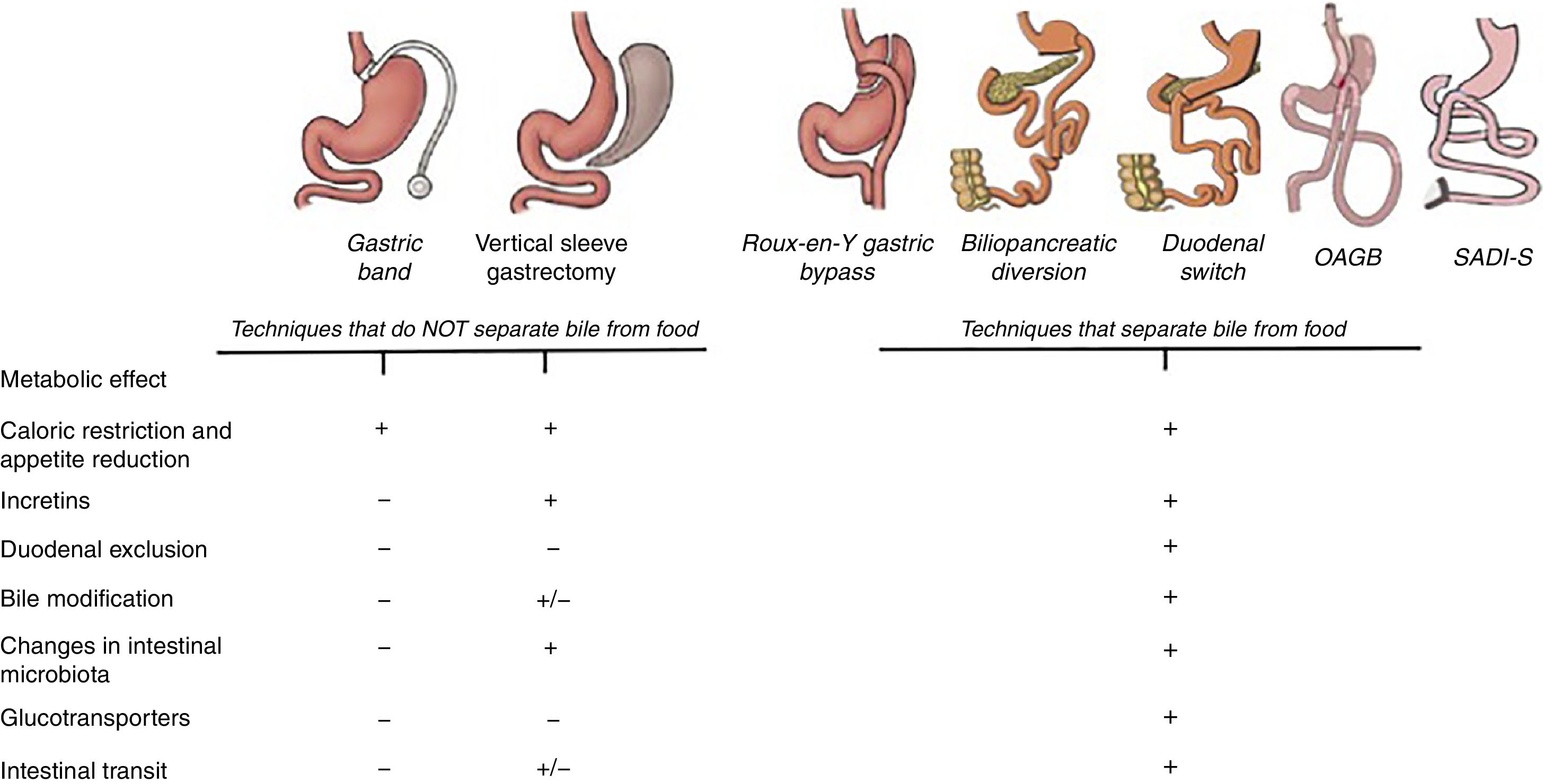
Bariatric and metabolic surgery is creating new concepts about how the intestine assimilates food. Recent studies highlight the role of the gastrointestinal tract in the genesis and evolution of type 2 diabetes. This article has been written to answer frequent questions about metabolic surgery results and the mechanisms of action. For this purpose, a non-systematic search of different databases was carried out, identifying articles published in the last decade referring to the mechanisms of action of metabolic techniques. Understanding these mechanisms will help grasp why some surgeries are more effective than others and why the results can be so disparate among patients undergoing the same surgical approach.
La cirugía bariátrica y metabólica está desarrollando nuevos conceptos sobre la asimilación y absorción de alimentos en el intestino. Estudios recientes han destacado la función del tracto gastrointestinal en la génesis y evolución de la diabetes mellitus tipo 2. En esta revisión pretendemos dar respuesta a preguntas frecuentes sobre los mecanismos de acción y los resultados de la cirugía metabólica. Realizamos una búsqueda bibliográfica no sistemática en diferentes bases de datos, identificando artículos publicados en la última década y referidos a los mecanismos de acción de la cirugía metabólica. Entender dichos mecanismos ayudará a comprender por qué unas cirugías son más efectivas que otras y por qué los resultados pueden llegar a ser tan dispares entre pacientes sometidos a la misma técnica quirúrgica.
