Cervical anastomotic leaks after esophagectomy are still a frequent and severe complication that needs an early diagnosis and an appropriate treatment. The aim of this study was to describe our experience with the management of this complication.
Patients and methodsRetrospective study (2003–2011) of a consecutive series of 77 patients with a cervical esophagogastric anastomosis, 18 of them (23.3%) presenting a leak. Fistulae were classified into four groups depending on clinical presentation, radiology (esophagogram or CT), surgical findings -in case of re-operation- and, since 2010, endoscopic examination. Type I leaks were an asymptomatic or radiographic leak, Type II had local signs limited to the neck, Type III was associated with respiratory symptoms due to a pleural or mediastinal collection, and Type IV with a systemic disorder secondary to gastric necrosis.
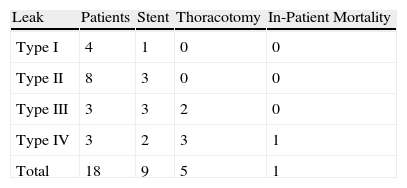
ResultsFour patients (22.2%) were classified as Type I, 8 (44.4%) as Type II, 3 (16.6%) as Type III, and 3 (16.6%) as Type IV. Eight patients were managed conservatively; in 9 a self-expanding stent was used, 5 required a thoracotomy, and one of them (Type IV) died. Leaks were related to a higher associated morbidity (61 versus 30%, P=.019) and a longer hospital stay (median of 28.5 vs 14 days, P=.009).
ConclusionsAlmost one quarter of cervical esophagogastric anastomoses present some kind of anastomotic leak. Although most of them can be treated conservatively or by endoscopy, they are associated with an increase in morbidity and mortality.
La dehiscencia de la anastomosis cervical tras esofagectomía sigue siendo una complicación frecuente y grave que precisa de un diagnostico precoz y un tratamiento apropiado. El objetivo de este estudio ha sido describir nuestra experiencia con esta complicación.
Pacientes y métodosEstudio retrospectivo (2003-2011) de una serie consecutiva de 77 pacientes con anastomosis esofagogástrica cervical, de los cuales 18 (23,3%) presentaron una dehiscencia anastomótica. Las fistulas se clasificaron en cuatro tipos según la presentación clínica, los datos radiológicos (esofagograma/tomografía computarizada), los hallazgos en las reoperaciones y, desde el año 2010, la exploración endoscópica. La fistula tipo I corresponde a una fuga subclínica, la tipo II presenta síntomas a nivel cervical, la tipo III se asocia con manifestaciones clínicas respiratorias secundarias a una colección pleural o mediastínica y la tipo IV con un cuadro sistémico secundario a una necrosis de la plastia.
ResultadosEn 4 pacientes (22.2%) la fistula se clasificó como tipo I, en 8 (44.4%) como tipo II, en 3 (16.6%) como tipo III y en otros 3 (16.6%) como tipo IV. Ocho pacientes fueron tratados de forma conservadora, en 9 se utilizó una prótesis autoexpansible, 5 requirieron toracotomía y un paciente (tipo IV) falleció. Los pacientes con fuga presentaron una mayor morbilidad (61 frente a 30%, p=0,019) y una estancia hospitalaria más larga (mediana de 28,5 vs. 14 días, p=0,009) en comparación con aquellos pacientes que no la desarrollaron.
ConclusionesCasi la cuarta parte de las anastomosis esofagogástricas cervicales presenta algún tipo de fuga anastomótica. Aunque la mayoría puede ser tratada de forma conservadora o endoscópicamente, su aparición está gravada con una elevada morbimortalidad.
We define anastomotic leak as the disruption of the anastomosis that leads to extravasation of the intraluminal content. For oesophageal anastomoses, we also include necrosis of the gastric conduit, where a leak may be a late manifestation.
The incidence of cervical anastomotic leak after oesophagectomy varies widely in the literature, with a range between 10% and 25% in reference centres.1,2 Its appearance is associated with a significant increase in perioperative morbidity, late stenosis3 and a mortality rate between 3% and 10%.4,5
Several factors have been associated with the development of anastomotic leak, such as the patient's nutritional status and comorbidities, anastomotic tension, ischaemia and tissue fragility, location (neck or chest) and neoadjuvant treatment, although the main factors are those related to an impaired oxygen perfusion.1,5–9
The leak may have different presentations and their severity is mainly defined by the location of the leak, its extension, the viability of the gastric conduit, the time period until treatment and systemic repercussions. Early identification and proper treatment can reduce morbidity and mortality.4
The objective of this study was to analyse our experience in the diagnosis and treatment of a series of patients with cervical anastomotic leaks.
