This study evaluated allogenic packed red blood cell (aPRBC) transfusion rates in patients undergoing resection for gastric cancer and the implementation of blood-saving protocols (BSP).
MethodsRetrospective study of all gastric cancer patients operated on with curative intent in Catalonia and Navarra (2011–2013) and included in the Spanish subset of the EURECCA Oesophago-Gastric Cancer Registry. Hospitals with BSP were defined as those with a preoperative haemoglobin (Hb) optimization circuit associated with restrictive transfusion strategies. Predictors of aPRBC transfusion were identified by multinomial logistic regression analysis.
ResultsA total of 652 patients were included, 274 (42.0%) of which received aPRBC transfusion. Six of the 19 participating hospitals had BSP and treated 145 (22.2%) patients. Low Hb level at diagnosis (10 vs 12.4g/dL), ASA score III/IV, pT3-4, open surgery, associated visceral resection, and having being operated on in a hospital without BSP were predictors of aPRBC transfusion, while low Hb level, associated visceral resection, and non-BSP hospital remained predictors in the multivariate analysis. In case of comparable risk factors for aPRBC transfusion, there was a higher use of preoperative intravenous iron treatment (26.2% vs 13.2%) and a lower percentage of transfusions (31.7% vs 45%) in hospitals with BSP.
ConclusionsThe perioperative transfusion rate in gastric cancer was 42%. Hospitals with BSP showed a significant reduction of blood transfusions but treated only 22% of patients. Main predictors of aPRBC were low Hb level, associated visceral resection, and undergoing surgery at a hospital without BSP.
Este estudio evaluó la tasa de transfusión de concentrados de hematíes alogénicos (TCHA) en la cirugía de resección del cáncer gástrico y la difusión de los protocolos de ahorro transfusional (PAT).
MétodosEstudio retrospectivo de todos los pacientes operados por adenocarcinoma gástrico con intención curativa en Cataluña y Navarra (2011–2013) e incluidos en el registro del grupo español EURECCA de cáncer esófago-gástrico. Los hospitales con PAT disponían de un circuito de optimización preoperatoria de la hemoglobina (Hb) y de política transfusional restrictiva. Los factores predictores de TCHA se identificaron mediante una regresión logística multinomial.
ResultadosSe incluyeron 652 pacientes, 274 (42%) de los cuales recibieron TCHA. Seis de los 19 hospitales disponían de PAT (22% de los pacientes). La Hb baja al diagnóstico (10 vs 12,4g/dL), una puntuación ASA III/IV, pT3-4, la cirugía abierta, la resección visceral asociada y haber sido atendido en un hospital sin PAT fueron factores predictores de TCHA, con la Hb baja, la resección visceral asociada y la intervención en un centro sin PAT persistiendo como predictores en el análisis multivariante. Hubo un mayor porcentaje de uso de hierro en el preoperatorio (26,2 vs 13,2%) y un menor porcentaje de transfusiones (31,7 vs 45%) en los hospitales con PAT.
ConclusionesLa tasa transfusional en la cirugía del cáncer gástrico fue del 42%. Los PAT resultaron eficaces pero su implementación fue solo del 22%. La Hb baja, la intervención en un centro sin PAT y la resección visceral asociada fueron predictores de transfusión.
At the time of their diagnosis, anaemia is present in up to 60% of patients with gastric cancer, reaching significantly low levels (serum haemoglobin [Hb] <10g/dL) in 40% of cases.1–3 As a result, perioperative transfusion rates are high, frequently higher than 30%.4–6 The transfusion of allogenic packed red blood cells (aPRBC) can compromise the immunity of cancer patients by possibly increasing postoperative morbidity and even negatively influencing tumour recurrence and long-term survival.7–9 The implementation of blood-saving protocols (BSP) that include the evaluation and treatment of preoperative anaemia, the use of intravenous (iv) iron and a restrictive transfusion policy, could help minimize perioperative aPRBC transfusion, but there are no studies that have confirmed this in large series of patients with gastric cancer.10,11 In the last decade, there has been a progressive centralization of the surgical treatment of esophagogastric cancer at referral hospitals in regions of Spain like Catalonia and Navarra, which has been accompanied by a decrease in postoperative morbidity and mortality,4,12 as observed in other countries.13–15 However, this centralization has not been accompanied by a harmonization of therapeutic protocols, so that only a few hospitals have BSP designed to optimize the use of aPRBC.16–19
The objective of the present study was to analyze the transfusion rate in patients undergoing gastric cancer surgery, as well as to evaluate the implementation and effect of BSP in our setting.
