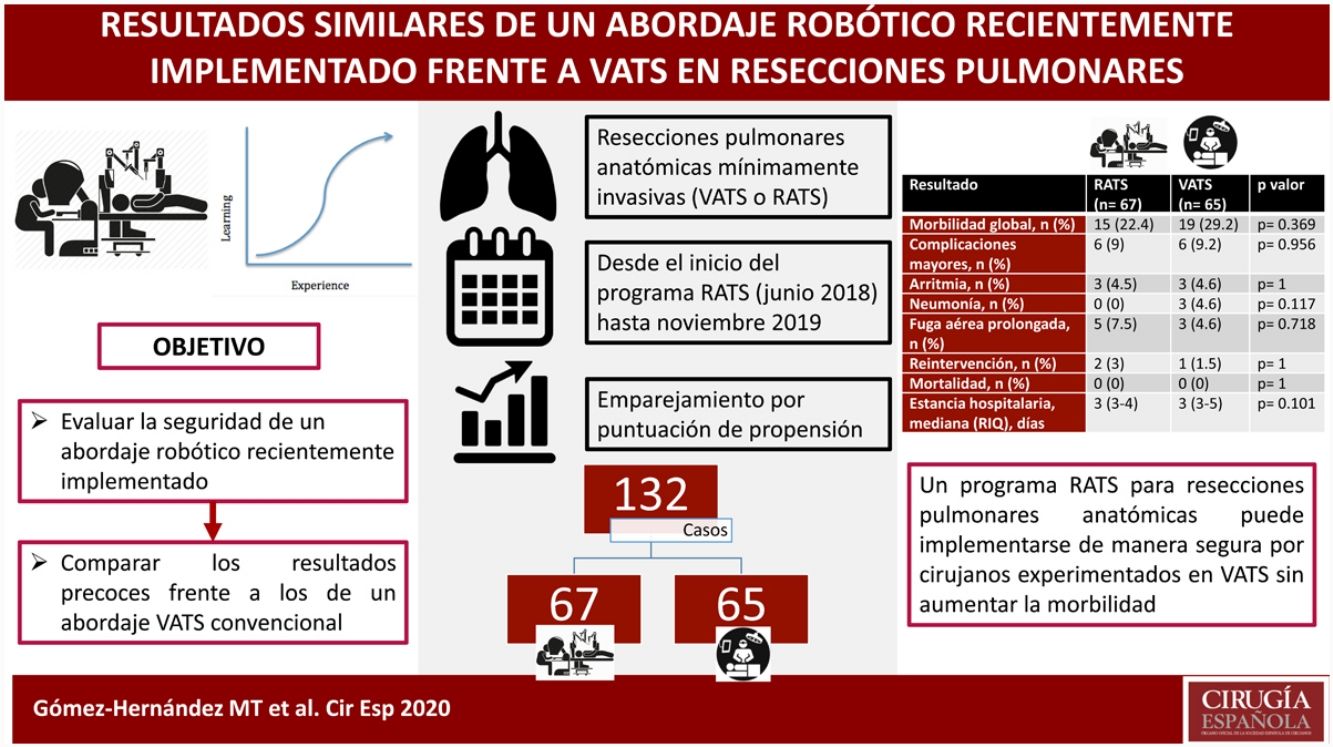
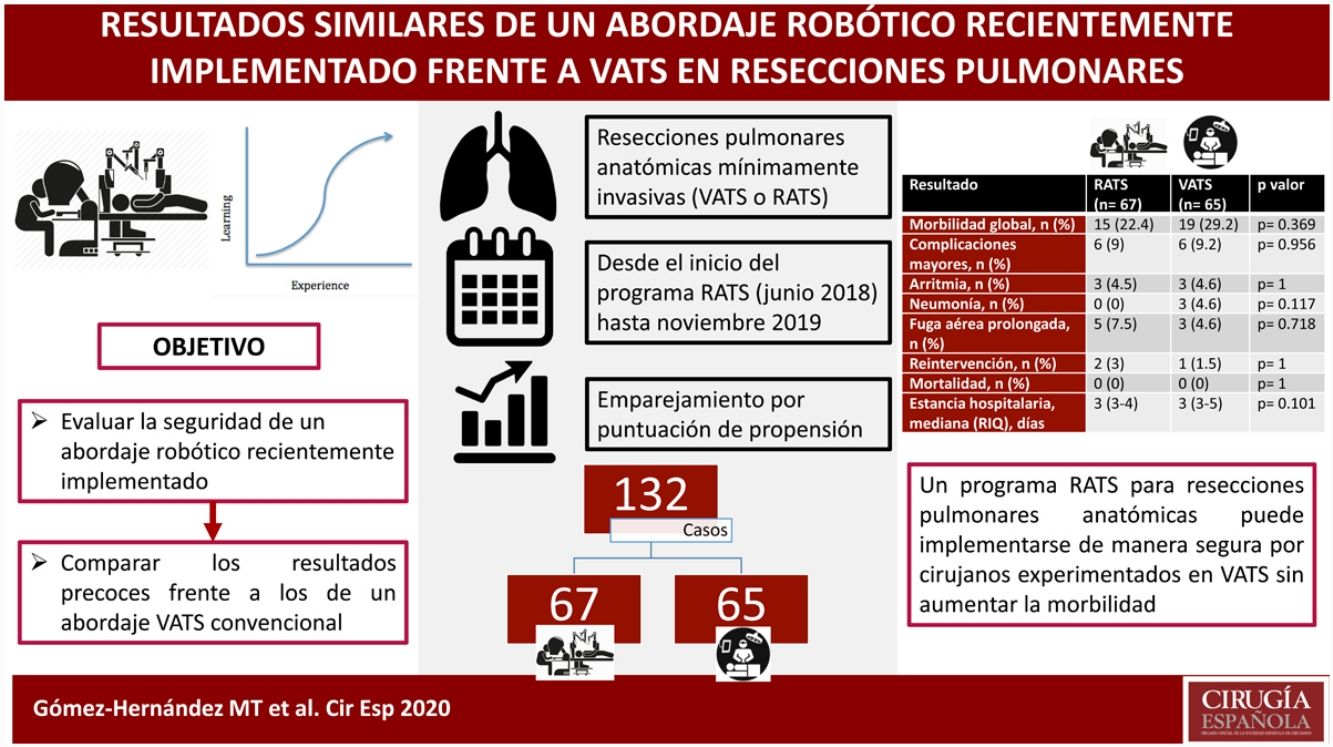
Outcomes after the introduction of surgical innovations can be impaired by learning periods. The aim of this study is to compare the short-term outcomes of a recently implemented RATS approach to a standard VATS program for anatomical lung resections.
MethodsRetrospective review of consecutive patients undergoing pulmonary anatomical resection through a minimally invasive approach since RATS approach was applied in our department (June 01, 2018, to November 30, 2019). Propensity score matching was performed according to patients’ age, gender, ppoFEV1, cardiac comorbidity, type of malignancy, and type of resection. Outcome evaluation includes: overall morbidity, significant complications (cardiac arrhythmia, pneumonia, prolonged air leak, and reoperation), 30-day mortality, and length of hospital stay. Data were compared by two-sided chi-square or Fisher's exact test for categorical and Mann–Whitney U test for continuous variables.
ResultsA total of 273 patients (206 VATS, 67 RATS) were included in the study. After propensity score matching, data of 132 patients were analyzed. The thirty-days mortality was nil. Overall morbidity (RATS: 22.4%, VATS: 29.2%; p=0.369), major complications (RATS: 9% vs VATS: 9.2%; p=0.956) and the rates of specific major complications (cardiac arrhythmia RATS: 4.5%, VATS: 4.6%, p=1; pneumonia RATS:0%, VATS:4.6%, p=0.117; prolonged air leak RATS: 7.5%; VATS: 4.6%, p=0.718) and reoperation (RATS: 3%, VATS: 1.5%, p=1) were comparable between both groups. The median length of stay was 3 days in both groups (p=0.101).