Type 2 diabetes mellitus (DM2) represents 90%–95% of all cases of diabetes diagnosed throughout the world. The International Diabetes Federation estimates that by 2040 there will be 642 million diabetics.1 Gastrointestinal surgery for the treatment of obesity and its comorbidities has proven to be the most effective therapy for the control of DM2, but many of the mechanisms of action are still unknown. Since most mediators of these surgical effects have not been identified, improvements to make them more effective and/or less invasive are not easy.
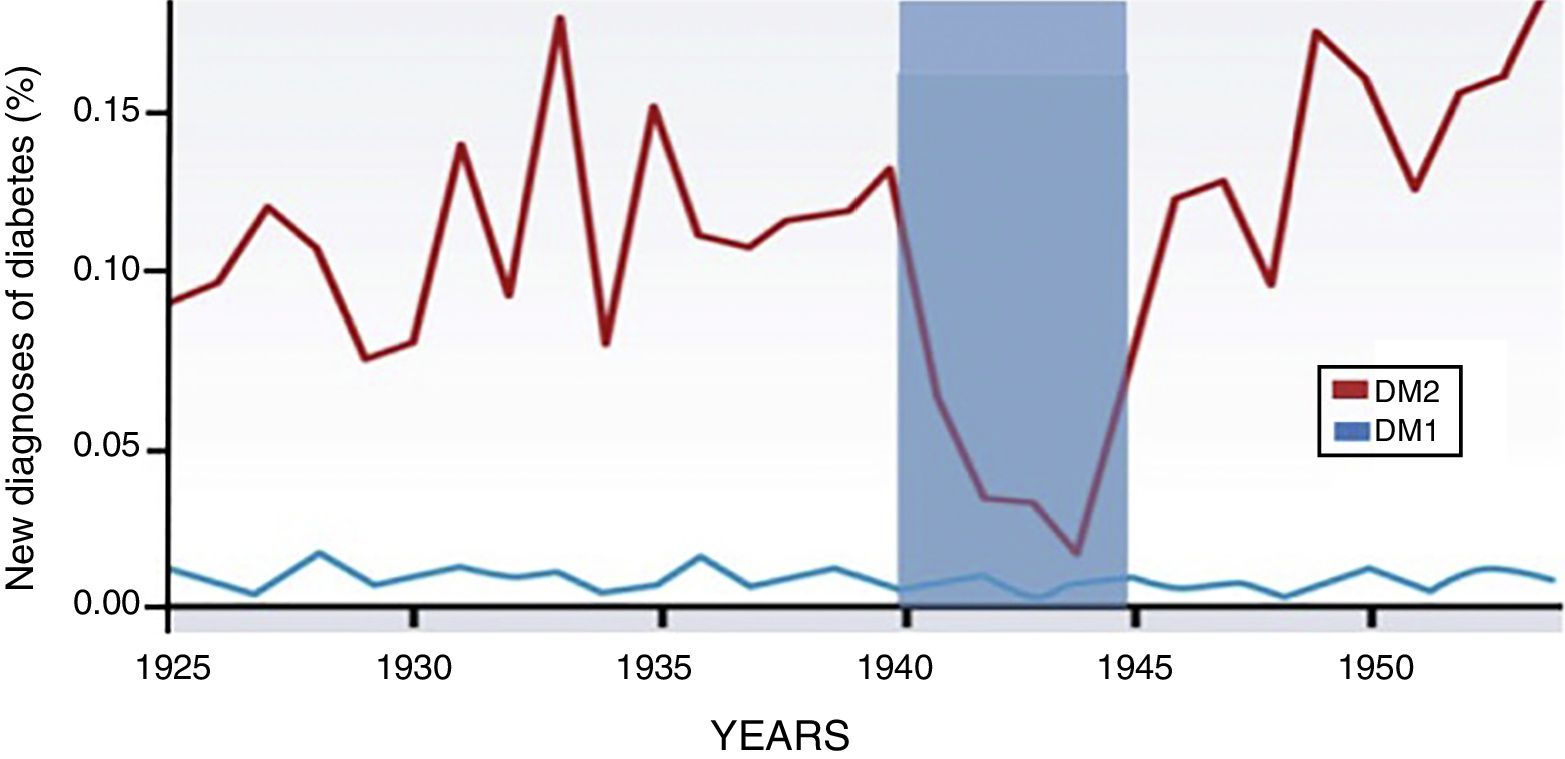
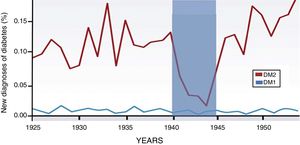
Through the years, the incidence of type 1 diabetes has not varied. However, the incidence and prevalence of DM2 is on the rise, and it is evident that this increase is associated with changes in diet2 (Fig. 1). Currently, nutritional patterns in developed countries show an increase in the consumption of hypercaloric products with a high glycemic index, low nutritional quality and in quantities not adapted to energy output. The ingested foods initiate their metabolic pathway in the gastrointestinal tract, so we need to stop seeing this system as merely a nutrient and waste manager, while emphasizing that it is an endocrine-metabolic organ in itself.3
Increase in the incidence of DM2 associated with eating habits. New cases of diabetes diagnosed in Norway from 1925 to 1955 (expressed as percentage of the population). The incidence of type 1 diabetes is maintained during the study period. However, DM2 increases progressively and only one fall of 85% is observed during the German occupation of 1940–1945. This graph shows how the genesis and development of DM2 are closely linked to food and, therefore, to the function of the gastrointestinal tract. The intestine has greater significance in this pathology, and pancreatic damage is secondary. Adapted from Ashcroft and Rossman.2
Although the number of bariatric-metabolic surgeries performed in the world is growing every day, the results of scientific research in this field are inconclusive and new hypotheses are proposed each day. In fact, basic researchers and clinicians often seem to be working in parallel, but not together, to answer the questions regarding the mechanisms of action of metabolic surgery.4
For this reason, we conducted a non-systematic search in the MEDLINE, Cochrane Library, SCOPUS, ISI Web of Science and Ovid databases, identifying articles published in the last decade, mainly in basic sciences and referring to the mechanisms of action of metabolic surgery, using the following keywords: “type 2 diabetes”, “bariatric surgery”, “intestinal adaptation”, “incretin effect”, “bile acids”, “microbiota”, “intestinal neoglucogenesis”, “glucotransporters”, “enteroplasticity” and “gut/bowel flow”. In this manner, we seek to answer some of the questions frequently asked by bariatric surgeons regarding why some surgeries are more effective than others and why the results can be so disparate among patients.