MethodsPatientsA retrospective analysis was conducted of a cohort composed of all patients who had undergone surgery with radical intention for gastric adenocarcinoma at the 19 hospitals authorized for this intervention in Catalonia and Navarra between January 2011 and December 2013. These medical centres were part of the Spanish group “EUropean REgister for Cancer CAre (EURECCA)” for the study of oesophago-gastric cancer.12 Patients were selected when there was information on Hb concentration at the time of diagnosis, before and after the intervention, as well as data on preoperative treatment with iv iron and APRBC. These data do not appear in the original EURECCA registry and were specifically requested in patients treated surgically during the study period, with the approval of the Ethics Committees of each of the hospitals participating in this project.
Main VariableThe main variable of the study was the transfusion rate, defined as the percentage of patients who received transfusions versus the total number of operated patients. The transfusion rate was divided according to whether the administration was performed preoperatively (aPRBC administered from diagnosis until the day of surgery), intraoperatively (during the surgery or in the immediate postoperative period during the patient's stay in recovery) or postoperatively (between the first postoperative day and hospital discharge). The mean transfusion was calculated, defined as the mean number of red blood cell concentrates administered to the transfused patients. The transfusion rate was defined as the quotient between the total units of packed red blood cells transfused in each period and the total number of patients operated on.
Data CollectionFor each patient, the following data were collected: age; sex; anaesthetic risk determined by the physical status classification of the American Society of Anesthesiologists (ASA); tumour location; Hb at the time of diagnosis, before and after surgery; treatment with neoadjuvant chemotherapy; administration of iv iron in the preoperative