ConclusionsA RATS program for anatomical lung resection can be implemented safely by experienced VATS surgeons without increasing morbidity rates.
La introducción de innovaciones quirúrgicas se asocia con períodos de aprendizaje que pueden afectar a los resultados. El objetivo de este estudio es comparar los resultados postoperatorios de un abordaje RATS para resecciones pulmonares anatómicas implementado recientemente frente a los de un abordaje VATS convencional.
MétodosRevisión retrospectiva de los pacientes sometidos a resección pulmonar anatómica mediante un abordaje mínimamente invasivo en nuestro centro desde el inicio del programa de cirugía RATS (junio de 2018) hasta noviembre de 2019. Los pacientes fueron emparejados por puntuación de propensión según variables de riesgo. Los resultados analizados fueron: morbilidad global, complicaciones (mayores, arritmia, neumonía, fuga aérea prolongada y reintervención), mortalidad a los 30 días y estancia hospitalaria. Los datos se compararon mediante la prueba de chi-cuadrado o la exacta de Fisher para variables categóricas y la prueba de U de Mann-Whitney para variables continuas.
ResultadosSe incluyeron en el estudio 273 pacientes (206 VATS, 67 RATS). Tras el emparejamiento, se analizaron los datos de 132 pacientes. La mortalidad a los 30 días fue nula. La morbilidad global (RATS: 22,4%, VATS: 29,2%; p=0,369), complicaciones mayores (RATS: 9%, VATS: 9,2%; p=0,956), arritmia (RATS: 4,5%, VATS: 4,6%, p=1); neumonía (RATS: 0%, VATS: 4,6%, p=0,117); fuga aérea prolongada (RATS: 7,5%; VATS: 4,6%, p=0,718) y reintervención (RATS: 3%, VATS: 1,5%, p=1) fueron comparables entre ambos grupos. La mediana de la estancia hospitalaria fue de 3 días en ambos grupos (p=0,101).
ConclusionesUn programa RATS para resecciones pulmonares anatómicas puede implementarse de manera segura por cirujanos experimentados en VATS sin aumentar los índices de morbilidad.
Video-assisted thoracic surgery (VATS) anatomic lobectomy for lung cancer, initially described in 1992,1,2 is the standard approach for lung resection in many centers due to reduced postoperative pain, faster recovery, fewer complications and better quality of life compared to conventional thoracotomy.3 Robotic-assisted (RATS) anatomical lung resection, reported one decade later,4,5 has started to develop recently.
The acquisition of new techniques, even for established consultants, involves some type of learning curve.4 Studies showed that learning curves generally ‘flatten out’ as experience increases, resulting in fewer complications, shorter operative time, and infrequent conversion to the standard procedure.5 Although some methods have been described to minimize the learning curve, such as training courses, cadaveric resection, and assistance from expert practitioners, ultimately, surgeons must gain proficiency and experience on suitable patients. The belief that learning curves may have a negative impact on patient outcomes,6 and that patients may be at a higher risk during this learning period. Moreover, there is a lack of professional and public tolerance for suboptimal results due to a learning curve7 to the extent that some patients may be reluctant to be operated using recently introduced surgical techniques.
Hence the implementation of a new surgical technique, such as RATS, may be challenging due to concerns about the potential excess morbidity associated with the learning curve. In this sense, although most practicing thoracic surgeons who use a robot platform are proficient thoracoscopic surgeons, the transition toward the open or VATS approach can be daunting.
We hypothesized that a proficient team in VATS surgery could implement a RATS program without the morbidity associated with the learning curve and with equal short-term outcomes from a 10-years VATS program for anatomical lung resection.
