The use of fluorescence in surgery has expanded and become widespread in recent years, which has led to a real technological phenomenon with the emergence of devices adapted for use in laparoscopic and robotic approaches. Fluorescence-guided surgery in the field of endocrine surgery is also on the rise. More and more articles describe its use in surgery of the thyroid, parathyroid and adrenal glands, although the series are still modest in size and protocols have not been standardized. There are currently several developing areas for the application of fluorescence in endocrine surgery, including the use of fluorescence with indocyanine green in adrenal gland surgery, the identification and prediction of parathyroid perfusion with indocyanine green, and autofluorescence of the parathyroid glands. The objective of this article is to review the current applications of fluorescence in endocrine surgery.
El uso de la fluorescencia en cirugía se ha ampliado y difundido en los últimos años, lo que ha constituido un auténtico fenómeno tecnológico ligado a la aparición de dispositivos adaptados a su utilización en los abordajes laparoscópicos y robóticos. La cirugía guiada por fluorescencia en el campo de la cirugía endocrina está igualmente en auge. Cada vez son más los artículos que describen su uso en la cirugía de las glándulas tiroides, paratiroides y suprarrenal, aunque con series aun modestas y con protocolos diversos no estandarizados. Existen actualmente diversas áreas de desarrollo de la aplicación de la fluorescencia en cirugía endocrina. Cabe destacar el uso de la fluorescencia con verde de indocianina en cirugía suprarrenal, la identificación y predicción de la perfusión paratiroidea con verde de indocianina, y la autofluorescencia de las glándulas paratiroides. El objetivo de este artículo es revisar las actuales aplicaciones de la fluorescencia en cirugía endocrina.
Fluorescence is the optical property of materials that present luminescence when excited by radiation of a given electromagnetic spectrum. The application of fluorescence in surgery provides the visualization of structures that are invisible to the naked eye in order to evaluate metabolic processes or tissue perfusion. After the administration of a fluorophore, light is used of an appropriate spectrum in conjunction with cameras using certain spectral filters. This process does not require a significant increase in surgical time or large equipment. The substance most widely used in fluorescence-guided surgery is indocyanine green (ICG). It is a sterile water-soluble tracer of 775Da developed by Kodak in 1955 and approved for clinical use by the FDA in 1959. It becomes excited by absorbing light from the near-infrared (NIR) spectrum and emits fluorescence of around 820nm in wavelength. After intravenous injection, it travels bound to plasma proteins, is rapidly filtered by the liver without being metabolized and has hardly any tissue absorption. ICG is eliminated by the biliary pathway in approximately 8min, depending on vascularization and liver function. If it is injected out of the bloodstream, it is drained through the lymphatic system, likewise linked to proteins. Its toxic dose is >5mg/kg, which is much higher than the clinical doses used. Adverse effects are uncommon (1.5/1000 patients) and are associated with hypersensitivity reactions. Its use is contraindicated in patients allergic to iodine due to the presence of sodium iodide in its composition.
ICG has been used in medicine since the middle of the 20th century to measure cardiac output, study retinal vessels or measure liver functional reserve. Its use in surgery has expanded and spread in recent years, resulting in a true technological phenomenon linked to the emergence of devices adapted for use in laparoscopic and robotic approaches.1–4 Fluorescence-guided endocrine surgery is also on the rise. There are more and more articles describing its use in surgery of the thyroid, parathyroid and adrenal glands, although the series have been small, using diverse non-standardized protocols. The objective of this article is to review and describe the current applications of fluorescence in endocrine surgery.
Material and MethodsA systematic review was conducted through the PubMed search engine of all published articles containing the keywords fluorescence, indocyanine green, near-infrared or autofluorescence, in addition to any of the following: adrenalectomy, adrenal, parathyroid or thyroidectomy. Dealing with novel techniques with a level of evidence that is still low, all the articles found were assessed, and those that provided more scientific evidence or more novel advances were commented.