period, type of iron and time elapsed between its administration and surgery; surgical technique (type of gastrectomy, laparoscopic access, associated visceral resection and extension of the lymphadenectomy); pathological stage (TNM classification of the Union for International Cancer Control [UICC] 7th edition); medical (pulmonary, cardiac) and surgical complications (anastomotic dehiscence, duodenal stump leak, surgical re-operation); length of hospital stay and in-hospital mortality.
The present study is observational and includes patients from hospitals with varying practices, both in the study of anaemia and in criteria for the indication of iron, type of iron used and blood transfusion criteria. Based on the recommendations for restrictive transfusion strategies, the hospitals that had BSP for gastric cancer surgery patients were differentiated, and it was evaluated whether they met the following three conditions: 1) systematic evaluation of Hb 2 to 4 weeks before surgery; 2) availability at the hospital of a protocol for the preoperative treatment of iron-deficiency anaemia with iv iron; and 3) existence of guidelines in the hospital, generally coordinated by the hospital transfusion committee, which support the practice of restrictive transfusion behaviour, with transfusion thresholds based on the recommendations and guidelines of scientific societies.
As of 2016 (and given the results of the present analysis), the EURECCA group has a common protocol with consensus recommendations for preoperative optimization and restrictive transfusion practices.
Statistical AnalysisData are expressed as mean±standard deviation or as frequencies and percentages. The Student's t test was used to compare quantitative variables and the chi-squared test for qualitative variables. The multivariate study of predictive factors of transfusion was performed using multinomial logistic regression. IBM SPSS (version 20) software was used for the calculations. A P level < .05 was considered statistically significant for the bilateral contrast tests.