MethodsStudy populationWe retrospectively reviewed data of consecutive patients who underwent pulmonary anatomical resection through a minimally invasive approach since RATS approach was applied in our department. Inclusion criteria: patients aged 18 years or older who underwent elective anatomical lung resection (anatomical segmentectomy, lobectomy or bilobectomy or pneumonectomy) by RATS or VATS from June 1, 2018, to November 30, 2019. Emergency procedures and patients who received induction therapies were excluded.
The selection criteria of patients being considered for resectional surgery was based on the physiologic evaluation recommended by evidence-based clinical practice guidelines.8 The preoperative physiologic assessment started with a cardiovascular assessment and spirometry to measure the forced expiratory volume in 1 second (FEV1) and the diffusing capacity for carbon monoxide (DLCO). Predicted postoperative (PPO) lung functions were calculated. If the ppoFEV1% and ppoDLCO% values were both >60%, the patient was considered at low risk of anatomic lung resection, and resectional surgery was indicated. If either ppoFEV1% or ppoDLCO% are within 60% and 30% predicted, a low-technology exercise test was performed for screening. If performance on the low-technology exercise test was satisfactory, patients were regarded as at low risk of anatomic resection, and surgical resection was indicated. A cardiopulmonary exercise test was indicated when the ppoFEV1% or ppoDLCO% (or both) were <30% or when the performance of the low technology exercise test was not satisfactory. Only patients with a peak oxygen consumption (VO2 peak)>10mL/kg/min or 35% predicted were considered for surgery. VO2 peak<10mL/kg/min or 35% predicted indicated a high risk of mortality and long-term disability for major anatomic resection. In these cases, surgical resection was not recommended.
Based on tumor characteristics, minimally invasive approach was recommended for all cases except when an extended resection (associated to chest wall, atrial, cave vein, diaphragm, vertebral resection, Pancoast tumors, sleeve resections, pleuropneumonectomy, or intrapericardial pneumonectomy) was potentially needed. In these cases, posterolateral or muscle-sparing thoracotomy was performed.
Operative techniqueAll VATS procedures were performed by a team of five – board-certified thoracic surgeons who performed at least 30 thoracoscopic lobectomies annually. VATS technique was perfectly standardized, and postoperative outcomes did not differ among the five surgeons. Whereas all RATS anatomical resections were performed by only two members of the same team, both competent in VATS anatomical resections with more than 200 previous cases and who initiated the RATS program in our center.
- a)
VATS approach: all five surgeons performed a three-port approach, the Copenhagen experience. A 4–5cm anterior utility incision is made in the fourth intercostal space, just anterior to the latissimus dorsi muscle. The wound is protected by a plastic soft tissue retractor kept in place by a ring in the chest cavity and one outside the skin. The cavity is evaluated with the camera (10mm, 30° angled video-thoracoscope). A low anterior 1cm camera port is positioned at the level of the top of the diaphragm and anterior to the level of the hilum and the phrenic nerve. The camera is operated by a team member standing at the ‘patient's anterior side, the same side of the surgeon. The final 1.5cm incision is positioned at the same level but more posterior in a straight line down from the scapula and anterior to the latissimus dorsi muscle. The vessels, the fissure, and the bronchus are then divided with appropriate endo-staplers. The specimen is removed using a bag through the utility incision. Finally, one 24F intercostal drain is placed in through the camera incision.
- b)
RATS approach: We used a 4-arm technique for robotic anatomical lung resections. An 8-mm robotic camera trocar is inserted in the eighth intercostal space (ICS) at the middle axillary line. The cavity is evaluated with the 0° angled camera. Two 12-mm robotic trocars are inserted in the eighth ICS at the level of the diaphragm at the anterior axillary line and the scapular line, respectively. An 8-mm robotic trocar is inserted in the eighth ICS at the level of the auscultatory triangle. Finally, a 10-mm auxiliary port is inserted in the ninth ICS just between the camera port and the first or third robotic port establishing a triangle. The position of this port depends on the target lobe that has to be resected between the camera and the anterior port for lower lobes and between the camera and the posterior port for upper lobes. We used CO2 insufflation pressure of 6–10mm Hg. The vessels, the fissure, and the bronchus are divided sequentially, with robotic or manual endo-staplers. Specimen removal is performed using a bag by slightly enlarging the anterior port. At the end, one 24F intercostal drain is placed in through the camera incision.