ResultsThe Use of Indocyanine Green in Adrenal Gland SurgerySince Gagner et al.5 described the first laparoscopic adrenalectomy in 1992, this technique has become the standard approach, and multiple publications have endorsed its use.6 ICG-guided adrenal gland was described in 2013 by Manny et al.7 with a series of 3 cases in which partial resection of the gland was performed using a robotic approach and the Firefly® device (Intuitive Surgical, Inc., Sunnyvale, CA, USA) after the administration of 2.5mg of intravenous ICG. They reported less fluorescence in adrenal masses compared to healthy glandular tissue, probably due to the different translocase enzyme expression. The pathology analysis demonstrated disease-free resection margins (one pheochromocytoma, one lipoadenoma and one follicular lymphoid hyperplasia). They concluded that this is a feasible, safe technique and that fluorescence with ICG could be useful to ensure the resection margins in partial adrenalectomy.
The first article on ICG adrenalectomy in an animal model was presented by Dip et al.8 in 2014. They described the perfect outline of the contour of the adrenal gland in 5 pigs after administration of ICG at a dose of 0.5mg/kg using the Karl Storz® laparoscopic device (Karl Storz Endoskope, Tuttlingen, Germany). The adrenal glands were clearly more hyperfluorescent than the surrounding fatty tissue, improving the differentiation between both structures compared to vision under white light, with statistically significant differences. In 2015, Sound et al.9 from the Dr. Berber group at the Cleveland Clinic (Ohio) described the use of ICG in a series with 10 total adrenalectomies using the Firefly® device for the robotic approach. The dose of infused ICG and the measurement of fluorescence at different times after infusion were recorded. It was determined that the distinction between glandular fluorescence and that of peri-glandular fatty tissue appears in the first minute after the infusion of ICG, is maximum after 5min, and still lasts 20min afterwards. DeLong et al.10 applied this technique to the conventional laparoscopic approach, also in 2015, in a series of 4 adrenalectomies guided by ICG with the Pinpoint® device (Novadaq Technologies, Inc., Mississauga, ON, Canada). They described the technique as feasible and safe, while defining the sequence of fluorescence observed. The adrenal arteries emit fluorescence first, followed by the adrenal parenchyma, and finally, the adrenal vein. Thus, the visualization of vascularization by fluorescence could improve its control in maneuvers prior to adrenalectomy, which is of special interest to avoid noradrenergic crises in the approach of pheochromocytoma or to help avoid intraoperative bleeding.
The largest study published to date on the use of fluorescence with ICG in adrenal surgery was presented by Dr. Berber's group. In it, Colvin et al.11 presented a series of 43 adrenalectomies by retroperitoneal or lateral transabdominal robotic approach. After administering an average of 3 injections of 5mg of ICG per patient, they compared glandular fluorescence with the surrounding fatty tissue, stratifying by tumor type. These authors demonstrated greater utility of the technique in cortical tumors, which were hyperfluorescent, compared to those of medullary origin, such as pheochromocytoma, hypofluorescent versus the rest of the gland, probably due to deficient expression of the translocase enzyme. This finding could have special interest in cortical-sparing adrenalectomy in bilateral pheochromocytomas.
Use of Indocyanine Green to Identify the Parathyroid GlandsMultiple strategies have been studied to improve the identification of the parathyroid glands. In 1971, Dudley12 described the use of methylene blue, although the appearance of toxic encephalopathy in certain patients reduced its use.13 Similarly, aminolevulinic acid has also been used, but the need to previously photosensitize the patient and protection from light for 48h after surgery has also limited its use.14 Imaging techniques to visualize the parathyroid glands, such as intraoperative ultrasound and scintigraphy, are the subject of multiple studies that have described sensitivities of 70%–85% for each technique.15 In this context, in 2014 Suh et al.16 presented a study in a canine model in which they objectively measured after surgery the intensity of fluorescence emitted by the parathyroid glands in 3 dogs at different increasing doses of intravenous ICG; these were clearly greater compared to those emitted by the thyroid glands. In 2015, again Sound et al.,17 from the Cleveland Clinic of Ohio, described for the first time the use of fluorescence with ICG for the identification of the parathyroid glands in humans, using the Pinpoint® device. They presented a series of 3 patients undergoing re-operation for persistent primary hyperparathyroidism. The study described the technique in detail and the ideal dose of ICG to administer (5mg), finding evidence of clear hyperfluorescence of the parathyroid glands from 2 to 20min post-infusion, which made their identification possible. The authors defined the technique as easy, fast, reproducible and safe. The same group presented a study in 2016 with 33 patients operated on for primary hyperparathyroidism in which 112 parathyroid glands were identified.15 After administration of 5mg of ICG, 93% (104) presented hyperfluorescence. The emission of fluorescence appeared 30–60s after infusion and lasted 20min. For the first time, they described a subjective graduation of the fluorescence based on the postoperative measurement of the area of the gland that was hyperfluorescent according to the following scale: 0 (no uptake), 1 (<30% of the volume of the gland), 2 (30%–70%) and 3 (>70%). Among multiple variables analyzed, age <60, preoperative serum calcium levels >11mg/dL and the maximum diameter of the parathyroid gland >10mm correlated with higher scores in the Parathyroid hyperfluorescence scale.