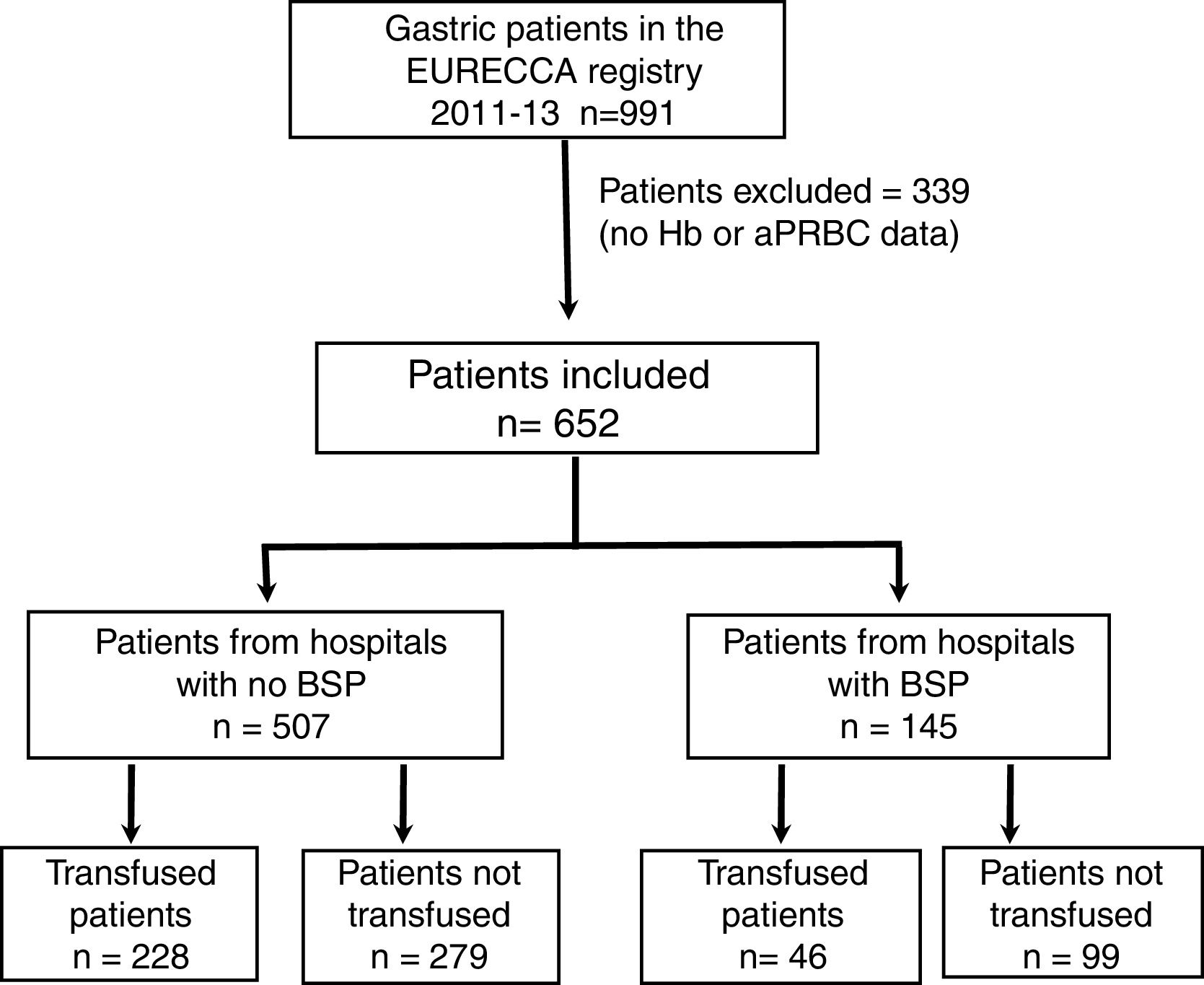
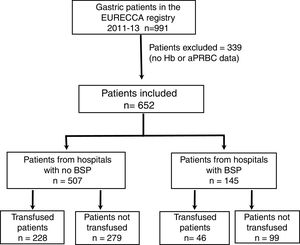
ResultsPatient CharacteristicsFrom January 2011 to December 2013, a total of 991 consecutive patients with gastric adenocarcinoma were included in the EURECCA registry. All of them were operated on with the intention of radical resection. 339 (34.2%) patients were excluded due to lack of Hb data, treatment with iv iron and/or aPRBC, so 652 patients were included in the analysis (Fig. 1).
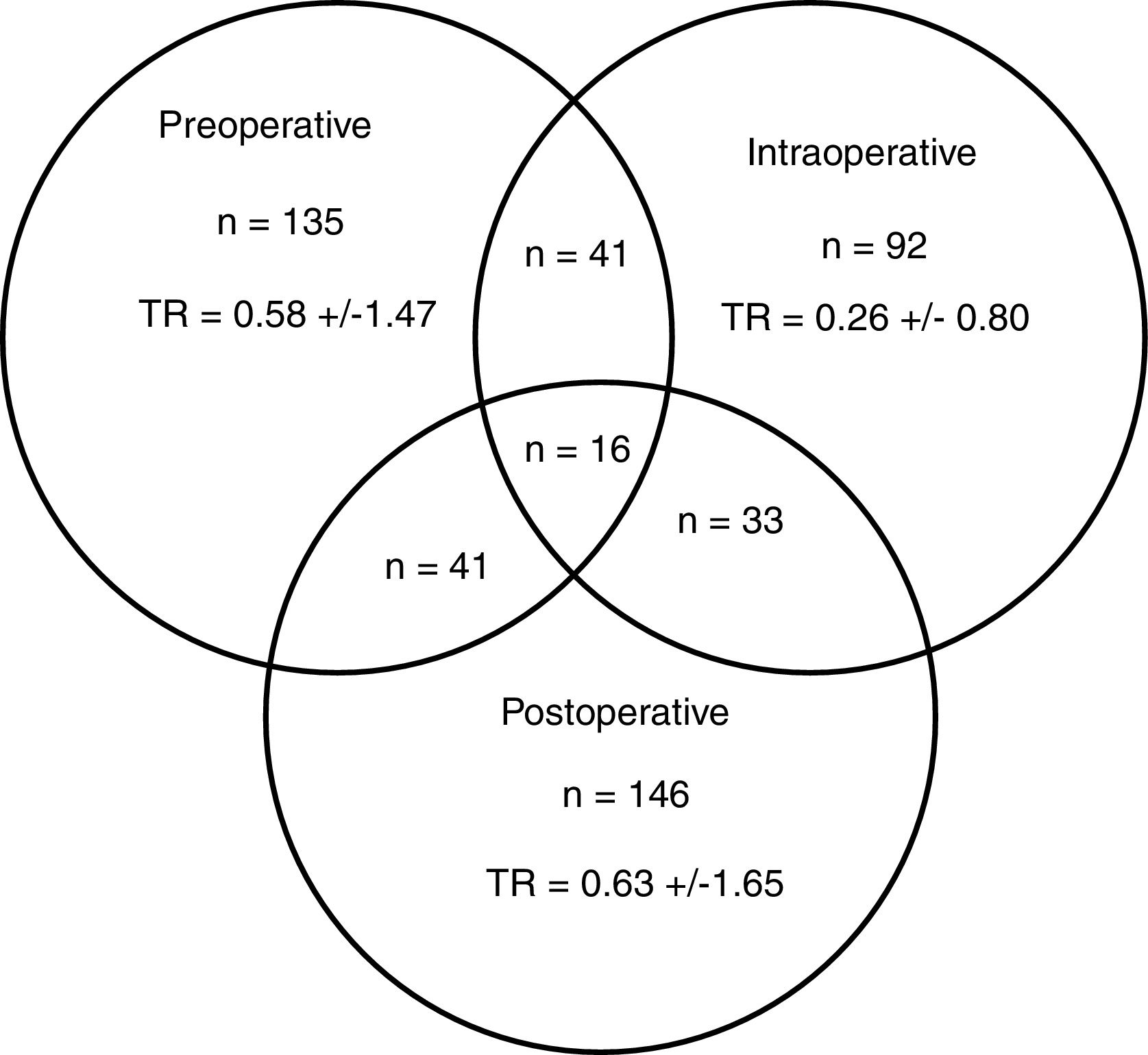
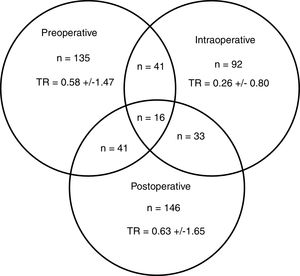
A total of 274 patients (42.0%) received transfusion, and 83 (30.3%) in more than one of the preoperative, intraoperative or postoperative periods (Fig. 2). The percentage of transfusions for these periods was 20.7, 14.1 and 22.4%, respectively. The mean transfusion in the preoperative, intraoperative and postoperative periods was 1.4, 0.6 and 1.5, respectively, and the transfusion rates were 0.58±1.47; 0.26±0.80 and 0.63±1.65. In 6 of the 19 participating centres, a BSP was available and 145 (22.2%) patients were treated surgically.
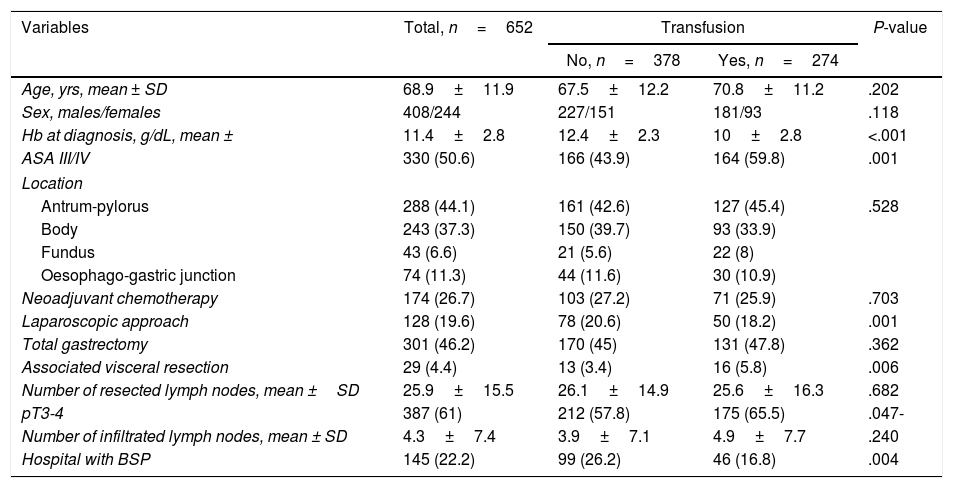
Predictive Factors for Transfusion of Allogenic Packed Red Blood CellsTable 1 shows the characteristics of the transfused patients (n=277) compared to the non-transfused patients (n=378). Transfused patients demonstrated lower Hb concentrations at the time of diagnosis (10 vs 12.4g/dL), a higher percentage of patients with ASA III/IV scores (59.8 vs 43.9%), lower use of the laparoscopic approach (18.2 vs 20.6%), greater number of associated visceral resections (5.8% vs 3.4%) and stages pT3-4 (65.5 vs 57.8%), as well as a lower frequency of intervention in hospitals with BSP (16.8 vs 26.2%); these differences were statistically significant. In the multivariate analysis, the independent factors of aPRBC transfusion were low Hb at the time of diagnosis (odds ratio [OR] 15.53, 95% confidence interval [CI] 7.17–33.62), treatment at a hospital without BSP (OR 2.13, 95% CI 1.37–3.34) and associated visceral resection (OR 1.59, 95% CI 1.08–2.36).
