Laparoscopic left-sided pancreatectomy (LLP) is an accepted technique for the treatment of benign and pre-malignant lesions of the left side of the pancreas, but there is still controversy on its use for malignant ones.
ObjectiveTo evaluate our results in LLP as a routine technique for primary lesions of the left pancreas.
Patients and methodsWe performed LLP in 15 patients for primary lesions of the pancreas from November 2007 to November 2011. An intra-abdominal drainage was left in all cases, and the recommendations of the International Study Group for Pancreatic Fistula were followed.
ResultsThe mean age of the patients was 64±13 years. Six radical spleno-pancreatectomies, 3 corporocaudal with preservation of the spleen, and 6 pure distal (4 with preservation of the spleen). There was one conversion. The mean surgical time was 230±69min. The mean post-operative stay was 8.1±7.6 days. At 90 days, complications were detected in 4 patients; 3 grade II and one grade V according to the modified classification of Clavien. There was one grade B pancreatic fistula. The diagnosis was a malignant neoplasm in 53% of cases. The number of resected lymph nodes in the cases where a radical resection was planned due to cancer was 21.7±11.5, there being negative margins in all cases.
ConclusionsLLP may be considered as a suitable technique for the treatment of primary pancreatic lesions, including malignant ones, provided that it is performed by groups with experience in pancreatic surgery and highly trained in laparoscopic surgery.
La pancreatectomía izquierda laparoscópica (PIL) es una técnica aceptada para el tratamiento de lesiones benignas y premalignas del páncreas izquierdo, existiendo todavía controversia en su uso para las malignas.
ObjetivoEvaluar nuestros resultados en la PIL como técnica de rutina para el tratamiento de las lesiones primarias del páncreas izquierdo.
Pacientes y métodosDesde noviembre de 2007 hasta noviembre de 2011 hemos intervenido 15 pacientes de PIL por lesiones pancreáticas primarias. En todos los casos se dejó un drenaje intraabdominal y se siguieron las recomendaciones de la International Study Group for Pancreatic Fistula.
ResultadosLa edad media fue de 64 ± 13 años. Se realizaron 6 espleno-pancreatectomías radicales, 3 córporo-caudales con preservación del bazo y 6 distales puras (4 con preservación del bazo). Hubo una conversión. El tiempo operatorio fue de 230 ± 69 minutos. La estancia media postoperatoria fue de 8,1 ± 7,6 días. A 90 días, según la clasificación modificada de Clavien se detectaron complicaciones en 4 pacientes: 3 grado II y 1 grado V. Hubo una fístula pancreática grado B. En el 53% de los casos el diagnóstico fue de neoplasia maligna. El número de ganglios resecados en los casos que se planificó una resección radical por carcinoma fue de 21,7 ± 11,5, siendo en todos los casos los márgenes negativos.
ConclusionesLa PIL puede considerarse como una técnica adecuada para el tratamiento de las lesiones primarias del páncreas, incluyendo las malignas, siempre que se realice por grupos con experiencia en cirugía pancreática y alta cualificación en cirugía laparoscópica.
Laparoscopic distal and subtotal pancreatectomy (left pancreatectomy, LP) is a formally accepted technique for the treatment of left-sided pancreatic pathology, especially cystic, benign or premalignant lesions and neuroendocrine tumours (NET).1–3 Several groups have shown that the advantages inherent to laparoscopy, previously tested for other surgical techniques, are also applicable in these cases.3,4 However, there is still controversy on its use for the curative treatment of malignant lesions, especially in invasive ductal carcinoma of the pancreas, for which a pancreatic resection with extended lymphadenectomy and appropriate safety margins is required.4,5 In addition, the technical difficulty involved in the laparoscopic approach for the pancreas, including distal pancreatectomy, makes LP not be considered currently as the technique of choice in most centres, and is only used for specific selected cases.3,4,6–8
The aim of our study is to evaluate the effectiveness and safety of LP, by analysing the results of our series, when it is considered as the routine technique for treating primary pancreatic lesions located in the body and tail of the pancreas, regardless of their degree of malignancy.