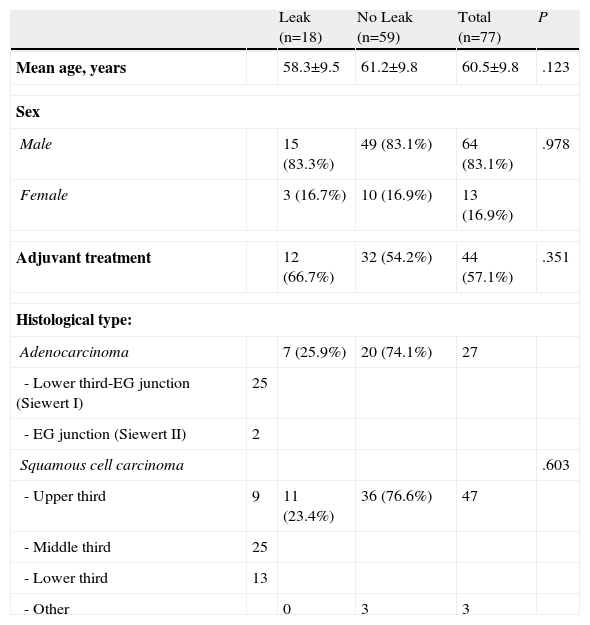
Patients and MethodsFrom 2003 to 2011, 112 patients with oesophageal neoplasm were treated in the Esophageal & Gastric Surgery Department of the Hospital Universitario Donostia of San Sebastián by the same surgical team. We performed a retrospective, longitudinal study including the 77 patients who underwent oesophagectomy with cervical oesophagogastric anastomosis. 18 patients presented anastomotic leak, which were the focus of our study. Table 1 shows the demographic data of these patients, the histological type and location of the tumours.
Demographic Data.
| Leak (n=18) | No Leak (n=59) | Total (n=77) | P | ||
| Mean age, years | 58.3±9.5 | 61.2±9.8 | 60.5±9.8 | .123 | |
| Sex | |||||
| Male | 15 (83.3%) | 49 (83.1%) | 64 (83.1%) | .978 | |
| Female | 3 (16.7%) | 10 (16.9%) | 13 (16.9%) | ||
| Adjuvant treatment | 12 (66.7%) | 32 (54.2%) | 44 (57.1%) | .351 | |
| Histological type: | |||||
| Adenocarcinoma | 7 (25.9%) | 20 (74.1%) | 27 | ||
| - Lower third-EG junction (Siewert I) | 25 | ||||
| - EG junction (Siewert II) | 2 | ||||
| Squamous cell carcinoma | .603 | ||||
| - Upper third | 9 | 11 (23.4%) | 36 (76.6%) | 47 | |
| - Middle third | 25 | ||||
| - Lower third | 13 | ||||
| - Other | 0 | 3 | 3 | ||
EG junction: oesophagogastric junction.
Minimally invasive techniques were used in 73 of 77 patients. In 65 patients, 3-field oesophagectomy was performed and in 12 patients we performed a transhiatal oesophagectomy. The 3-field technique started with thoracoscopy. Until 2007, this was performed in the left lateral decubitus position, and afterwards in prone decubitus. Three trocars were used (2 of 10mm and one of 5mm), respectability was evaluated, and then the arch of the azygos vein was transected and the oesophagus was resected en bloc. At this point, using laparoscopy, gastric mobilisation and tubulization were performed along the greater curvature by means of a blue load endoscopic liner stapler (3.5mm) leaving a 4–5cm conduit.
The laparoscopic transhiatal technique reproduced the classic procedure described by Orringer.10 In both cases, the specimen is extracted through a left lateral cervicotomy and the oesophagogastric anastomosis is performed. As a general rule, if the plasty fits comfortably in the neck, a modified Collard type anastomosis11 (49 cases) or an Orringer10 type anastomosis (9 cases) is performed. If the plasty did not easily reach, a hand-sewn end-to-end anastomosis (19 cases) is performed in a single plane with interrupted absorbable sutures.
