirs is rare.1 However, this entity involves high morbidity and potential mortality, which can delay cancer treatment in affected patients. Extravasation is considered an oncological accident whose reported incidence ranges from 0.1% to 6%.2,3 Once the adverse event occurs, there is currently no effective treatment capable of interrupting the progression of the drug or the consequent tissue necrosis.
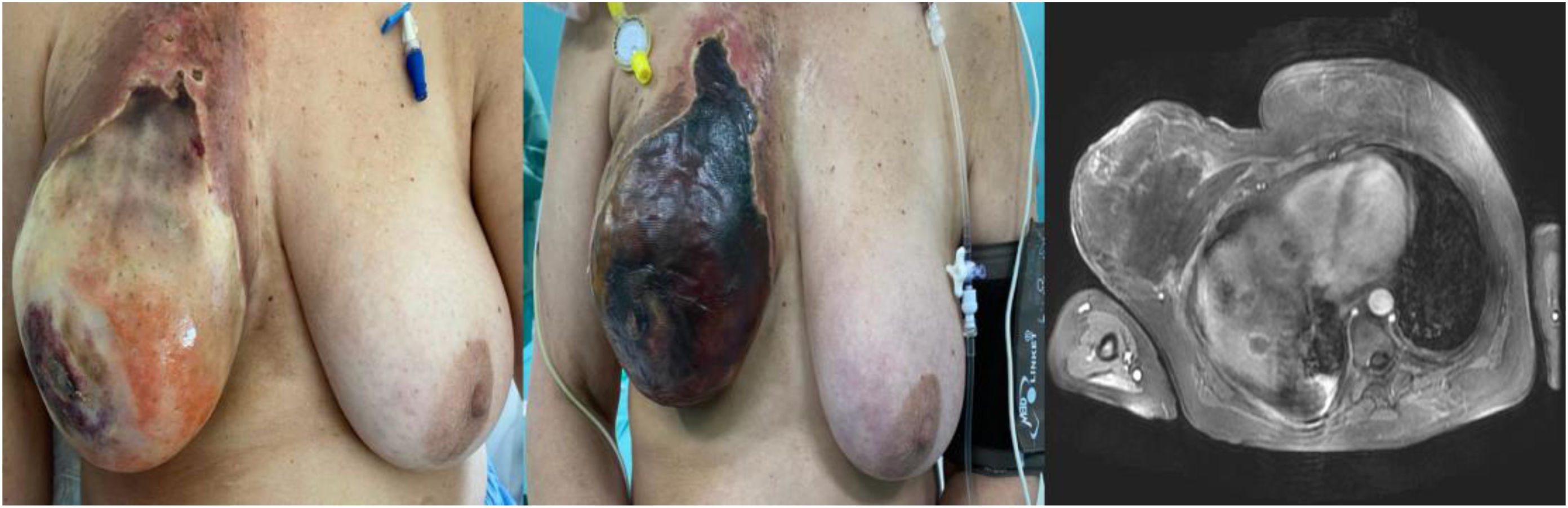
We present the case of a 56-year-old woman with a personal history of left ovarian cystadenocarcinoma and endometrial adenocarcinoma, who exhibited tumor progression after several lines of chemotherapy treatment. A new line of treatment was initiated with doxorubicin and trabectedin. After its administration through a subcutaneous reservoir in the right thoracic region, reflux of clear fluid was observed as well as a sudden increase in the volume of the ipsilateral breast. A few hours later, the patient presented a moderately erythematous right breast, which had increased in size.
Given the suspicion of subcutaneous extravasation, local measures were applied, including fomentations and dry cold compresses. The erythema progressed in intensity and abundant blisters developed, affecting the entire surface of the breast. Because of this event, chemotherapy treatment was interrupted indefinitely.
Magnetic resonance imaging (MRI) revealed areas that appeared to be small collections in the right mammary gland, pectoral and ipsilateral intercostal muscles, measuring 12.5 × 7.2 × 18.5 cm. The findings were consistent with an extensive area of necrosis (Fig. 1).