In 2016, Vidal Fortuny et al.,18 in Geneva, Switzerland, infused ICG in patients scheduled for subtotal parathyroidectomy once surgical clips were placed that divided each of the 4 parathyroid glands into 2 differentiated zones. In this way, they preserved the average parathyroid gland that emitted the most fluorescence, resecting the remaining 3 and a half glands. Thus, they tried to guarantee the perfusion of the glandular remnant in order to avoid postoperative hypoparathyroidism. The same hypothesis and methodology were used by the same group shortly afterwards19 and also with the Pinpoint® device, in which 13 patients underwent subtotal parathyroidectomy. According to the authors, their results showed that a well-vascularized glandular remnant could avoid persistent hypoparathyroidism. These were the first studies that found a correlation between the perfusion or fluorescence of the parathyroid glands and their subsequent function, without simply being based on the identification of the glands, which has constituted a substantial change for later studies. With this new study hypothesis, the same year, Vidal Fortuny et al.20 published a series of 36 patients who had undergone total thyroidectomy with measurement of parathyroid fluorescence using the Pinpoint® device. To this end, they created a subjective measurement scale that classifies each gland with values 2 (hyperfluorescence, well vascularized gland), 1 (parathyroid slightly or heterogeneously fluorescent, partially vascularized gland) or 0 (non-fluorescent, non-vascularized parathyroid). All patients were treated with oral calcium and vitamin D in the postoperative period, in accordance with hospital protocol. Thirty patients had at least one parathyroid gland with a score of 2 (well vascularized) and none of them had postoperative hypoparathyroidism. Two of the 6 patients without any gland with a score of 2 on the fluorescence scale had transient hypoparathyroidism. The authors showed the existence of an excellent correlation between perfusion and parathyroid function by which a single well-vascularized gland could avoid postoperative hypoparathyroidism. Similarly, Zaidi et al.,21 again from the Cleveland Clinic, used the parathyroid fluorescence scale previously described by their group15 in which they also contemplated the size of the hyperfluorescent area measured postoperatively. Thus, they contemplated the 4 possibilities described: 0 (non-fluorescent parathyroid), 1 (<30% of the volume of the gland is hyperfluorescent), 2 (30%–70%) and 3 (>70%). Using the same Pinpoint® device, 27 total thyroidectomies were performed with visualization of 85 parathyroid glands. Differences were found, although these were not statistically significant in the values of postoperative PTH in patients with less than 2 glands scored with a value of 1 according to the scale mentioned.