Patients and MethodsSince November 2007, the laparoscopic approach has been systematically considered for performing subtotal and distal pancreatectomies (LP). LP is considered any resection that requires a glandular section to the left of the portal vein, either in the pancreatic neck, body or tail. In this context subtotal pancreatectomy (STP) is defined as complete resection of the body and tail of the pancreas with parenchymal section at the pancreatic neck, and distal pancreatectomy (DP) when transection is carried out at the body or tail of the pancreas. Pancreatic parenchymal section was performed with an endoscopic linear stapler with staple depth of 2.5mm (blue load) except in two cases, in which due to difficulty in placing the stapler, a bipolar sealing device was used: one, for a lesion located in the neck, and the other, for a well-circumscribed lesion very close to the splenic hilum. In 5 cases, the pancreatic transection edge was reinforced a hand-sewn continuous suture using 3/0 monofilament. In all cases, a low pressure aspiration drain was left by the transected edge of the pancreatic parenchyma. Amylase determination was performed from the third day onward only if the drain was productive at that time. It was withdrawn if it was not productive or if amylase determination was negative. When there was a preoperative suspicion of malignant pancreatic neoplasm, a pancreatosplenectomy was scheduled with en bloc standard lymphadenectomy, in accordance with RAMPS technique (radical antegrade modular pancreatosplenectomy) previously described by Strasberg9 and by our group using a fully laparoscopic approach.10 All patients underwent intraoperative anatomopathological analysis of the resected specimen and of its pancreatic and retroperitoneal margins.
We followed the recommendations of the ISGPF (International Study Group for Pancreatic Fistula) for the diagnosis and classification of postoperative pancreatic fistula.11 We used the modified Clavien classification for pancreatic resection surgery for the diagnosis and grading of postoperative complications,12 which were recorded at 90 days postoperatively.
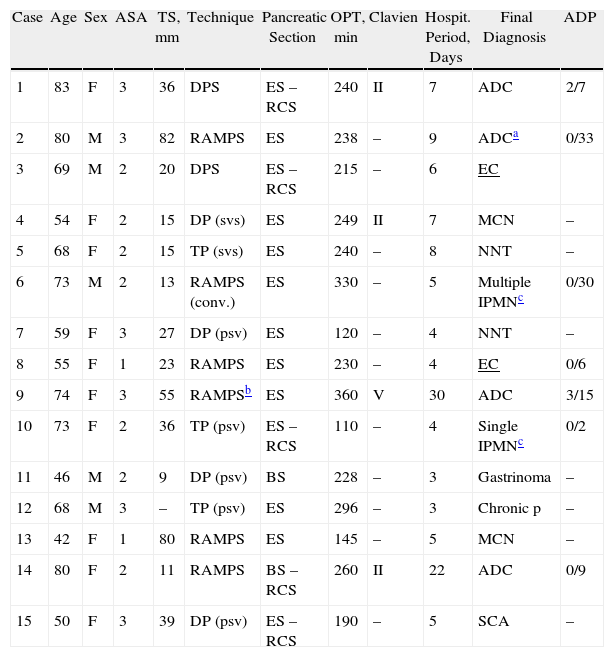
ResultsUp to November 2011, 22 LPs were performed. The study excluded 7 patients, of which 6 had direct open surgery and one after an exploratory laparoscopy to rule out peritoneal carcinomatosis which would have contraindicated proceeding with resection: 2 patients in which the pancreatic resection was performed secondarily to another major procedure (gastrectomy, nephrectomy) in collaboration with other units or Departments; 2 cases of bulky endocrine carcinomas with suspected infiltration of the coeliac trunk and 3 patients previously operated on conventionally: 2 with a pancreaticoduodenectomy who had a second primary neoplasm of the pancreatic remnant at 26 and 57 months, one of which initial exploratory laparoscopy was performed, and another, previously operated in another centre, of a pancreatic pseudocyst cystjejunostomy whose final diagnosis after surgical resection was ductal adenocarcinoma. Therefore, the final study population of this study included 15 patients who underwent an LP for primary pancreatic lesions located on the left-side of the pancreas. Table 1 shows the demographic and clinical characteristics of these patients arranged chronologically.