Perioperative management was uniform for all patients throughout the study period. Antibiotic prophylaxis comprised a single dose of cefazoline 2g repeated after 6h if surgery continued. All surgical approaches were minimally invasive and varied among VATS and RATS according to the robot availability. Systematic nodal dissection was performed according to the guidelines of the European Society of Thoracic Surgeons (ESTS).9 Patients were extubated in the operating room and, after 6h in the recovery room, transferred to the thoracic ward. At the beginning of the procedure, the surgeons inserted through a paravertebral catheter postoperative analgesia infused with bupivacaine and fentanyl infusion until drain was removed or a maximum of three days postoperatively and with oral paracetamol and non-steroid anti-inflammatory drugs thereafter. Nursing care was homogeneous in all cases and included incentive spirometry and early mobilization. All of the patients participated in our specific pre- and postoperative chest physiotherapy intensive program.10
Statistical analysisAnalyzed data included baseline demographic and characteristics of patients and postoperative outcomes (operative morbidity, major complications, 30-day mortality, and length of hospital stay). Operative morbidity was defined, and any postoperative complication occurring during hospitalization or within the first 30 days after the intervention was included: respiratory failure (the need for mechanical ventilation for more than 24h or the need for reintubation at any time), acute respiratory distress syndrome, atrial arrhythmia, ventricular arrhythmia, atelectasis requiring bronchoscopy, pneumonia, pulmonary thromboembolism, acute myocardial infarction, renal failure, stroke, prolonged air leak (defined as an air leakage into the pleural drainage lasting more than 5 days after surgery), hemothorax, pneumothorax with or without air leak requiring drainage, bronchial fistula, wound dehiscence, wound hematoma, empyema, chylothorax, recurrent nerve paralysis, and phrenic nerve paralysis. These complications were already defined according to the joint report of variable definitions agreed by the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons.11 All complications were re-classified according to the Dindo et al. systematic classification of postoperative morbidity12 as minor (I–II) and major (IIIa–V). 30-Day mortality was defined as any postoperative death occurring during hospitalization or within the first 30 days after surgery.
As specific postoperative adverse events we evaluated: arrhythmia, pneumonia, prolonged air leak and reoperation.
Propensity-score matching was performed by using a multivariable logistic model to estimate the probability of patients who underwent RATS or VATS procedures. The following covariates were used for matching: age, gender, predicted postoperative forced expiratory volume in one second (ppoFEV1), cardiac comorbidity (including coronary artery disease, any previous cardiac surgery, current treatment for arrythmia or current treatment for cardiac failure), type of malignancy, and type of resection.
We measured discrete variables as proportions and percentages and compared them by using the chi-square test or Fisher's exact test when expected frequencies were below 5. We also compared continuous variables by using the Mann–Whitney U test. All tests were two-sided, with statistical significance set at a p-value of less than 0.05. These criteria were pre-specified before starting the analysis. Statistical analyses were performed using SPSS software, version 26 (IBM Corp, Chicago, Illinois, 2019).
ResultsA total of 273 patients received an elective anatomical lung resection through a minimally invasive approach during the study period: 206 by VATS and 67 RATS. No pneumonectomies or bilobectomies with a minimally invasive approach were performed during the study period. Three robotic anatomical resection cases (4.5%) were converted to VATS due to bronchial injury, arterial bleeding and parenchymatous air leak. While fifteen VATS anatomical resections (7.3%) were converted to an open approach due to arterial injury or risk of bleeding (4 cases), oncological reasons (5 cases), incomplete pulmonary collapse (3 cases) and adhesions (3 cases).
Patient characteristics before matching are summarized in Table 1. After propensity score matching, data of 132 patients were analyzed. Patient populations were similar after statistical matching (Table 2).