The bilateral axillary-mammary robotic approach with ICG fluorescence using the Firefly® device was described in 2016. Yu et al.22 compared a series of 22 patients operated on with this technique with a control group of 44 patients operated without fluorescence, using statistical matching techniques with propensity score matching. The authors described a lower rate of incidental parathyroidectomies in the ICG group, although they did not find significant differences in the rate of postoperative hypoparathyroidism. In 2017, Lang et al.23 presented the first study described with objective and intraoperative assessment of fluorescence intensity in thyroid surgery. They described a series of 70 patients who had undergone total thyroidectomy with identification of the 4 parathyroid glands using the SPY® Fluorescent Imaging System device (Novadaq Technologies, Inc., Mississauga, ON, Canada). In each patient, the intensity of tracheal fluorescence was observed, giving it a value of 100%, based on which the relative fluorescence of each parathyroid was evaluated. A glandular intensity of >150% for at least one parathyroid gland was defined as the best predictor value with a postoperative hypocalcemia rate of 0%, compared to 81.8% in patients with glandular fluorescence intensity ≤150%. Again, the Vidal Fortuny et al. group24 published in 2018 the most recent article on parathyroid fluorescence with ICG. They compared the rate of postoperative hypoparathyroidism in 196 patients undergoing total thyroidectomy divided into 3 groups. One hundred and forty-six patients presented at least one parathyroid gland with a fluorescence index of 2 according to the already mentioned scale by the same author.20 This group was randomized into 2 subgroups of 73 patients each. In one of them, the standardized postoperative doses of calcium and vitamin D were administered according to the protocol of the hospital, and serum levels of calcium and PTH were measured on postoperative day+1 (control group). In the other subgroup, no follow-up lab work was done, nor was any postoperative preventive treatment administered (intervention group). The remaining 50 non-randomized patients presented a fluorescence index <2 in all their parathyroid glands and received treatment to prevent hypocalcemia (nonrandomized group). The results showed a non-inferiority of the intervention group in terms of hypocalcemia compared to the control group (P=.012), while the nonrandomized group had a higher rate of postoperative hypoparathyroidism compared to the randomized group (P=.007). The study concluded that fluorescence with ICG predicts postoperative parathyroid function and vascularization, and that the presence of at least one well vascularized gland avoids the need for follow-up PTH and calcium studies as well as a preventive treatment protocol with calcium and vitamin D.
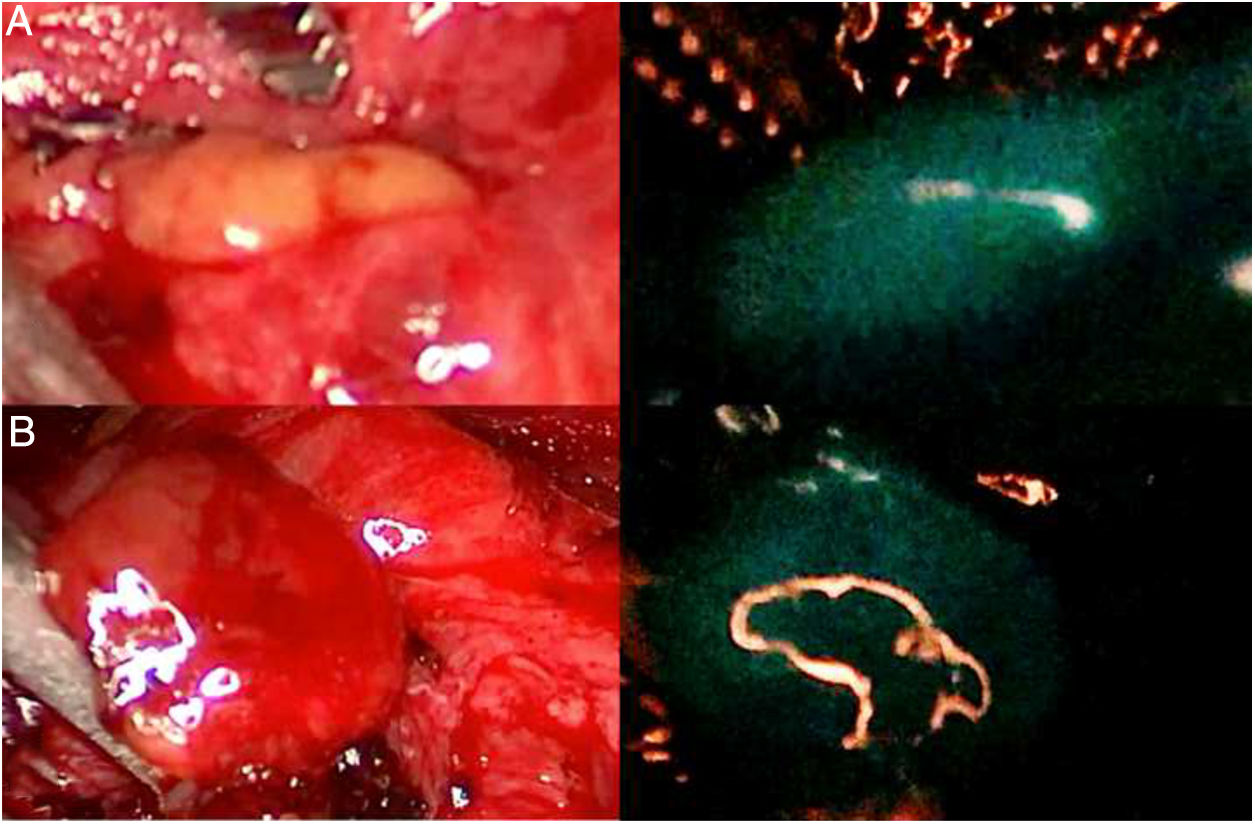
Use of Autofluorescence in the Identification of the Parathyroid GlandsIn 2011, Paras et al.25 described autofluorescence of the parathyroid glands when illuminated with NIR light, with an emission around 820nm, and clearly superior to that of the thyroid gland. Although the nature of the endogenous fluorophore is unknown, calcium or vitamin D receptors have been postulated as possible culprits. In 2015, the same group performed a study with 264 parathyroid glands from 137 patients in whom autofluorescence was measured.26 Nearly 