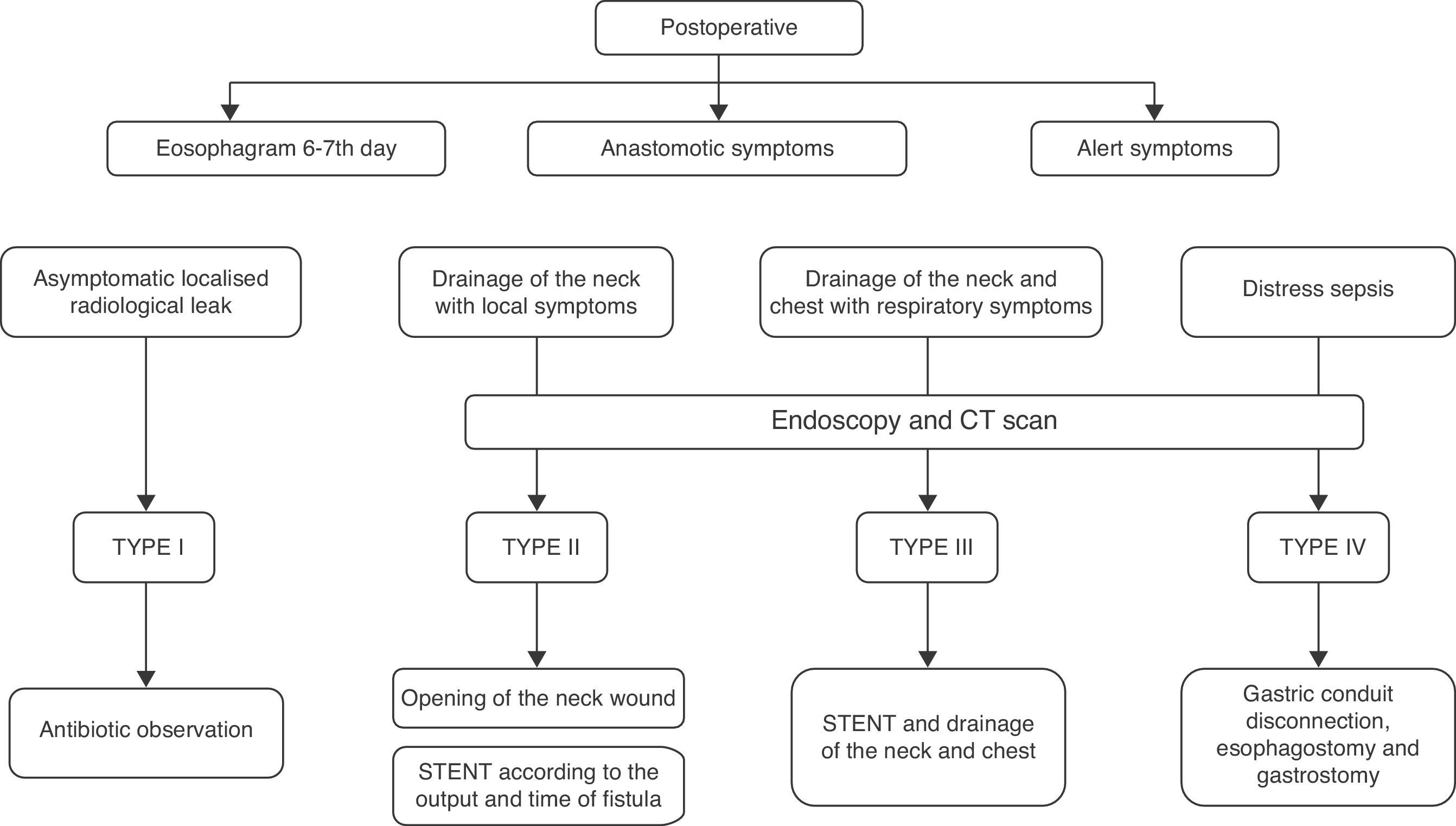
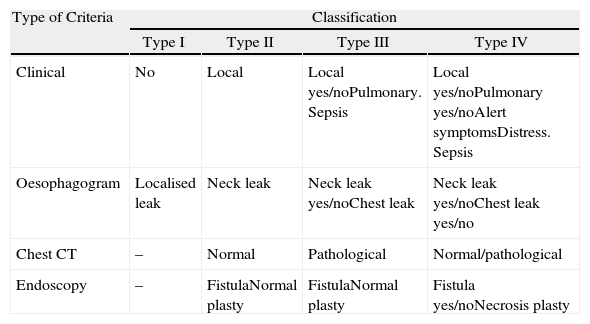
Diagnosis and Classification of Anastomotic LeaksNormally, a radiological contrast study is carried out on the sixth postoperative day. When a complication was suspected, a computed tomography (CT) scan with contrast was performed and, since 2010, endoscopy is also used. With the introduction of endoscopy, we have adopted a classification based on clinical (local or systemic involvement), radiological (extension of the fistula) and endoscopic criteria (size of the leak and involvement of the gastric conduit), inspired by a series of recently published articles1,4,12 (Table 2). Using this classification patients who developed an anastomotic leak were classified according to their clinical features, radiological data and surgical findings in the case of reoperations even though endoscopy was not used systematically until 2010.