Patients demographics and baseline characteristics before propensity-score matching.
| Characteristics | RATS (n=67) | VATS (n=206) | p-value |
|---|---|---|---|
| Age, mean±SD, years | 61.54±10.18 | 67.57±9.17 | p=0.000 |
| Male sex, n (%) | 30 (44.8) | 159 (77.2) | p=0.000 |
| ppoFEV1%, mean±SD, % | 84±22.97 | 77.5±19.2 | p=0.026 |
| BMI, mean±SD | 26.15±4.85 | 26.72±4.29 | p=0.362 |
| Cardiac comorbidity, n (%) | 9 (13.4) | 39 (18.9) | p=0.304 |
| CKD*, n (%) | 1 (1.5) | 8 (3.9) | p=0.461 |
| Tumoral size, n (%) | |||
| <4cm | 61 (91) | 181 (87.9) | p=0.476 |
| Type of malignancy, n (%) | p=0.988 | ||
| Primary neoplasm of the lung | 48 (71.6) | 147 (71.4) | |
| Metastases other than lung | 8 (11.9) | 26 (12.6) | |
| No lung cancer | 11 (16.4) | 33 (16) | |
| Pathological stage N1–N2, n (%) | 7 (10.4) | 25 (12.1) | 0.709 |
| Type of resection, n (%) | p=0.061 | ||
| Lobectomy | 49 (73.1) | 172 (83.5) | |
| - RUL | 13 (19.4) | 81 (39.3) | |
| - ML | 9 (13.4) | 7 (3.4) | |
| - RLL | 11 (16.4) | 25 (12.1) | |
| - LUL | 5 (7.5) | 35 (17) | |
| - LLL | 11 (16.4) | 24 (11.7) | |
| Segmentectomy | 18 (26.9) | 34 (16.5) | |
| - Culmen | 1 (1.5) | 11 (5.3) | |
| - Lingula | 1 (1.5) | 3 (1.5) | |
| - S6 | 10 (14.9) | 13 (6.3) | |
| - Basal pyramid | 6 (9) | 7 (3.4) | |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; SD: standard deviation; ppoFEV1: predicted postoperative forced expiratory volume in one second; BMI: body mass index; CKD: chronic kidney disease; RUL: right upper lobectomy; ML: middle lobectomy, RLL: right lower lobectomy; LUL: left upper lobectomy; LLL: left lower lobectomy.
CKD*: defined as glomerular filtration rate (GFR) <60ml/min/1.73m2 for 3 months.
Patients demographics and baseline characteristics after propensity-score matching.
| Characteristics | RATS (n=67) | VATS (n=65) | p value |
|---|---|---|---|
| Age, mean±SD, years | 61.54±10.18 | 64.71±10.47 | p=0.080 |
| Male sex, n (%) | 30 (44.8) | 33 (50.8) | p=0.491 |
| ppoFEV1%, mean±SD, % | 84±22.97 | 82.6±18.55 | p=0.703 |
| BMI, mean±SD, kg/m2 | 26.15±4.85 | 26.72±4.41 | p=0.549 |
| Cardiac comorbidity, n (%) | 9 (13.4) | 10 (15.4) | p=0.749 |
| CKD*, n (%) | 1 (1.5) | 3 (4.6) | p=0.362 |
| Tumoral size, n (%) | |||
| <4 cm | 61 (91) | 58 (89.2) | p=0.727 |
| Type of malignancy, n (%) | p=0.717 | ||
| Primary neoplasm of the lung | 48 (71.6) | 44 (67.7) | |
| Metastases other than lung | 8 (11.9) | 11 (16.9) | |
| No lung cancer | 11 (16.4) | 10 (15.4) | |
| Pathological stage N1–N2, n (%) | 7 (10.4) | 8 (12.3) | 0.736 |
| Type of resection, n (%) | p=0.475 | ||
| Lobectomy | 49 (73.1) | 51 (78.5) | |
| - RUL | 13 (19.4) | 10 (15.4) | |
| - ML | 9 (13.4) | 1 (1.5) | |
| - RLL | 11 (16.4) | 4 (6.2) | |
| - LUL | 5 (7.5) | 21 (32.3) | |
| - LLL | 11 (16.4) | 15 (23.1) | |
| Segmentectomy | 18 (26.9) | 14 (21.5) | |
| - Culmen | 1 (1.5) | 5 (7.7) | |
| - Lingula | 1 (1.5) | 2 (3.1) | |
| - S6 | 10 (14.9) | 4 (6.2) | |
| - Basal pyramid | 6 (9) | 3 (4.6) | |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; SD: standard deviation; ppoFEV1: predicted postoperative forced expiratory volume in one second. BMI: body mass index; CKD: chronic kidney disease; RUL: right upper lobectomy; ML: middle lobectomy, RLL: right lower lobectomy; LUL: left upper lobectomy; LLL: left lower lobectomy.