100% of the glands were identified in each patient with this technique, independently of the thyroid or parathyroid disease that motivated the surgery, with the exception of those patients with secondary hyperparathyroidism in whom the detection rate was clearly lower (53.8%; P=.008). In addition, a BMI>25kg/m2, preoperative serum calcium>10.5mg/dL and vitamin D<30ng/mL were also associated with lower intensity of parathyroid autofluorescence (P=.0018; P=.012; P=.026, respectively). There were no differences according to age, sex, ethnicity or preoperative PTH levels. In 2016, Falco et al.27,28 presented the first study with a commercial device, the Fluobeam 800® (Fluoptics, Grenoble, France). They compared the fluorescence of the parathyroid glands with the thyroid gland and surrounding fatty tissue in 74 patients, finding a greater intensity of fluorescence in the parathyroid glands (P<.0001). They also compared the number of visible glands with infrared light and white light, finding a greater number of patients in whom 4 parathyroid glands were found with infrared light (μ=3.7glands/patient) than with white light (μ=2.5), with statistically significant differences (P<.001). The same device was used by De Leeuw et al.29 in 2016 when analyzing the sensitivity of the test in 28 ex vivo samples from parathyroidectomy with a result of 94.1%, the parathyroid still being autofluorescent even one hour after excision and fixed in formaldehyde. Thus, autofluorescence is independent of vascularization and gland function. In the same way, they analyzed the use of the device in 81 in vivo parathyroid glands in 35 patients, with a sensitivity of 98.8% in the detection of the glands. According to the authors, brown adipose tissue and thyroid colloid nodules could give false positive results.
Similar results have been obtained in recent studies in which other devices have been used to measure autofluorescence. Kim et al.30 described the use of an infrared lamp (INFRALUX-300®, Daekyung Electro Medical Co., Pocheon City, Gyeonggi-Do, Korea) added to the NIR imaging system to improve visualization of parathyroid tissue in 8 patients. The same year, Shinden et al.31 used the Photodynamic Eye® (PDE) commercial device (Hamamatsu Photonics, Hamamatsu, Japan) designed for surgery with ICG, finding greater intrinsic fluorescence in the parathyroid glands of 17 patients than in the thyroid, lymph nodes and surrounding fat, both in vivo and ex vivo. In 2017, Ladurner et al.32 used the Karl Storz® device in 30 patients in whom 34 of the 42 parathyroid glands showed autofluorescence, while 8 parathyroid glands encompassed by adipose tissue could not be located with infrared light alone. At this point, in 2017 Kahramangil and Berber33 compared the visualization of parathyroid glands after intravenous infusion of ICG with parathyroid autofluorescence in 44 patients undergoing thyroidectomy (22 patients with each technique). The percentages of parathyroid glands identified were 95% in the ICG group and 98% in the autofluorescence group, with no significant differences. In contrast, the percentage of glands visualized before being identified with white light visible with the naked eye was 6% in the ICG group and 52% in the autofluorescence group, with statistically significant differences (P<.001). The authors concluded that both techniques have a similar parathyroid detection rate, but that autofluorescence is faster than ICG.