New Concepts in Type 2 Diabetes Mellitus in Metabolic SurgeryIncretins and Anti-IncretinsThe incretin effect or “hindgut” theory (distal intestine) is the mechanism of action of the most well-known metabolic surgery. It is postulated that the rapid arrival of poorly digested food to the distal intestine promotes increased secretion of intestinal hormones, called incretins. Understanding the incretin effect led to the development of antidiabetic drugs like GLP-1 analogs (glucagon-like peptide type 1) or the inhibitors of the enzyme that degrades these hormones (inhibitors of dipeptidyl peptidase-4, or anti-DPP4). All bariatric techniques modulate incretin levels to some extent and, depending on this response, the metabolic effect is higher or lower, temporary or prolonged. For a time, surgeons limited themselves to demonstrating which of the existing techniques caused more elevated incretin levels in order to “cure” DM2. Over the years, the incretin effect has been shown to be just one link in the resolution of the disease. Studies in humans and animals have demonstrated that glycemia can be improved without the need to increase incretins.5,6 In addition, Habener et al.7 have recently suggested that it is the GLP-1 produced in the pancreas, and not the GLP-1 secreted in the intestine, which actually stimulates insulin secretion. Another extended theory is based on the opposite assertion: the existence of the so-called “anti-incretins”, described in several articles by Rubino.8,9 This author has pointed out that every event in the organism has a counterregulatory response. So, if there is an incretin effect that regulates hyperglycemia, there should be a counterregulatory mechanism that prevents hypoglycemia. This author suggests that the imbalance between both mechanisms could lead to the development of DM2. In this way Rubino has suggested the existence of a peptide with an antagonistic effect to incretins, the so-called “anti-incretins”. The existence of anti-incretins is based on the “foregut” theory (proximal intestine), since it is assumed that these “X” peptides are generated in the duodenum. Therefore, an overproduction of “anti-incretins” could stimulate the factors causing DM2. For this reason, Rubino proposes duodenal exclusion techniques to control the “anti-incretin” effect. However, the molecules or peptides that would explain the “anti-incretin” effect are still unknown, although Salinari et al.10 have provided indirect data about their existence.
More Evidence About the Importance of the Proximal IntestineMany hypotheses have been proposed regarding duodenal exclusion, but few have been confirmed. It is known that one of the main factors in the genesis and progression of DM2 is the loss of feedback signals between the intestine and the liver, which is the main producer of endogenous glucose. In the liver, gluconeogenesis is activated long before the nutrients reach the portal system; in fact, it is activated when food circulates through the duodenum.11 Thus, duodenal exclusion is key in the metabolic improvement of diabetes and, therefore, endoscopic procedures like the “Endobarrier” method have found their basis.12 On the other hand, there is the belief that the signals between the proximal bowel and the liver differ between healthy and diabetic phenotypes. In 2012, Salinari et al.13 published a study demonstrating that duodenal exclusion improves glucose metabolism in non-obese diabetic rats (Goto-Kakizaki), but not in normal rats, suggesting that the diabetic phenotype responds differently to intestinal manipulation. Meanwhile, Known et al.14 described that, in patients undergoing gastric bypass as treatment for stomach cancer, insulin resistance improved in diabetics but did not change in non-diabetics. Together, these findings suggest that the duodenum and proximal jejunum may contribute to glucose homeostasis differently in diabetic versus non-diabetic states. Other studies with Goto-Kakizaki rats demonstrated that duodenal exclusion reduced postprandial blood glucose levels, without increasing insulin or elevating incretins.15,16 In fact, in one of these studies, this effect occurred without the need for gastric resection or the derivation of large portions of the malabsorptive bowel, which suggests that changes in the first portion of the small intestine are key.16
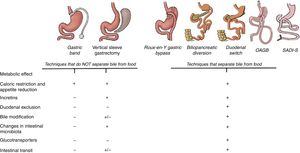
Importance of Bile: Bile Acids and Sodium ConcentrationBariatric techniques that promote biliary bypass (separating bile from food) tend to have a better metabolic response than restrictive techniques alone. Bile is a complex fluid that acts differently depending on the intestinal portion. The bile present in the duodenum is different qualitatively and quantitatively from the bile present in the ileum, since along the intestine there are several circuits in charge of reabsorbing the bile acids and returning them to the enterohepatic circulation.17,18 The alteration of the intestinal flow of the diversion techniques changes the normal circulation of bile, and therefore modifies the reabsorption of bile acids, which justifies the increase of serum bile acids (SBA) in the circulation.19,20 SBA suppress the expression of multiple genes involved in hepatic gluconeogenesis, therefore an increase in plasma SBA decreases hepatic gluconeogenesis and consequently lowers blood glucose. On the other hand, the SBA induce the secretion of incretins directly in the distal intestine through the stimulation of certain G-type membrane proteins coupled to a receptor known as TGR5.21 The effect of SBA on incretins was demonstrated in a study that administered bile acids rectally (taurocholic acid).22 In this study, it was observed that the secretion of GLP-1 and insulin increased in a dose-dependent manner when taurocholic acid was administered. However, by blocking the GLP-1 receptors, hardly any changes in glycemia were observed. That is why the effect of bile acids seems to be associated with the incretin effect.