CKD*: defined as glomerular filtration rate (GFR) <60ml/min/1.73m2 for 3 months.
Clinical outcomes and adverse events of the unmatched and the propensity-matched cohorts for this study are summarized in Tables 3 and 4 and Tables 5 and 6, respectively. No perioperative death was observed in the series. Overall, no difference was observed in clinical outcomes and postoperative complications between both cohorts. However, a trend toward lower operative morbidity and pneumonia was found in the RATS cohort compared to the VATS cohort.
Summary of clinical outcomes: unmatched analysis.
| Clinical outcome | RATS (n=67) | VATS (n=207) | p value |
|---|---|---|---|
| Operative morbidity, n (%) | 15 (22.4) | 57 (27.7) | p=0.394 |
| Major complications, n (%) | 6 (9) | 18 (8.7) | p=0.956 |
| 30-Day mortality, n (%) | 0 (0) | 0 (0) | p=1 |
| Length of hospital stay, median (IQR), days | 3 (3–4) | 3 (3–5) | p=0.027 |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; IQR: interquartile range.
Adverse events: unmatched analysis.
| Adverse event | RATS (n=67) | VATS (n=207) | p value |
|---|---|---|---|
| Arrythmia, n (%) | 3 (4.5) | 7 (3.4) | p=0.711 |
| Pneumonia, n (%) | 0 (0) | 5 (2.4) | p=0.339 |
| Prolonged air leak, n (%) | 5 (7.5) | 18 (8.7) | p=0.744 |
| Reoperation, n (%) | 2 (3) | 7 (3.4) | p=1 |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
Overview of clinical outcomes: matched analysis.
| Clinical outcome | RATS (n=67) | VATS (n=65) | p value |
|---|---|---|---|
| Operative morbidity, n (%) | 15 (22.4) | 19 (29.2) | p=0.369 |
| Major complications, n (%) | 6 (9) | 6 (9.2) | p=0.956 |
| 30-Day mortality, n (%) | 0 (0) | 0 (0) | p=1 |
| Length of hospital stay, median (IQR), days | 3 (3–4) | 3 (3–5) | p=0.101 |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; IQR: interquartile range.
Adverse events: matched analysis.
| Adverse event | RATS (n=67) | VATS (n=65) | p value |
|---|---|---|---|
| Arrhythmia, n (%) | 3 (4.5) | 3 (4.6) | p=1 |
| Pneumonia, n (%) | 0 (0) | 3 (4.6) | p=0.117 |
| Prolonged air leak, n (%) | 5 (7.5) | 3 (4.6) | p=0.718 |
| Reoperation, n (%) | 2 (3) | 1 (1.5) | p=1 |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
Surgical innovation seeks to reduce aggressiveness and improve postoperative outcomes. After implementing new procedures, surgeons, anesthetists, and nursing teams must integrate new techniques into their practice, and this can have an impact on outcomes, as has been studied in diverse surgical specialties.6,13–17 Thus, the effect of the application of new techniques on the outcomes must be measured and reported.7
This study aimed to evaluate the safety of a recently implemented RATS approach by comparing short-term outcomes to those of a 10-years VATS program for anatomical lung resections. Propensity-score matching and focusing only on minimally invasive techniques provided a reasonable basis for comparing two similar approaches.