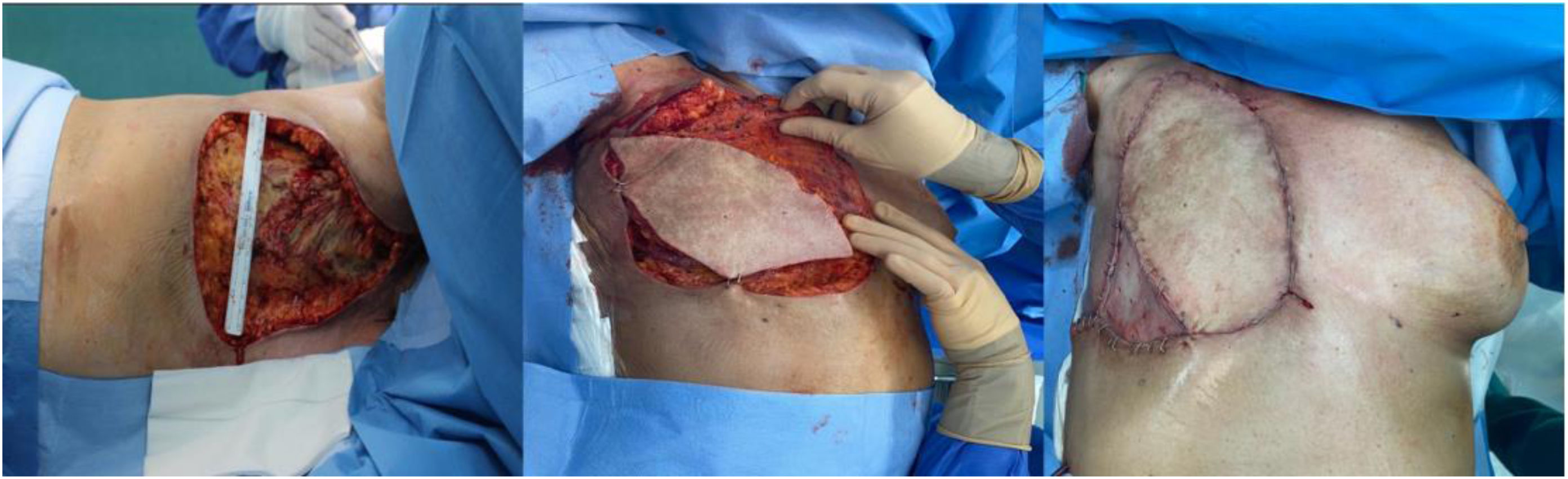
After the necrosis stopped spreading and spontaneous regeneration of the bed was deemed impossible one month after the adverse event, debridement of the devitalized tissue was indicated. We carried out an extended radical mastectomy, including all the pectoralis major muscle as well as part of the pectoralis minor and intercostal muscles, resulting in a defect measuring 22 × 16 cm. Immediate reconstruction involved a pedicled latissimus dorsi myocutaneous flap, based on the thoracodorsal artery and tunneled through the axillary region until it reached the anterior chest wall. In addition, a partial free skin graft was used in the lower-outer quadrant of the defect, using the thigh as the donor site (Fig. 2).
In the immediate postoperative period, we observed dehiscence of one of the ends of the flap, in addition to the donor area, which was probably related to the poor quality of the adjacent tissues and accentuated patient fragility. This complication was resolved during the hospital stay with targeted treatment, including washing with 0.5% chlorhexidine, Vaseline gauze and antiseptic ointments (Nitrofural), with no need for revision surgery.
Three months after extravasation and after the resolution of this condition, the patient died due to oncological progression after the forced suspension of chemotherapy treatment.
Currently, there is no consensus regarding either the initial management or the reconstruction of iatrogenic extravasations, making this condition a medical-surgical challenge.4,5
Once the adverse event occurs, conservative treatment is initially recommended with local measures, such as the application of fomentations or topical dimethyl sulfoxide (DMSO),4 which is capable of preventing the appearance of tissue necrosis in up to 91.8% of cases.6 In cases of large-volume extravasation, intravenous dexrazoxane is suggested as the most specific treatment.7 The use of intralesional granulocyte-macrophage colony-stimulating factor (GM-CSF),8 as well as hyaluronidase,9 has also been described.
However, once tissue necrosis has set in, treatment will be based on radical debridement of the devitalized tissue and subsequent reconstruction of the resulting defect, which is coordinated with a multidisciplinary team. The therapeutic arsenal is extensive, with options that vary in complexity, including skin grafts, local, regional or free flaps, and microsurgical techniques. The latissimus dorsi myocutaneous flap is considered the gold standard for coverage of the anterior thoracic region. In this case, we consider this technique superior to a free flap, since the potential recipient vessels close to the defect could be affected and not be valid for reconstruction.
In our experience, the latissimus dorsi pedicled flap, described by Tansini in 1906,10 is an extremely useful reconstructive option for anterior chest wall defects. It provides a large amount of tissue due to its versatility both in its composition (muscular, myocutaneous or osteomyocutaneous) and in its orientation. This flap is considered a workhorse in reconstructive surgery, since it can be used in the reconstruction of any area of the body using microsurgical techniques.10 Its vascularization depends on a dominant pedicle (thoracodorsal artery and vein) and a secondary blood supply from posterior intercostal perforators.10 The advantages of this repair technique over others are its reliable pedicle and the fact that it provides a large amount of well-vascularized tissue. In cases like this, it enables us to provide stable, good-quality coverage, while reducing the risks of local and systemic infection in an immunocompromised patient.