Comparison of the Characteristics of Transfused and Non-transfused Patients.
| Variables | Total, n=652 | Transfusion | P-value | |
|---|---|---|---|---|
| No, n=378 | Yes, n=274 | |||
| Age, yrs, mean ± SD | 68.9±11.9 | 67.5±12.2 | 70.8±11.2 | .202 |
| Sex, males/females | 408/244 | 227/151 | 181/93 | .118 |
| Hb at diagnosis, g/dL, mean ± | 11.4±2.8 | 12.4±2.3 | 10±2.8 | <.001 |
| ASA III/IV | 330 (50.6) | 166 (43.9) | 164 (59.8) | .001 |
| Location | ||||
| Antrum-pylorus | 288 (44.1) | 161 (42.6) | 127 (45.4) | .528 |
| Body | 243 (37.3) | 150 (39.7) | 93 (33.9) | |
| Fundus | 43 (6.6) | 21 (5.6) | 22 (8) | |
| Oesophago-gastric junction | 74 (11.3) | 44 (11.6) | 30 (10.9) | |
| Neoadjuvant chemotherapy | 174 (26.7) | 103 (27.2) | 71 (25.9) | .703 |
| Laparoscopic approach | 128 (19.6) | 78 (20.6) | 50 (18.2) | .001 |
| Total gastrectomy | 301 (46.2) | 170 (45) | 131 (47.8) | .362 |
| Associated visceral resection | 29 (4.4) | 13 (3.4) | 16 (5.8) | .006 |
| Number of resected lymph nodes, mean ±SD | 25.9±15.5 | 26.1±14.9 | 25.6±16.3 | .682 |
| pT3-4 | 387 (61) | 212 (57.8) | 175 (65.5) | .047- |
| Number of infiltrated lymph nodes, mean ± SD | 4.3±7.4 | 3.9±7.1 | 4.9±7.7 | .240 |
| Hospital with BSP | 145 (22.2) | 99 (26.2) | 46 (16.8) | .004 |
ASA, American Society of Anesthesiologists; SD, standard deviation; Hb, haemoglobin; BSP, blood-saving protocol. Data expressed as frequencies and percentages in parentheses, unless indicated otherwise.