But bile is not only relevant for the bile acid content, but also because of sodium, as it is the bodily fluid with the highest concentration of sodium.23 Baud et al.24 described that the absorption of glucose from the intestinal lumen to the blood circulation is altered by gastric bypass due to changes in the sodium-rich bile flow. In this study, they focused on the activity of glucose transporters that are found in the microvilli of the enterocytes (luminal pole of the intestine). In the intestinal lumen, the main glucose transporter is sodium-glucose cotransporter type 1 (SGLT1), an active type that uses an electrochemical gradient, by which two sodium ions stimulate the passage of a glucose molecule to the enterocyte. Therefore, any surgical or endoscopic procedure that excludes bile (sodium) from part of the intestinal tract entails lower SGLT1 cotransporter activity and therefore a decrease in glucose absorption.
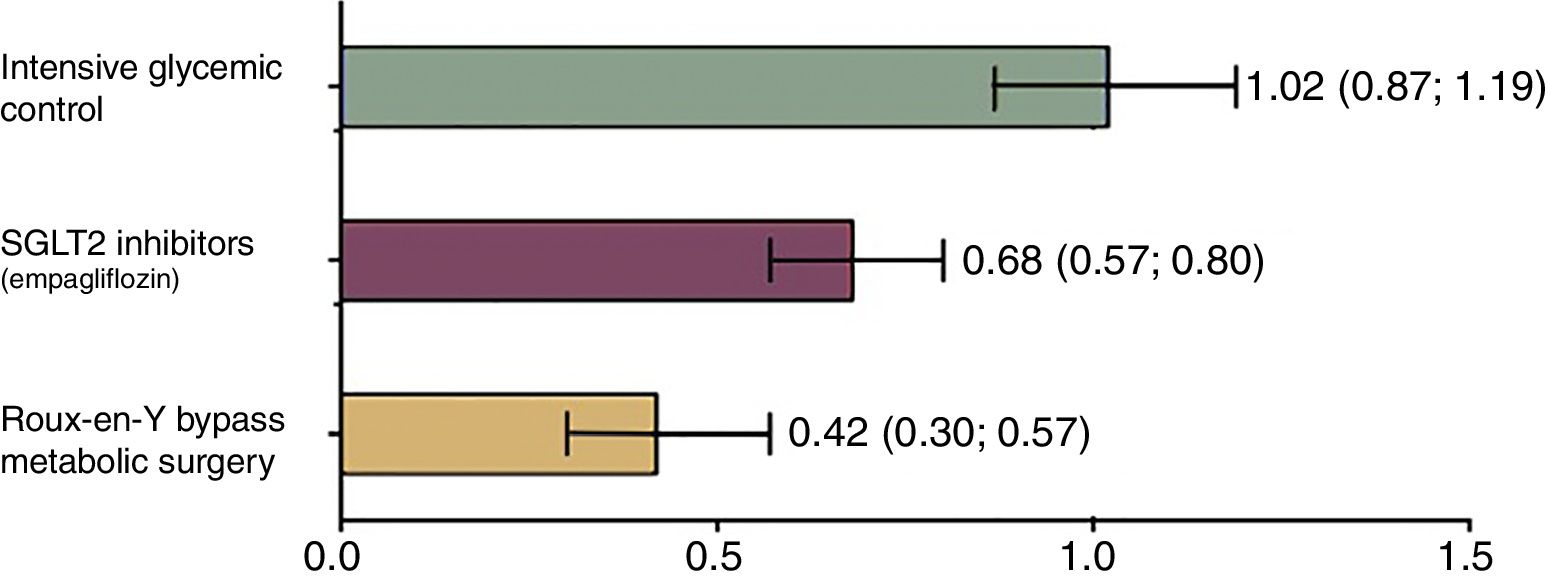
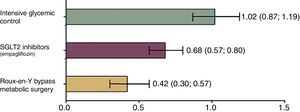
Currently, drugs that act as selective inhibitors of SGLT1 are being developed. These drugs promise to reduce the absorption of glucose from the diet, which is why they will be key in the treatment of DM2 and obesity.25 Currently, only selective sodium-glucose cotransporter type 2 (SGLT2) inhibitors are available, which are the main cotransporters that operate in the kidney and favor glucose excretion in the urine.26 These drugs (including dapagliflozin, canagliflozin and empagliflozin lower blood glucose levels without inducing insulin secretion, regulate glycosylated hemoglobin and prevent cardiovascular events.27 However, their effectiveness is even lower than that of surgery28 (Fig. 2). Similarly, the non-selective inhibitors of both transporters (SGLT1/SGLT2) are being developed and have not yet been commercialized. These include ertugliflozin, remogliflozin and sotagliflozin. It is expected that, in the future, they will offer a therapeutic alternative in diabetes due to their dual-acting capacity to reduce blood glucose.29
Relative risk of mortality in patients with DM2 versus controls according to the treatment applied. Mortality was analyzed according to all the causes observed between a control group that did not receive any medical intervention and 3 groups that received different therapies. Group 1: intensive glycemic management with standard measures (diet and lifestyle changes). Group 2: control with SGLT2 inhibitors that represent the latest trend in the treatment of diabetes. Group 3: patients undergoing metabolic surgery (Roux-en-Y gastric bypass). Group 1 presented a relative risk of mortality similar to the control group that did not receive any therapy. Meanwhile, the group that underwent surgery showed this to be the best option to reduce the risk of mortality, and even better than the new therapies. Adapted from Baud et al.28