In 2018, Benmiloud et al.34 published a cohort study conducted by 2 surgeons in 2 consecutive time periods in which one of the surgeons (surgeon 1) used autofluorescence to identify the parathyroid glands in the second time period (NIR+group). Comparing these results with the rest, the authors found that the NIR+group presented a lower rate of postoperative hypocalcemia (P<.01), a higher rate of parathyroid identification (P<.05) and a lower rate of parathyroid autotransplantation (P<.05). The most recently published study on parathyroid autofluorescence is by Kahramangil et al.,35 a multicenter study that analyzes its use in 210 patients. Autofluorescence detected 98% of the glands, and 46% of them (one gland per patient, on average) were not initially visible before complete dissection of the surrounding fatty tissue.
DiscussionThe use of ICG in endocrine surgery is a safe and effective technique. The recommended dose is 5mg, both in adrenal and thyroid surgery, although its administration can be repeated on several occasions, if necessary. In the field of adrenal surgery (Fig. 1), ICG shows clear adrenal hyperfluorescence in the first minute, maximum after 5min and visible up to 20min after infusion, which allows it to be perfectly differentiated from the surrounding fatty tissue and ensure its dissection and complete resection. In addition, the visualization of adrenal vascularization with ICG, although inconstant, can provide greater control during dissection maneuvers and thus reduce the rates of hemorrhagic complications, as well as those derived from glandular manipulation. It is worth mentioning that tumors of the adrenal cortex appear to be hyperfluorescent, while spinal tumors and malignant tumors would be hypofluorescent compared to the rest of the gland, which is of special interest in partial resections. Similarly, the use of ICG in cervical endocrine surgery (Fig. 2) shows a parathyroid fluorescence clearly superior to the thyroid and visible from 30 to 60s to 20min after infusion. Young age, high levels of preoperative calcemia and a high glandular size could correlate with greater parathyroid hyperfluorescence. There is also an interesting correlation between hyperfluorescence and postoperative parathyroid function. The presence of at least one well-vascularized parathyroid gland could avoid postoperative hypofunction, and postoperative follow-up lab tests and prophylactic treatment may be unnecessary. Several subjective scales have been developed to measure parathyroid fluorescence with promising results, but there are still few methods for objective intraoperative grading. The discovery of parathyroid autofluorescence (Fig. 3), without the need for the administration of any exogenous tracer, and clearly superior to the thyroid, presents an important change in the field of fluorescence-guided endocrine surgery. Parathyroid autofluorescence is not related to glandular function, vascularization, or disease. Secondary hyperparathyroidism, high BMI, high serum calcium levels and low preoperative vitamin D levels have been associated with lower autofluorescence, with no differences according to age, sex, ethnicity or preoperative PTH levels. This technique seems to have a similar rate of parathyroid identification as ICG, but it provides faster glandular detection and may help reduce the rate of postoperative hypocalcemia.