Classification of Cervical Anastomotic Leaks.
| Type of Criteria | Classification | |||
| Type I | Type II | Type III | Type IV | |
| Clinical | No | Local | Local yes/noPulmonary. Sepsis | Local yes/noPulmonary yes/noAlert symptomsDistress. Sepsis |
| Oesophagogram | Localised leak | Neck leak | Neck leak yes/noChest leak | Neck leak yes/noChest leak yes/no |
| Chest CT | – | Normal | Pathological | Normal/pathological |
| Endoscopy | – | FistulaNormal plasty | FistulaNormal plasty | Fistula yes/noNecrosis plasty |
Complications in patients with leaks compared to patients without a leak were classified according to the criteria proposed by Clavien for surgical complications,13,14 which consider both the severity of the complication and the type of action required.
In case where stent placement was indicated as a treatment for the anastomotic leak, the Interventional Radiology Department used the self-expanding stent type SX-ELLA Oesophageal Biomed® HV (Hradec Kralove, Czech Republic), which is non-absorbable, coated and metallic and of 20mm diameter with a length ranging between 85mm and 135mm. The prosthesis was removed endoscopically after 6–8 weeks.
Statistical AnalysisContinuous variables were expressed as medians and interquartile range (25–75 percentile). We used Student's t-test to compare the distributions of continuous data and the chi-square test to compare qualitative variables. Statistical analysis was performed using the Statistical Package for Social Sciences 17.0 (SPSS, Inc., Chicago, IL, USA).
ResultsEighteen (23.3%) of 77 patients with cervical oesophagogastric anastomosis developed a leak, which in accordance with the previously proposed classification, were divided into the following types:
- -
Type I: 4 patients (22.2%). These were detected on the follow-up oesophagogram performed on the sixth postoperative day. None of the patients showed associated symptoms.
- -
Type II: 8 patients (44.4%). These cases only presented local symptoms in the cervical wound at about the first week of the postoperative period, and the fistula drained either spontaneously or after the surgical wound was opened.
- -
Type III: 3 patients (16.6%). Systemic affectation appeared between the sixth and thirteenth postoperative day. The expansion of the leak into the thorax produced a significant respiratory compromise in these patients.
- -
Type IV: 3 patients (16.6%). These patients had severe systemic deterioration and haemodynamic instability. In 2 patients, necrosis of the conduit was discovered during thoracotomy and, in one of them, by endoscopy.
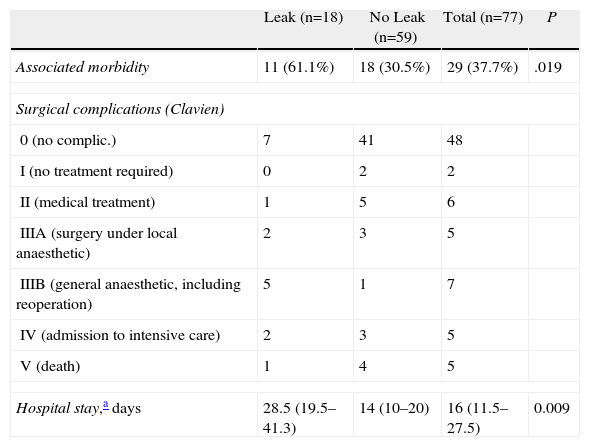
Table 3 shows the associated morbidity, hospital stay and complexity of surgical complications according to the modified Clavien classification13,14 in patients with and without anastomotic leak. Associated morbidity, defined as “other complications excluding leak”, was quantitatively higher with statistical significance in patients with a leak, as well as the median hospital stay. Qualitatively, using the modified Clavien classification, the severity of complications was similar in both groups. Although patients with a leak required more surgical procedures, patients without a leak had a higher mortality rate.