Characteristics and Results of the Patients Operated on Laparoscopic Pancreatectomy.
| Case | Age | Sex | ASA | TS, mm | Technique | Pancreatic Section | OPT, min | Clavien | Hospit. Period, Days | Final Diagnosis | ADP |
| 1 | 83 | F | 3 | 36 | DPS | ES – RCS | 240 | II | 7 | ADC | 2/7 |
| 2 | 80 | M | 3 | 82 | RAMPS | ES | 238 | – | 9 | ADCa | 0/33 |
| 3 | 69 | M | 2 | 20 | DPS | ES – RCS | 215 | – | 6 | EC | |
| 4 | 54 | F | 2 | 15 | DP (svs) | ES | 249 | II | 7 | MCN | – |
| 5 | 68 | F | 2 | 15 | TP (svs) | ES | 240 | – | 8 | NNT | – |
| 6 | 73 | M | 2 | 13 | RAMPS (conv.) | ES | 330 | – | 5 | Multiple IPMNc | 0/30 |
| 7 | 59 | F | 3 | 27 | DP (psv) | ES | 120 | – | 4 | NNT | – |
| 8 | 55 | F | 1 | 23 | RAMPS | ES | 230 | – | 4 | EC | 0/6 |
| 9 | 74 | F | 3 | 55 | RAMPSb | ES | 360 | V | 30 | ADC | 3/15 |
| 10 | 73 | F | 2 | 36 | TP (psv) | ES – RCS | 110 | – | 4 | Single IPMNc | 0/2 |
| 11 | 46 | M | 2 | 9 | DP (psv) | BS | 228 | – | 3 | Gastrinoma | – |
| 12 | 68 | M | 3 | – | TP (psv) | ES | 296 | – | 3 | Chronic p | – |
| 13 | 42 | F | 1 | 80 | RAMPS | ES | 145 | – | 5 | MCN | – |
| 14 | 80 | F | 2 | 11 | RAMPS | BS – RCS | 260 | II | 22 | ADC | 0/9 |
| 15 | 50 | F | 3 | 39 | DP (psv) | ES – RCS | 190 | – | 5 | SCA | – |
ADC: adenocarcinoma; ADP: positive lymph nodes/total resected ratio (only for malignant neoplasms); SCA: serous cystadenoma; EC: endocrine carcinoma; ES: endoscopic stapler; DPS: distal pancreatectosplenectomy; MCN: mucinous cystic neoplasm; TP: body and tail pancreatectomy with spleen preservation; DP: distal pancreatectomy with spleen preservation; psv: preservation of splenic vessels; RAMPS: radical antegrade modular pancreatectosplenectomy; RCS: reinforcement of continuous suture; BS: bipolar sealer; svs: splenic vessel section; IPMN: intraductal papillary mucinous tumour; NNT: non-functioning neuroendocrine tumour; TOP: operation time; TS: tumour size.
The patients were 10 women and 5 men with a mean age of 64±13 years. The surgical techniques were: 6 RAMPS, 3 STP with preservation of the spleen (2 with preservation of the splenic vessels), 4 DP with preservation of the spleen (3 with preservation of the splenic vessels) and 2 distal pancreatosplenectomies (DSP). The mean operative time was 230±69min. In one case, a 270° fundoplication was added due to the presence of a type III hiatal hernia.
A patient who had had previous episodes of acute pancreatitis due to multiple intraductal papillary mucinous tumours (IPMT) in the body and tail of the pancreas required conversion to open surgery after the section of the pancreatic neck, section of the splenic vessels at their origin and mobilisation of the whole body and lymphadenectomy, due to suspected infiltration in the transverse mesocolon at the splenic angle. Definitive diagnosis confirmed the existence of IPMT with carcinoma in situ foci with all resection margins being negative and without mesocolon infiltration.