Millions of microorganisms coexist in the intestine that are symbiotically related with the host. The bacterial flora deal with functions that the intestine cannot perform, such as the synthesis of certain vitamins and the metabolism of some complex polysaccharides. Likewise, they keeps the intestinal immune system active.30,31 The type of intestinal microbiota (protein composition and exogenous genetic load) is determined in part by the type of nutrients ingested.30 It has been hypothesized that if a person is fed a high-fat diet, this can increase the proportion of endotoxin-producing bacteria and generate an “internal metabolic endotoxemia”. This endotoxemia is a chronic inflammatory state that induces insulin resistance.31
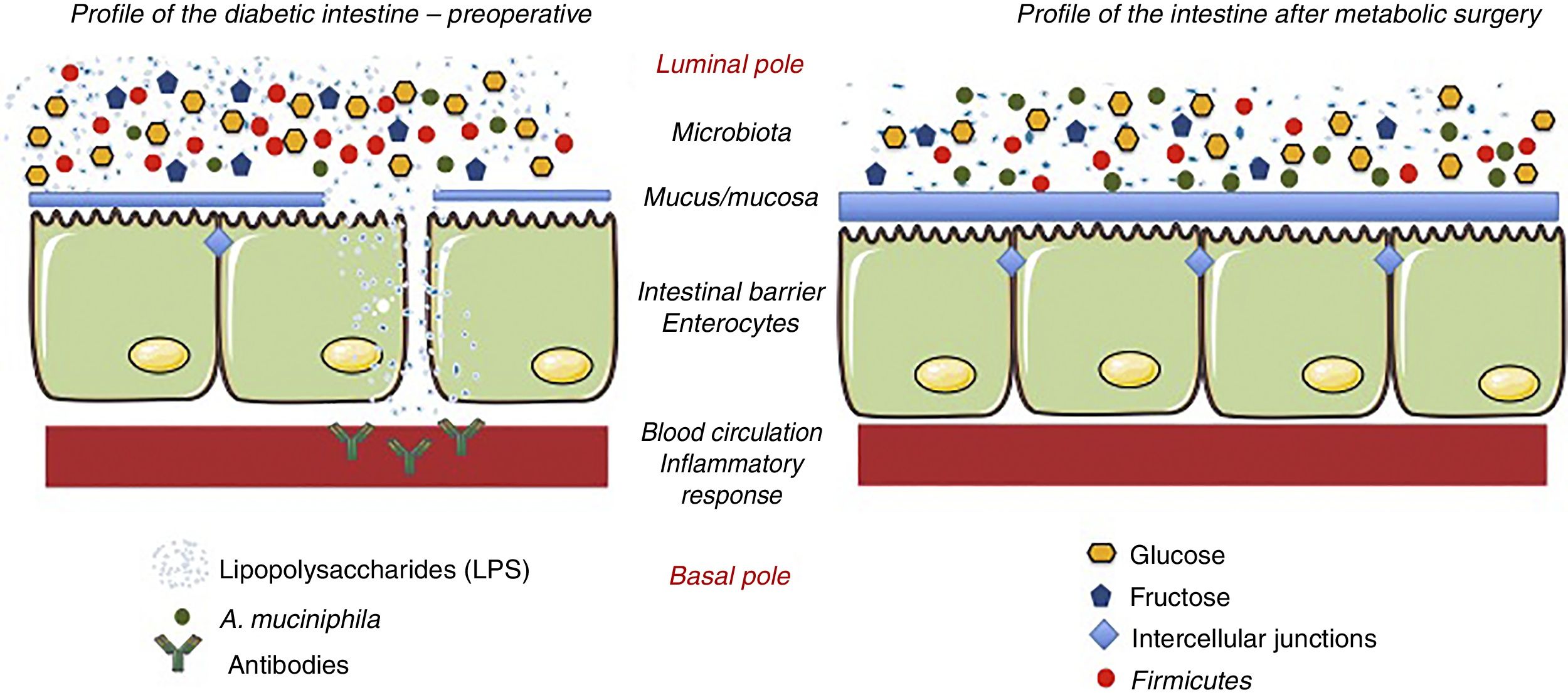
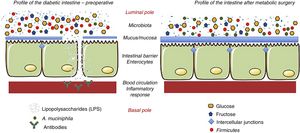
The effect of surgery on the intestinal flora is evident, since, by changing the anatomy of the gastrointestinal tract, the quantity and quality of the nutrients change.32–35 The most notable change produced after surgery is the change in the Firmicutes/Bacteroidetes ratio. Thus, after surgery, the Firmicutes (gram-negative bacteria) decrease and the Bacteroidetes (gram-positive bacteria) increase. The Firmicutes are characterized by having high levels of lipopolysaccharides (LPS), a component of the bacterial membrane that promotes inflammation.36 This inflammation seems to be associated with permeability problems, similar to what occurs in cases of food intolerance (lactose, gluten, etc.). LPS alter carbohydrate metabolism through a chronic inflammatory response.37 Reducing the proportion of Firmicutes lowers the degree of inflammation, so that modifying the Firmicutes/Bacteroidetes ratio has a beneficial effect per se, regardless of weight loss. Membrez et al.38 reported that the use of antibiotics in obese mice almost completely eliminated the intestinal flora and fasting blood glucose improved, even though obesity persisted. Another hypothesis suggests that the presence of a specific bacterium is responsible for the beneficial metabolic effect: Akkermansia muciniphila (A. muciniphila).39 It is believed that A. muciniphila has anti-inflammatory effects in humans, and studies have shown inverse relationships between the colonization of this bacterium and intestinal inflammatory conditions, such as obesity and diabetes.40 In other words, obese and diabetic individuals have a lower proportion of this bacterium, and there is less mucus on the surface of the intestinal mucosa. In contrast, when this bacterium has been administered to obese and/or diabetic individuals, they recover the integrity of the intestinal barrier and improve their metabolic profile.41 This same phenomenon occurs in patients undergoing metabolic surgery, where the presence of this bacterium increases the thickness of the mucus and the levels of inflammation decrease42 (Fig. 3).