Patients’ Postoperative Course.
| Leak (n=18) | No Leak (n=59) | Total (n=77) | P | |
| Associated morbidity | 11 (61.1%) | 18 (30.5%) | 29 (37.7%) | .019 |
| Surgical complications (Clavien) | ||||
| 0 (no complic.) | 7 | 41 | 48 | |
| I (no treatment required) | 0 | 2 | 2 | |
| II (medical treatment) | 1 | 5 | 6 | |
| IIIA (surgery under local anaesthetic) | 2 | 3 | 5 | |
| IIIB (general anaesthetic, including reoperation) | 5 | 1 | 7 | |
| IV (admission to intensive care) | 2 | 3 | 5 | |
| V (death) | 1 | 4 | 5 | |
| Hospital stay,a days | 28.5 (19.5–41.3) | 14 (10–20) | 16 (11.5–27.5) | 0.009 |
With regard to the type of anastomosis, leakage occurred in 9 of 49 Collard anastomosis (18.4%) in 4 of 9 Orringer anastomosis (44.4%), and in 5 of 19 manual anastomosis (26.3%).
Type I leaks were treated conservatively by observation except in one case, which was resolved by stenting (Table 4). All the patients had a favourable recovery.
Of the 8 patients with Type II leak, 5 were treated conservatively and a stent was placed in 3. The indication for stent placement, once the neck wound was treated locally, was established with very subjective criteria, depending mainly on the debit of the leak and the fistula resolution time. The patients had a favourable recovery and, at the time of discharge, all were able to tolerate oral intake.
A stent was placed in the 3 patients with Type III leak, although two of them required, in addition, a thoracotomy for proper thoracic drainage. The 3 patients had a favourable recovery.
In the first 2 patients, before 2010, with a Type IV leak, an initial attempt to control the anastomotic leak by stent placement was used. However, in both patients, the symptoms worsened and there was a deterioration of their general condition, and they underwent reoperation, with necrosis of the gastric conduit confirmed at thoracotomy. One of these patients underwent a bipolar oesophageal exclusion and survived, in the other patient the exclusion was not performed and an attempt was made to control the leak by placing a new stent, but after a prolonged stay in hospital, the patient eventually died. The third patient, after 2012, whose necrosis was discovered using endoscopy, underwent immediate reoperation with a bipolar oesophageal exclusion with oesophagostomy and gastrostomy. The 2 patients with bipolar exclusion in the series who survived underwent subsequent reconstructive surgery using a coloplasty.
In 9 patients a stent was used to close the fistula. There were no problems with stent placement. In the 7 cases of Type I, II and III leaks, the stent was able to seal the fistula and, after its removal, closure was checked endoscopically. In the 2 cases of Type IV leaks prior to 2010, and in those in which endoscopy was not performed, the stent did not work, therefore delaying the correct decision.
In-hospital mortality in patients with a leak was 5.5%, but we have to account for another death after discharge. This patient had a Type II leakage in whom a stent was placed; the patient died of aspiration pneumonia.
At follow-up, 6 of 18 patients (33.3%) who had a leak developed a stenosis of the anastomosis and required pneumatic dilatations. In patients without leak, this complication occurred in 5 of 59 patients (8.5%). The mean amount of dilatation sessions needed 3.
DiscussionAlthough anastomotic leak is one of the most frequent and severe complications following oesophagectomy, at present no consensus has been reached on its definition and classification,1,4,7,15,16 which makes for disparate results in the literature. Some authors define leaks on the basis of radiological findings, while others do so on the basis of their clinical impact.2,7,8,15–17
In 4 extensive series,18–21 with 3129 patients undergoing surgery, the leak rate fluctuated between 12% and 21%. In our series, the incidence of cervical leak after oesophagectomy for cancer is 23.3%, but two-thirds of the leaks are Type I and II, which have no systemic repercussions and are easier to treat.
The variability of the results is associated with the great variability in treatments, since the management of leaks in general, and the use of endoprostheses22 in particular, frequently involves intuitive decisions that do not follow a pre-established strategy. In our series, a patient with Type I fistula was overtreated with a stent when simple observation may have been sufficient. The analysis of our experience has led us to recognise the need to design a protocol in order to provide early diagnosis of this complication and to improve results.