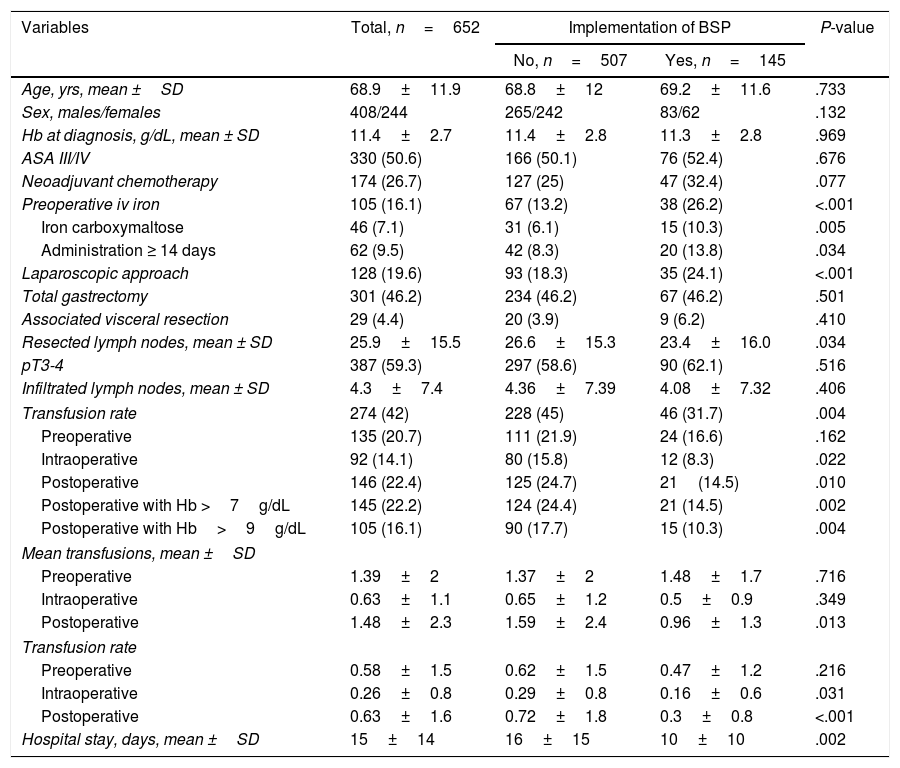
Table 2 compares the data of patients treated at hospitals with BSP (n=145) and without BSP (n=507). Both groups of patients were comparable in age, sex, ASA III/IV score, Hb at the time of diagnosis, tumour stage (pT and pN), use of neoadjuvant chemotherapy, type of gastrectomy and associated visceral resection. However, fewer laparoscopic resections and less extensive lymphadenectomies were performed in hospitals with BSP. Hospitals with BSP prescribed more iv iron preoperatively (26.2 vs 13.2%), especially ferric carboxymaltose, and administered more frequently ≥ 14 days before the intervention (13.8 vs 8.3%). The hospitals with BSP showed a lower overall transfusion rate (31.7% vs 45%). When analysing the aPRBC transfusions by periods, no significant differences were observed in the preoperative period, but the intraoperative transfusion rate (8.3 vs 15.8%) and postoperative transfusion rate (14.5 vs 24.7%) were lower in hospitals with BSP. Specifically, fewer postoperative aPRBC were prescribed for patients with Hb >7g/dL (14.5 vs 24.4%) and Hb >9g/dL (10.3 vs 17.7%). The mean postoperative transfusion was also lower in the hospitals with PAT, as were the intra- and postoperative transfusion rates. All this allowed us to estimate an overall savings of 102 units of RBC in hospitals with BSP. The mean hospital stay was also shorter at the hospitals with BSP (10±10 vs 16±15 days, P=.002).
Comparison Between Patients Treated at Hospitals With and Without Blood-saving Protocols.
| Variables | Total, n=652 | Implementation of BSP | P-value | |
|---|---|---|---|---|
| No, n=507 | Yes, n=145 | |||
| Age, yrs, mean ±SD | 68.9±11.9 | 68.8±12 | 69.2±11.6 | .733 |
| Sex, males/females | 408/244 | 265/242 | 83/62 | .132 |
| Hb at diagnosis, g/dL, mean ± SD | 11.4±2.7 | 11.4±2.8 | 11.3±2.8 | .969 |
| ASA III/IV | 330 (50.6) | 166 (50.1) | 76 (52.4) | .676 |
| Neoadjuvant chemotherapy | 174 (26.7) | 127 (25) | 47 (32.4) | .077 |
| Preoperative iv iron | 105 (16.1) | 67 (13.2) | 38 (26.2) | <.001 |
| Iron carboxymaltose | 46 (7.1) | 31 (6.1) | 15 (10.3) | .005 |
| Administration ≥ 14 days | 62 (9.5) | 42 (8.3) | 20 (13.8) | .034 |
| Laparoscopic approach | 128 (19.6) | 93 (18.3) | 35 (24.1) | <.001 |
| Total gastrectomy | 301 (46.2) | 234 (46.2) | 67 (46.2) | .501 |
| Associated visceral resection | 29 (4.4) | 20 (3.9) | 9 (6.2) | .410 |
| Resected lymph nodes, mean ± SD | 25.9±15.5 | 26.6±15.3 | 23.4±16.0 | .034 |
| pT3-4 | 387 (59.3) | 297 (58.6) | 90 (62.1) | .516 |
| Infiltrated lymph nodes, mean ± SD | 4.3±7.4 | 4.36±7.39 | 4.08±7.32 | .406 |
| Transfusion rate | 274 (42) | 228 (45) | 46 (31.7) | .004 |
| Preoperative | 135 (20.7) | 111 (21.9) | 24 (16.6) | .162 |
| Intraoperative | 92 (14.1) | 80 (15.8) | 12 (8.3) | .022 |
| Postoperative | 146 (22.4) | 125 (24.7) | 21(14.5) | .010 |
| Postoperative with Hb >7g/dL | 145 (22.2) | 124 (24.4) | 21 (14.5) | .002 |
| Postoperative with Hb>9g/dL | 105 (16.1) | 90 (17.7) | 15 (10.3) | .004 |
| Mean transfusions, mean ±SD | ||||
| Preoperative | 1.39±2 | 1.37±2 | 1.48±1.7 | .716 |
| Intraoperative | 0.63±1.1 | 0.65±1.2 | 0.5±0.9 | .349 |
| Postoperative | 1.48±2.3 | 1.59±2.4 | 0.96±1.3 | .013 |
| Transfusion rate | ||||
| Preoperative | 0.58±1.5 | 0.62±1.5 | 0.47±1.2 | .216 |
| Intraoperative | 0.26±0.8 | 0.29±0.8 | 0.16±0.6 | .031 |
| Postoperative | 0.63±1.6 | 0.72±1.8 | 0.3±0.8 | <.001 |
| Hospital stay, days, mean ±SD | 15±14 | 16±15 | 10±10 | .002 |
ASA, American Society of Anesthesiologists; SD, standard deviation; Hb, haemoglobin; BSP, blood-saving protocol.