Hypothesis about the mechanisms of action that explain the effects of metabolic surgery associated with the microbiota. Metabolic surgery changes the disposition of food in the gastrointestinal tract. In turn, these changes alter the bacterial flora or microbiota. The main change is the reduction of lipopolysaccharide-producing Firmicutes. When lipopolysaccharides decrease, the inflammatory response associated with diabetes decreases. Similarly, the hyperplasia-hypertrophy of the intestinal epithelium after surgery and the presence of A. muciniphila improve the integrity of the intestinal barrier function. Adapted from Cani et al.36 through Servier Medical Art by Creative Commons Attribution 3.0.
In any event, in the microbiota hypothesis, it is difficult to discern whether the effects are the cause or a consequence of modifying the relationships between the host and the microorganisms, or whether it is solely the bacteria that lead to the genesis of the disease.36,43 Future studies in proteomics, genomics and metabolomics will provide much information.
Hypotheses That Support Intestinal GluconeogenesisThe small intestine also contributes to the synthesis of glucose through a process called intestinal gluconeogenesis.44 The observation that the small intestine is able to synthesize glucose and release it into the portal circulation has helped in the understanding of diabetes. However, it is still a little known process. This mechanism involves glucose-6-phosphate synthase (G6P-asa) and phosphoenolpyruvate carboxykinase (PEPCK), enzymes that are found in high concentrations in the liver, but are absent in the normal intestine.45 However, after surgical gastrointestinal rearrangement, a notable elevation of both enzymes has been observed in segments of the jejunum and ileum. The release of intestinal glucose into the portal flow can be interpreted in hepatic receptors as glucose from food, thus altering the regulatory signals of hepatic gluconeogenesis.46 The debate about the existence of intestinal gluconeogenesis (as well as renal) has been accepted in the study of patients with liver transplantation.47 The production of endogenous glucose is essential in the anhepatic phase during liver transplantation, and the evidence that organs such as the kidney or intestine contribute to this process is undeniable.