A large number of classifications have been used to assess the severity of leaks.1,4,7,12,15 Among them, Veeramootoo et al.4 have considered clinical, analytical and radiological criteria; DeMeester's23 group mainly uses endoscopy and Luketich's3 group classification is based on clinical and endoscopic criteria.
Clinical manifestations may be very disparate, from an asymptomatic patient in Type I leaks to very severe symptoms such as distress or septic shock in Type IV leaks. This variation depends on the size of the leak, how it spreads and the presence of ischaemia or necrosis of the gastric conduit.17 Symptoms and signs such as supraventricular tachycardia, auricular fibrillation, altered mental condition, increased oxygen requirements, fever, hypotension, decreased urine output, and/or sepsis alert us to the possible presence of an anastomotic leak and should cause higher levels of suspicion of this diagnosis.1
An oesophagogram is the most commonly used procedure to assess the viability of an oesophageal anastomosis. In our centre, routine follow-up is performed on the sixth postoperative day, and it allows the location and study of the type of dissemination of a leak. Some authors claim that this is not necessary, given the frequency of false negatives.4,24 Contrast CT scans can prove information on the extension of the leak and its intrathoracic consequences. However, neither the oesophagram nor a normal CT scan rule out an ischaemic gastric conduit.12
The introduction of endoscopy in the diagnosis of anastomotic leaks has allowed a more accurate assessment of the ischaemia/necrosis of the gastric conduit and the magnitude of the anastomotic disruption.25 Ischaemia/necrosis is one of the most significant risk factors in the production of anastomotic leaks4 and this may be enhanced by altered gastric blood flow after the construction of the conduit, by anastomotic tension, early conduit distension, haemodynamic instability (including the use of vasopressors) or by decreased oxygen supply (acute anaemia and hypoxaemia).16 There is reluctance in the use of endoscopy as a diagnostic method in these patients,12,16 as it may cause injury to the newly created anastomosis due to physical trauma caused by the endoscope or air insufflation during examination. Its introduction in our protocol has allowed us to diagnose a Type IV fistula on the fifth postoperative day and treat it immediately.12,23
Moreover, as noted, the associated morbidity and hospital stay in our series are significantly increased in patients with anastomotic leaks. Obviously, in patients with confirmed leaks, there is a greater need to use or extend invasive procedures such as drains, tubes or iv access that result in an increased stay in intensive care and therefore overall hospital stay.17,26 However, as seen in Table 3, patients without leaks are not exempt from serious complications and mortality is mainly due to causes other than the leak, mostly respiratory problems.
The type of anastomosis performed influenced the incidence of leaks. The anastomosis proposed by Collard presented fewer leaks (18.4%) and, conversely, the anastomosis described by Orringer has yielded poor results (44.4%). The cause of this difference is perhaps due to the narrow gastric conduits that are poorly suited to the Orringer type anastomosis,10 and we have abandoned this type of anastomosis.
The incidence of late stenosis in our series was 33.3% in patients with leaks and 8.5% in patients without leaks. This figure is significantly lower than previously described in the literature, where nearly two-thirds of patients with a leak will require one or more pneumatic dilatations, compared to 21% of patients without a leak.3
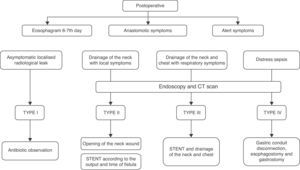
Our current algorithm for the diagnosis and treatment of leaks is shown in Fig. 1. The use of endoprostheses and drainage of collections have a prominent role in the control of both the source of the leak and its consequences. In Type IV leaks, the algorithm suggests immediate takedown when there is systemic repercussion and ischaemia. We believe that their consistent application, in particular with the use of endoscopy for early diagnosis, although the treatment performed was not substantially modified, most likely would have reduced delays in the sequence of actions taken.
In conclusion, anastomotic leak has a significant impact on the patient's perioperative course. The review of our patients and experience acquired has allowed us to systematise the diagnostic and treatment processes and develop an algorithm proposal that requires validation in a prospective series.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Larburu Etxaniz S, et al. Fístula cervical postesofagectomía: diagnóstico y tratamiento. Cir Esp. 2013;91:31–7.