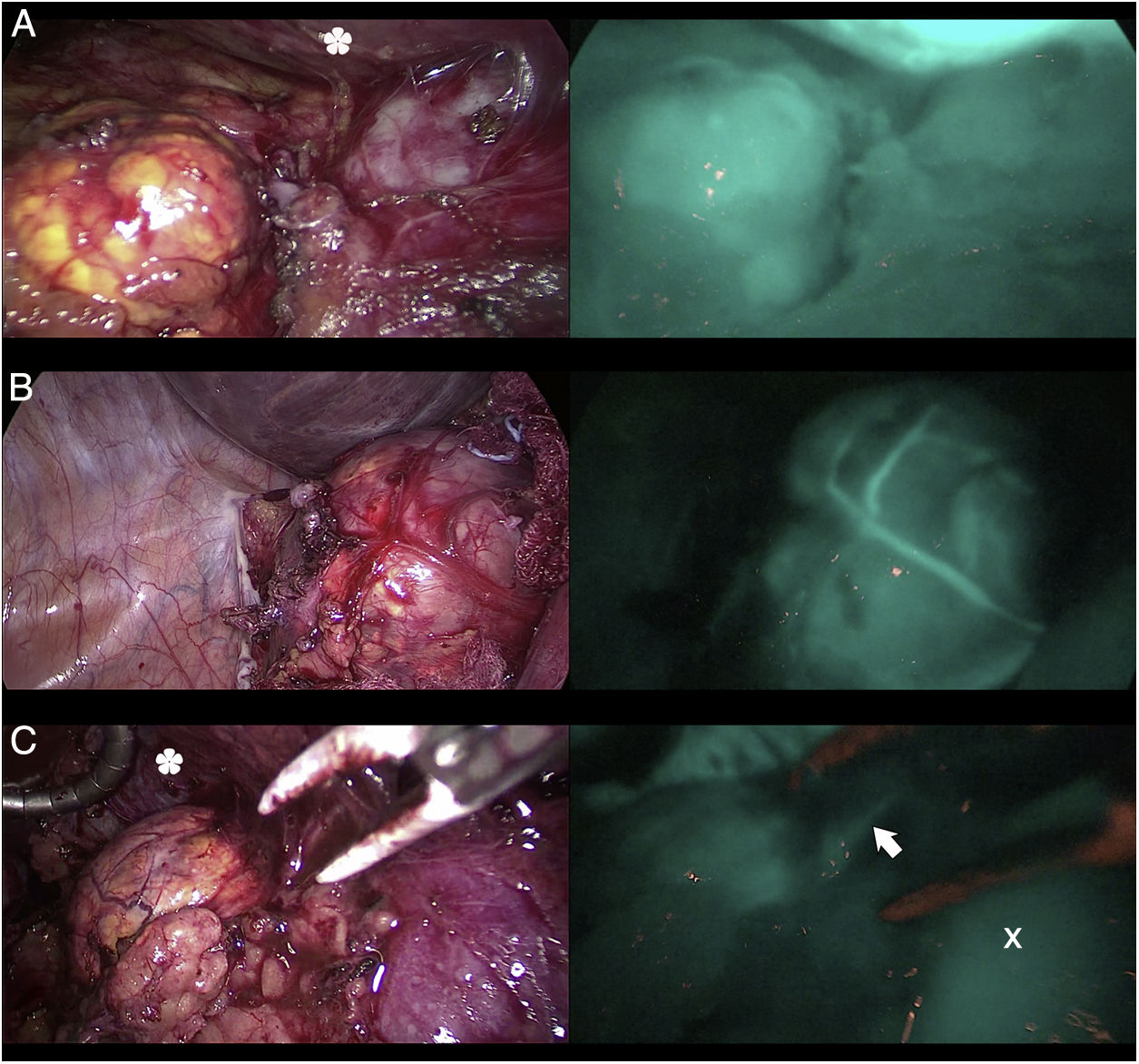
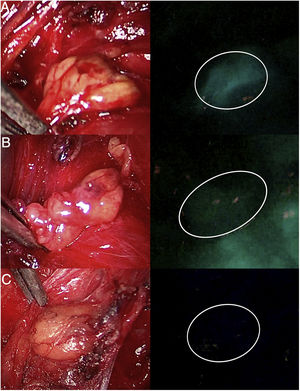
Three cases (A, B and C) of anterior laparoscopic adrenalectomy with Karl Storz® Image 1 HD device. In the left column, the adrenal glands are illuminated with white light and in the right column under NIR light after infusion of 5mg of intravenous ICG. Adrenal hyperfluorescence was observed in all 3 cases, clearly greater than in the surrounding fatty tissue. The white arrow indicates the right suprarenal vein. Hepatic hyperfluorescence is marked with *. The inferior vena cava is marked with an X.