The mean hospital stay was 8.1±7.6 days. Eleven patients (73%) were admitted for less than one week. Complications were detected in 4 patients: 3 grade II and one grade V. One patient with a history of ischaemic heart disease underwent transfusion of packed red blood cells. A low-output pancreatic fistula (type B) was diagnosed, which required total parenteral nutrition due to delayed gastric emptying. One patient was re-admitted for an infection of a trocar site wound requiring “bedside” debridement and antibiotic treatment. A patient with a history of kidney failure and chronic metabolic acidosis secondary to bilateral ureterohydronephrosis due to radical cystectomy and previous ureterosigmoidostomy, died after presenting a progressive and severe re-exacerbation of chronic renal failure without a clear precipitating cause, not attributable to any intraabdominal complications (unremarkable abdominal CT), and who did not undergo haemodialysis by the Nephrology Service due to a combination of advanced age and T3N1 pancreatic carcinoma. No complications related to the spleen were detected in any of the 7 cases in which it was preserved (5 with preservation of splenic vessels). One patient was found to have a sterile collection at 4 months, which was percutaneously drained, with normal determination of amylase levels.
The final diagnoses are shown in Table 1. In 8 patients, the diagnosis was malignant neoplasm. The average number of lymph nodes resected in cases of ductal carcinoma was 16±12, although in the 4 cases where a RAMPS was planned due to suspected malignancy it was 21.7±11.5. In all cases, both the pancreatic section margin and the retroperitoneal margins were negative.
DiscussionQuality criteria for left-sided pancreatic resections using a conventional approach are variable, even in high-volume centres.13–16 Previously published morbidity and mortality figures vary between 10%–47% and 0%–6%, respectively. The rates published in the DISPACT study deserve special mention.17 This prospective, randomised multicentre study included 450 patients, with the primary objective of evaluating two techniques of pancreatic transection.17 It strictly complies with the criteria established by the ISGPF in the diagnosis of pancreatic fistula,11 and it found a total rate of postoperative complications of 70%, with 45% of patients with at least one serious complication, 6% of wound infection and a mortality rate within 90 days of 3%. These data are probably the most reliable approximate reference of the results of LP by conventional approach. Stutchfield et al.18 addressed the quality standards to which the laparoscopic approach should aspire, considering as acceptable rates of morbidity, clinically relevant pancreatic fistula and mortality that do not exceed 39%, 8%, and 3%, respectively. In the Spanish national registry of pancreatic resections by laparoscopy,2 rates of complications related to pancreatectomy of only 16% are presented and no cases of mortality are reported, although it is a voluntary registry and only 6% of cases were treated for pancreatic carcinoma. The existing comparative studies between the laparoscopic approach and open surgery show no significant differences in terms of operation time, pancreatic fistula rate, mortality and even oncological radicality, and instead find advantages in favour of the laparoscopic approach in terms of blood loss reduction, postoperative hospital stay, total complications and a quicker postoperative recovery.3,7,8,19–22 In our series, the mean hospital stay was 8.1 days, slightly higher than that established by other groups,3,6,21–23 but less than the 15 days reported in conventional surgery17 and similar to or lower than that of other series.19,24,25 We note that in our series, the mean hospitalisation stay was prolonged especially in elderly patients (Table 1), and the mean age of our patients was clearly higher, with almost 10 years of difference in some cases, than most other series.2,3,18,22 In general, our results regarding the hospital stay/pancreatic fistula are more similar to those of the Asian literature than of those of Western literature, where surprising figures are reported of a postoperative stay of 4–6 days and pancreatic fistula rates of up to 23%.3,6,21,22 In terms of our mortality rate, we believe that it is more attributable to a likely poor selection of cases for surgical resection than of the type of approach selected. Moreover, our rates of complications related to the technique, reoperation and transfusion, are comparable to those of most published LLP series, which confirms the safety and reproducibility of the technique.