Our results show that the implementation of a RATS anatomical resection can be accomplished safely with similar or improved postoperative results compared to the running VATS program. Therefore, the robotic-assisted technique is a similar, alternative minimally invasive approach for pulmonary resection to offer patients without compromising safety.
Several studies have compared robotic-assisted lung resections to open approaches21–23 or VATS.13–16,18-20 Robotic-assisted thoracic surgery (RATS) has been associated with shorter lengths of stay and lower rates of mortality and morbidity compared with thoracotomy. Whereas, comparative studies of RATS and VATS approaches have provided mixed results. The differences in the clinical outcomes between both approaches are inconsistent, and there is no proven benefit of one technique over the other. However, none of the studies have independently considered the period needed to acquire the proficiency to perform robotics efficiently.
A large study from The Society of Thoracic Surgeons database found that robotic-assisted lobectomy operative times were longer, but the complication rate, hospital length of stay, lymph node upstaging, and 30-day mortality were equivalent with thoracoscopic lobectomy.16 Swanson and colleagues14 reviewed minimally invasive lobectomy procedures from the Premier database from 2009 to 2011 and found that RATS procedures cost more, trended slightly toward longer operating room times, with similar morbidity outcomes. However, a more recent analysis showed that robotic lobectomy had fewer complications and shorter length of stay than VATS procedures.20 Moreover, when surgical outcomes are limited to surgeons performing 20 or more procedures yearly, the robotic-assisted approach is associated with a lower conversion-to-open rate and lower 30-day complication rate than VATS, with a mean operative time difference of 25min.15
A similar study was carried out by Lee et al.,13 who analyzed perioperative outcomes of 69 patients who underwent minimally invasive lobectomy (35 robotic-assisted and 34 VATS). All procedures were performed by a single surgeon proficient in VATS lobectomy, initiating a RATS lobectomy program. The researchers showed longer operating room time and equivalent mortality and complication rates. In our study, we have not measured operating time as a limitation, as we highlight below, but outcomes are quite the same after VATS and RATS lung resection, and RATS does not compromise patients’ safety at all.
Similarly, Louie et al.17 found that early experience with robotic lobectomy and segmentectomy resulted in similar outcomes compared with VATS cases. Although they included a small number of cases (52 robotic procedures and 35 VATS procedures) performed by four surgeons who vary widely in their level of training and experience in VATS and laparoscopy, the results could be influenced by the difference in the degree of surgeons’ expertise and skills.
One might think that patient selection criteria for a robotic-assisted anatomical lung resection could be more restrictive than for VATS regarding tumors during the implementation period. However, as our analysis was limited only to minimally invasive approaches, we consider that we eliminated the bias of patient selection based on tumor staging and that patient selection criterion was similar for both case series. On the other hand, although initial reports showed that the learning curve of robotic anatomical lung resection was in the range of 14–40 cases,21–23 we considered a more extended RATS implementation period and included a larger number of patients in the study (a total of 67 cases performed by two surgeons: 49 robotic-assisted lobectomies and 18 robotic-assisted segmentectomies). The studies mentioned above are restricted to lobectomy, whereas Zhang et al.24 recently showed that technical competency for robotic-assisted segmentectomy is achieved after more than 40 procedures.
Operating time and conversion rates were not included among the aims of this study. Although it could be an essential factor to consider in the learning curve analysis, surgical outcomes -morbidity and mortality- regarding the safety of the implemented program are the most relevant aspects that should be communicated.
In summary, the implementation. of a RATS program for anatomical lung resection is safe since postoperative outcomes, including postoperative morbidity, 30-day mortality, and length of stay, are equivalent to those of a well-established VATS program.
Funding sourceThis research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflict of interestThe authors declare that they have no conflict of interest directly or indirectly related to the contents of the manuscript.