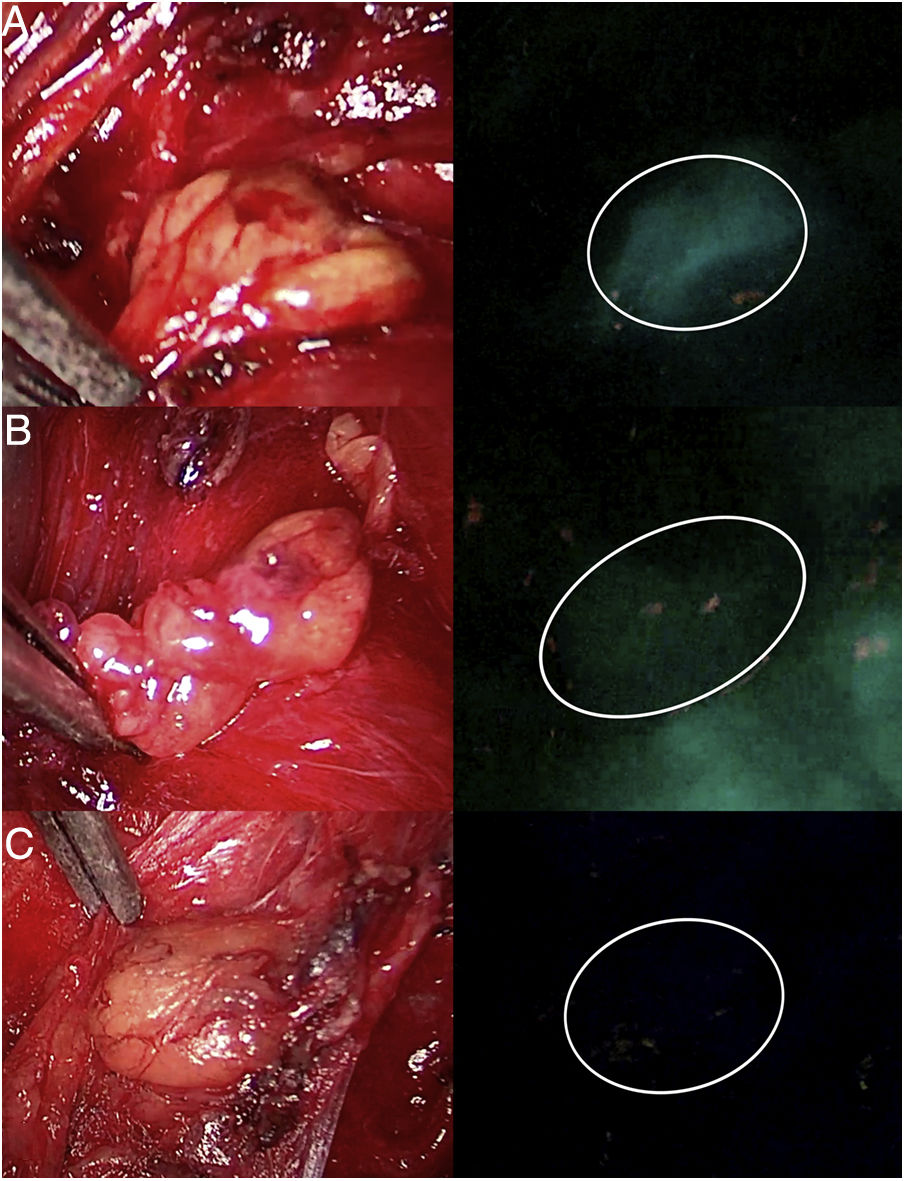
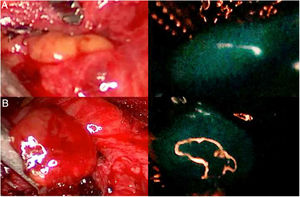
Three parathyroid glands (A, B and C) visualized under white light (left column) and under NIR light (right column) with the Image 1 HD device by Karl Storz®, after infusion of 5mg of intravenous ICG. Parathyroid gland A presents homogeneous hyperfluorescence. The gland marked B shows a patchy or heterogeneous fluorescence due to probable insufficient vascularization. Parathyroid C does not show fluorescence, so its vascularization and function are clearly anticipated to be altered.
Fluorescence-guided surgery has been making significant advances that may modify the concept of endocrine surgery in the future. Although more studies are required to provide greater statistical evidence for these new improvements, current results are promising.
Conflict of InterestsNone of the authors has a conflict of interests, nor has any funding been received.
Please cite this article as: Bonnin-Pascual J, Álvarez-Segurado C, Jiménez-Segovia M, Bianchi A, Bonnin-Pascual F, Molina-Romero FX, et al. Aportaciones de la fluorescencia a la cirugía endocrina. Cir Esp. 2018;96:529–536.