Transfusion rate, patients transfused/total patients operated; Mean transfusion, total number of packed red blood cell units transfused/total patients transfused; Transfusion rate, total number of packed red blood cells transfused/total patients operated. Data are expressed as frequencies and percentages in parentheses, unless otherwise indicated.
In the patients treated with iv iron and not transfusions (n=64), a significant increase in Hb was observed between the diagnosis and the surgical intervention (9.8±1.8 to 10.8±1.6g/dL; P<.001). This increase was not observed in patients not treated with IV iron or transfused preoperatively (n=453), in whom Hb ranged from 12.4±2 to 11.9±3g/dL (P=.532).
DiscussionThe present population-based study shows that the transfusion rate of patients undergoing surgery for gastric adenocarcinoma in our setting is 42%. Although it is a high rate, it is comparable the rates of several studies included in a recent meta-analysis6 and similar to the rate observed in the same territory when the centralization of oesophago-gastric cancer surgery had not been completed.4 Several studies show that concentrating cancer surgeries, which increases the volume and specialization in authorized hospitals, favourably affects the results.14,20 Specifically, data from the EURECCA registry of oncological gastrectomies performed between 2011 and 2013 in the 19 authorized hospitals in Catalonia show a significant decrease in complications and postoperative mortality compared to previous data available from the same territory, with dispersed activity in 69 hospitals.4,12 However, despite the improvement of these quality of care indicators, the transfusion rate has not been reduced, probably because centralization has not been accompanied by rationalization and harmonization of the protocols. Along these lines, Simunovic et al.21 demonstrated, by comparing the centralization process for pancreatic cancer surgery in 2 regions of Canada, that the increase in hospital volume was not enough to improve the quality care parameters if it was not accompanied by interventions to update the processes. Precisely, the EURECCA registry, started in 2011 for esophagogastric cancer, aims to compare data and results from different European territories in order to achieve greater standardization of therapeutic protocols.12,15,22
The transfusion of aPRBC can compromise the immunity of patients, which could negatively influence postoperative morbidity.23,24 In addition, the immunosuppression derived from aPRBC has been related to an increased risk of long-term tumour recurrence in patients operated on for cancer, regardless of tumour stage.6,8,9,25,26 Also, considering the condition of human blood being a limited resource, it is recommended that healthcare institutions promote BSP based on the preoperative optimization of Hb and restrictive use of transfusions.16–19 The present study shows that only 22% of the patients were treated at hospitals with BSP, in which the optimization with iv iron and transfusion are protocolized and supervised. In these hospitals, less aPRBC transfusions were used, despite the fact that their patients were comparable to the rest in terms of Hb levels at the time of diagnosis, ASA, oncological stage, tumour location and type of gastrectomy. Furthermore, in hospitals with BSP, fewer minimally invasive surgeries were practiced, which is an approach that is associated with less transfusion need in the literature and in the present study.27,28 Despite the low regional implementation of BSP, the estimated global savings at hospitals with a protocol was 102 units of packed red blood cells. In order to extend the results of these few hospitals, the member hospitals of the EURECCA group have agreed on a common BSP in esophagogastric cancer, whose implementation has been evaluated prospectively since March 2017.