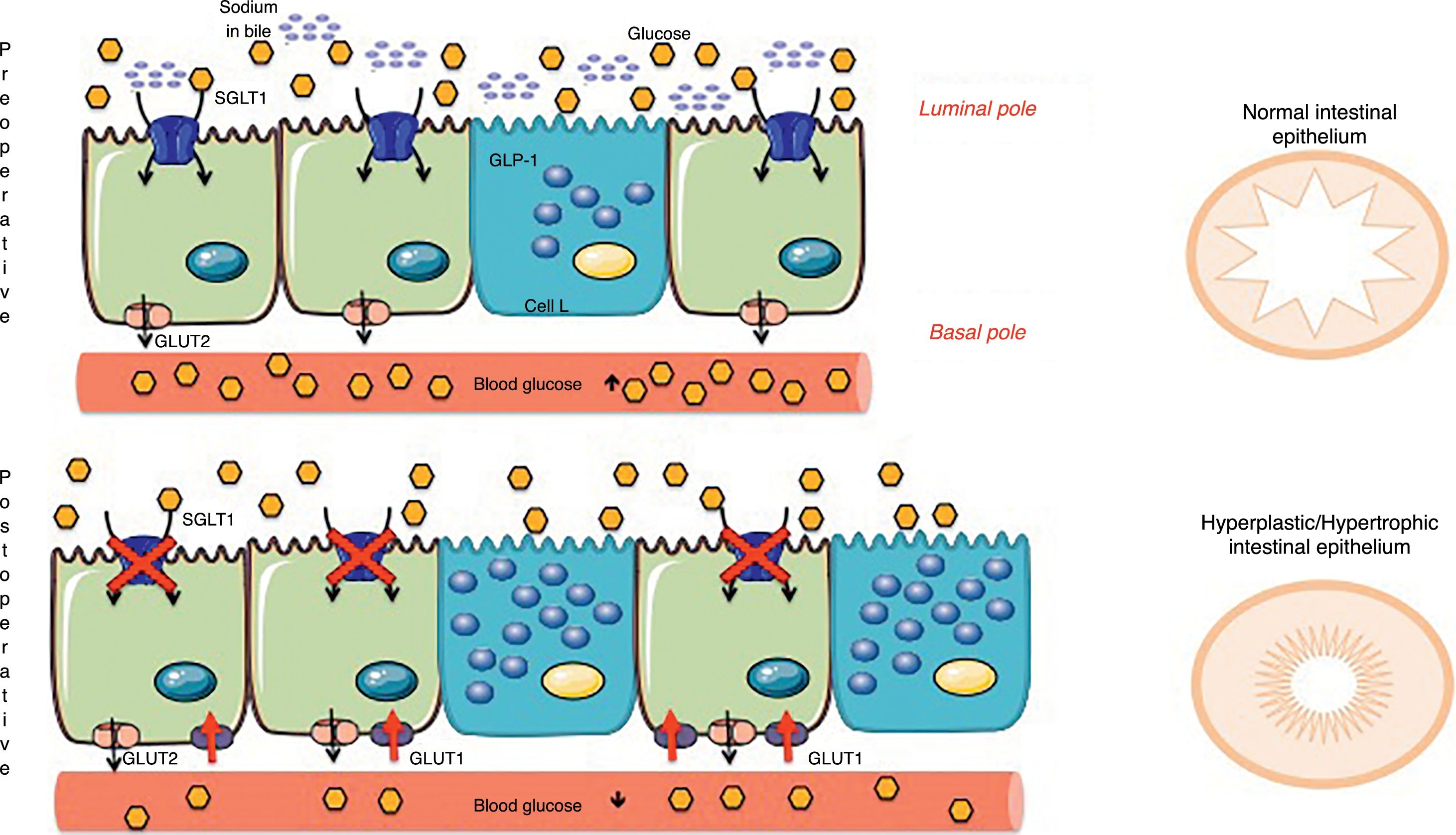
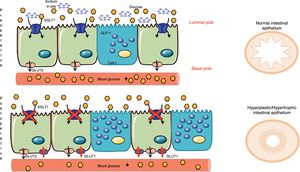
Concepts of Intestinal AdaptationAmong the new hypotheses about the intestine that explain the metabolic effect of surgery are old concepts derived from short bowel syndrome and bowel adaptations due to multiple resections.48 The intestinal mucosa modifies the cell turnover signaling, apoptosis and hyperplasia due to the change of nutrients in the intestinal lumen. These nutrients not only act as fuel, but also as signaling molecules of different metabolic pathways and, consequently, directly influence intestinal adaptation.49 This is how the concept of intestinal adaptation arises, where the most obvious changes after metabolic surgery are hyperplasia and mucosal hypertrophy.3,50 Previously, the study by Baud et al.24 was mentioned, which demonstrated that gastric bypass modifies sodium content and therefore glucose absorption, which should be present at the same time in the intestinal lumen (apical pole of the enterocyte). Thus, bypass surgeries further alter glucose absorption, since SGLT1 is unable to obtain glucose from the intestinal lumen, and the mucosa therefore undergoes hyperplasia/hypertrophy. However, the new thickened epithelium continues to have a fuel deficit in the diet, which is why another compensatory mechanism must be activated. Saeidi51 and Cavin52 describe a restructuring of the intestinal glucose transporters, but explained from the basolateral membrane (basal pole of the enterocyte). The new intestinal mucosa must satisfy the growing bioenergetic requirement, and that is why in the basal pole (in contact with the bloodstream) there are glucose transporters called glucose transporters type 1 (GLUT1). These passive transporters, which do not require energy, are not common in the adult intestine, but their expression increases after an intestinal bypass to the point that their concentration is the second after the brain (the organ with the most GLUT1 in the body).51 That is why the intestine, for its maintenance, extracts the glucose it needs from the blood flow, causing a rapid and considerable drop in blood glucose (Fig. 4). The Cavin studies52,53 also describe the increase in enteroendocrine cells (incretin-producing L and K cells) after surgery. However, one must consider that enterocytes are the most numerous cell group in the intestine and, although the increase in cells secreting GLP-1/GIP is undeniable, the changes in enterocytes are thought to be quantitatively more important. Cavin et al.53 made comparisons between different types of surgery, especially between gastric bypass and vertical sleeve gastrectomy (VSG). Although there is an increase in incretin-secreting cells after VSG, the same degree of hyperplasia-hypertrophy in the intestine is not observed as with bypass surgeries and, therefore, it is concluded that in the VSG there is no extra demand for glucose from the intestine that needs to be covered from the blood circulation. This may explain why relapses of DM2 are more frequent in patients undergoing VSG than bypass. Even so, Cavin emphasizes that the intestinal absorption of alimentary glucose is delayed in VSG, probably because some of the components of the gastric juices modifying the biliary composition.53 However, in the absence of studies with long-term results, it is still unknown whether the metabolic benefit of VSG will be lasting or if it will be affected by a new adaptation of the digestive tract.54
Mechanisms of intestinal adaptation after bariatric surgery. Diversion surgeries (those that separate bile from food) have a malabsorptive component associated with the sodium-glucose transporter in the apical membrane of the enterocyte (SGLT1), which decreases the capacity for glucose absorption from food (Baud, 2016). To compensate for this phenomenon, the intestine becomes hyperplastic and hypertrophic. However, this process is not enough, and therefore the enterocyte awakens a passive transporter from the embryonic stage, called GLUT1, which is expressed in the basolateral membrane (Saeidi51 and Cavin52). This GLUT1 transporter captures the glucose in the blood to provide energy to the new cells and the glycemia therefore drops. Adapted from Cavin et al.52 through Servier Medical Art by Creative Commons Attribution 3.0.