The most common and important determinant of postoperative outcome in PI, both open and laparoscopic, is pancreatic fistula. The DISPACT18 study found a rate of pancreatic fistula around 30%, using as a means of pancreatic section both the linear stapler (177 patients, 32%) and hand-sewn sutures (175 patients, 28%). In addition, we must add 19% of intra-abdominal abscess or collections. Thaker et al.26 in a prospective study comparing stapled pancreatic section alone or adding reinforcement with absorbable mesh sleeves on the stapled line, found a decrease in the rate of pancreatic fistula from 36% to 3.5%. Meanwhile, Abu Hilal et al.6 also found a decrease in the rate of pancreatic fistula from 50% to 9% if the stapled line is reinforced with sutures. With regard to the purely laparoscopic approach, Fernández-Cruz et al.23, in their retrospective analysis of 82 patients consecutively operated on, have a rate of clinically relevant pancreatic fistula rate below 10%, using the endoscopic linear stapler as the only method of pancreatic section. Other series have shown rates ranging between 10% and 50%.3,6,21,22,24,27 In our series, pancreatic fistula was not detected in any of the 13 patients in which pancreatic section was performed with the endoscopic stapler, although in 4 of them, determined by the quality of the staples, thickness and consistency of the pancreas, the section margin was reinforced with sutures. We believe that this low rate of pancreatic fistula could be explained because most of the pancreatic sections were performed in the neck (73%), where the parenchyma is thinner and stapling is often more effective and safe, but it would certainly not be reproducible if all sections had been carried out at the body of the pancreas.
LP is not a simple technique nor easy to implement, and this is demonstrated by the fact that, in most of the series, operating times are not usually lower than 3h, especially considering that most are not oncological resections.3,19,21,23,25 However, the conversion rates published are relatively low,3,21–23 usually below 15%, which seems to show that this is a reproducible and standardisable technique. Although in selected cases, especially in those in which lesions are small, benign, and located in the tail of the pancreas, very common features published in most series,3,22,23,25 resection is usually technically less complex when considering this approach as a standard for most cases, in lesions of any nature and location, expertise in both pancreatic surgery and advanced laparoscopic surgery is required, and this is the most limiting factor for a high percentage of surgeons. Unlike in most published series, but in line with the DISPACT study, in our study, 53% of resections were performed for malignant neoplasms, with a complete resection of the body and tail of the pancreas performed in 73% of cases. Moreover, in the last 5 of the 7 patients in whom the spleen was preserved, splenic vessels were maintained, which significantly lengthened the operating time. This would explain our operating time being longer than that reported in other series.21,23 In this regard, we believe that the term distal pancreatectomy with or without splenectomy, is confusing and hinders the comparison between series, since it includes in the same group complex resections such as the RAMPS10 and other technically less demanding resections such as that of the pancreatic tail, and as such, it should be specified if it is a STP or a pure DP.
The use of this approach in malignant neoplasms has been questioned, because of doubts about a lower quality of the oncological resection. In our series, both the mean number of resected lymph nodes and the analysis of the resection adjust to the quality standards desired.4,28 They are consistent with the results published by other groups,21,27 confirming that optimal oncological radicality is feasible and can be standard in the laparoscopic approach. In general, our results, both with respect to indications and to the postoperative and oncological results, would support the view expressed previously by other groups with more experience in the use of laparoscopy as a method of choice in LP,29 who consider the laparoscopic approach to be that of choice in benign or non-invasive lesions of the pancreas and a safe alternative to open surgery for malignant lesions.
In conclusion, LP can be considered the technique of choice in the treatment of benign and premalignant left-sided lesions of the pancreas. Provided it is performed by groups of experts in pancreatic surgery with a high qualification in laparoscopic surgery, it can also be regarded as an alternative to open surgery for the treatment of a number of cases of malignant neoplasms.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Poves I, et al. Resultados del abordaje laparoscópico en la pancreatectomía izquierda. Cir Esp. 2013;91:25–30.