The first pillar of a BSP is the preoperative optimization of anaemia by stimulating erythropoiesis, where iv iron plays a relevant role. Preoperative anaemia affected 55.2% of the patients in the present study, a percentage similar to reports from other registries.2,7,29 Treatment with iv iron, especially in its carboxymaltose form, has been shown to improve Hb, decreasing the transfusion rate and hospital stay after elective surgery for colorectal cancer.30,31 This benefit has only been shown for gastric cancer in the postoperative period.32 The data of the present study show that iv iron improved the Hb levels of surgical patients. Despite this benefit, preoperative iv iron therapy was indicated only in 16.1% of the cases, which implies a wide margin for improvement. Even in hospitals with BSP, iv iron was administered to only 26.2% of the patients, which can be explained in part by the pressure of care and the urgent intervention of the neoplasms. However, a margin of 2 weeks has been shown to be sufficient and the minimum time recommended to improve Hb, with an average dose of 1000mg of iv iron.31 Other possible causes of the poor implementation of preoperative iron therapy may be its cost and the conditions required for its administration.33 However, despite coming from altruistic donations, aPRBC also present high costs associated with the processes of blood collection, analysis, preparation, storage and administration. Multiple studies have shown that optimization with iv iron is a cost-effective measure, especially in its carboxymaltose form.34,35
In addition to favouring preoperative optimization, BSP support restrictive transfusions, trying to minimize aPRBC use in stable and non-bleeding patients, with thresholds established in accordance with the recommendations of clinical practice guidelines: aPRBC in patients with Hb <7g/dL with no cardiovascular risk factors; in patients with risk factors, aPRBC with Hb >7g/dL and <9g/dL; in cases with Hb ≥9g/dL, transfusion would not be indicated in the absence of active haemorrhage.17 In this regard, our registry shows that patients with Hb >7g/dL and Hb >9g/dL treated at hospitals with BSP presented a lower risk of being transfused, despite the fact that in both hospital groups patients presented a similar age and anaesthetic risk.
The main limitation of this study is its retrospective and descriptive nature, which prevents establishing the unequivocal causal relationship between aPRBC transfusion and risk factors. In addition, the EURECCA registry did not collect objective measurements of patient comorbidity at that time, with the exception of the ASA score, which limits the understanding of the criteria for the indication of transfusion. What is most interesting about the study is that it is based on a population registry, meaning that is provides reliable information on the situation of transfusions in our setting.
In conclusion, 42% of the patients were transfused in the perioperative period of oncological gastrectomy, a rate that has not decreased in the last 15 years despite the centralization of the pathology. Treatment with preoperative iv iron improved Hb, but it was only administered in 16% of the patients. In hospitals with BSP, there was a greater use of preoperative iv iron therapy and lower intraoperative and postoperative transfusion rates. The predictive factors of aPRBC transfusion included low Hb at the time of diagnosis, surgery at a centre without a BSP, and associated visceral resection.
FundingFunded in part with a 2016 grant from the Societat Catalana de Cirurgia for multicentre studies. The laboratory Vifor Pharma Spain, S.L. collaborated economically in the creation of the multicentre database EURECCA Spain and in its logistical support.
Contribution of the AuthorsJavier Osorio: study design, data collection, analysis and interpretation of the results, article composition.
Carlos Jericó: data collection, article composition.
Elisenda Garsot: data collection, critical review and approval of the final version.
Alexis Luna: data collection, critical review and approval of the final version.
Mónica Miró: data collection, critical review and approval of the final version.
Maite Santamaría: data collection, critical review and approval of the final version.
Eva Artigau: data collection, critical review and approval of the final version.
Joaquín Rodríguez-Santiago: data collection, critical review and approval of the final version.
Sandra Castro: data collection, critical review and approval of the final version.
Josep Feliu: data collection, critical review and approval of the final version.
Aurora Aldeano: data collection, critical review and approval of the final version.
Carles Olona: data collection, critical review and approval of the final version.
Dulce Momblan: data collection, critical review and approval of the final version.
David Ruiz: data collection, critical review and approval of the final version.
Gonzalo Galofré: data collection, critical review and approval of the final version.
Inmaculada Pros: data collection, critical review and approval of the final version.
Xabier García-Albéniz: study design, analysis and interpretation of the results, critical review and approval of the final version.
Miguel Lozano: data collection, critical review and approval of the final version.
Manuel Pera: study design, data collection, analysis and interpretation of the results, critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Prof. Lluís Grande for his valuable comments and suggestions, Dr. Marta Pulido for editing and reviewing the manuscript, and Marta Gimeno for managing the data and maintaining the EURECCA registry in Spain.
Please cite this article as: Osorio J, Jericó C, Miranda C, Garsot E, Luna A, Miró M, et al. Conducta transfusional perioperatoria en la cirugía del cáncer gástrico: análisis del grupo español del registro EURECCA de cáncer esófago-gástrico. Cir Esp. 2018;96:546–554.