The western diet is rich in easily assimilated carbohydrates (mainly liquids), fats and “refined grain” foods. “Refined grain” foods are defined as those that have been stripped of their starchy endosperm, germ and bran in the milling process; as a result, they have a substantial loss of fiber, vitamins, iron, magnesium, and other dietary components. As a result, refined grain products are nutritionally inferior, have a higher starch content, are less dense, do not favor intestinal transit and are less satiating than their “wholegrain” counterparts.55
Intestinal transit is favored as long as there is a peristaltic gradient from cranial to caudal to ensure that the intestinal content is driven toward the lower intestinal portions. In fact, the food that enters the jejunum induces a vagal reflex that slows peristalsis to enable the digestion-absorption of food.56 If the food that enters the jejunum does not favor peristalsis, its passage through the intestine is further delayed, which is usually accompanied by signs and symptoms associated with constipation.57
After surgery, especially after gastric bypass, poorly digested foods are frequently moved on to the intestine due to accelerated gastric emptying.58 Similarly, the more malabsorptive the technique, the greater the amount of large molecules in the intestine, and this will also lead to the dragging of water and ions. All this increases the peristalsis and accelerates the arrival of intestinal content to the colon, which justifies the episodes of diarrhea in some patients after the intervention. Accelerated intestinal transit is considered a major determining factor in the effectiveness of surgery.59 In this sense, the Nguyen et al.60 group indicated that after a gastric bypass, the speed of intestinal transit increases, which generates malabsorption whenever the speed is not less than 4kcal/min. If the exposure is greater, the absorption of glucose is higher, which can considerably reduce the effectiveness of the surgery.59,60
ConclusionsThe incidence and prevalence of DM2 has been increasing significantly, and for now metabolic surgery is the only procedure with long-term solid results. The benefits of surgery go beyond the secretion of incretins and there are other factors that also influence the improvement of blood glucose regardless of weight loss. This review has described a series of mechanisms of action that explain how glycemia decreases after surgery, and most of these mechanisms are associated with changes that occur in the intestine especially (Fig. 5). Understanding these mechanisms is essential when choosing the surgical technique, and diversion procedures are the most recommended in diabetic patients. Restrictive techniques or VSG are not contraindicated, but these options need to be assessed individually. Unfortunately, the absence of randomized trials and clinical trials limits the conclusions about which is the best surgical option among the different diversion techniques. The development of research, both in the clinical setting and in basic sciences, is essential, but even more important is the effective communication between the two fields.
Main mechanisms that explain the control of blood glucose levels in different bariatric surgery techniques. All the techniques have a mechanical restrictive effect that leads to decreased appetite and caloric intake. Until now, the metabolic effect was mainly attributed to the incretin effect that, with the exception of the gastric band, is observed in all the techniques. However, the diversion techniques, in addition to all the above, also modify the intestinal structure and convert the intestine into a system that consumes glucose from the blood. For this reason, excluding the duodenum and separating the bile from food achieves a greater reduction in blood glucose.
The authors have no conflicts of interests to declare.
Please cite this article as: Zubiaga L, Vilallonga R, Ruiz-Tovar J, Torres A, Pattou F. Importancia del tracto gastrointestinal en la diabetes de tipo 2. La cirugía metabólica es más que incretinas. Cir Esp. 2018;96:537–545.