The treatment of rectal cancer via laparoscopy is controversial due to its technical complexity. Several randomized prospective studies have demonstrated clear advantages for the patient with similar oncological results to those of open surgery, although during the learning of this surgical technique there may be an increase in complications and a worse prognosis.
ObjectiveOur aim is to analyze how the learning curve for rectal cancer via laparoscopy influences intra- and postoperative results and oncological markers. A retrospective review was conducted of the first 120 patients undergoing laparoscopic surgery for rectal neoplasia. The operations were performed by the same surgical team with a wide experience in the treatment of open colorectal cancer and qualified to perform advanced laparoscopic surgery. We analyzed sex, ASA, tumor location, neoadjuvant treatment, surgical technique, operating time, conversion, postoperative complications, length of hospital stay, number of lymph nodes, stage and involvement of margins.
ResultsSignificant differences were observed with regard to surgical time (224min in the first group, 204min in the second group), with a higher rate of conversion in the first group (22.5%) than in the second (11.3%). No significant differences were noted for rate of conservative sphincter surgery, length of hospital stay, post-surgical complications, number of affected/isolated lymph nodes or affected circumferential and distal margins.
ConclusionsIt is possible to learn this complex surgical technique without compromising the patient's safety and oncological outcome.
El tratamiento del cáncer de recto por laparoscopia es controvertido por su complejidad técnica. Estudios prospectivos aleatorizados han demostrado claras ventajas para el paciente, con resultados oncológicos equiparables a la cirugía abierta, aunque durante el aprendizaje de esta cirugía puede existir un aumento de las complicaciones y peor pronóstico.
ObjetivoNuestro objetivo es analizar cómo influye la curva de aprendizaje del cáncer de recto por vía laparoscópica en los resultados intra y postoperatorios, así como en los marcadores oncológicos.
Pacientes y métodosSe realizó una revisión retrospectiva de los 120 primeros pacientes intervenidos de neoplasia de recto por vía laparoscópica. La población a estudio se ordenó cronológicamente por fecha de intervención y se dividió en un primer grupo que contenía las 40 primeras intervenciones, y un segundo grupo que contenía las 80 siguientes. Las intervenciones fueron realizadas por el mismo equipo quirúrgico con una amplia experiencia en el tratamiento del cáncer colorrectal abierto, además de estar capacitados para realizar cirugía laparoscópica avanzada. Se analizaron sexo, ASA, localización del tumor, neoadyuvancia, técnica quirúrgica, tiempo operatorio, conversión, complicaciones postoperatorias, estancia hospitalaria, número de ganglios, estadio y afectación de márgenes.
ResultadosSe observaron diferencias significativas en cuanto a tiempo quirúrgico (224min en el primer grupo, 204min en el segundo grupo), con una mayor tasa de conversión en el primer grupo (22,5%) frente al segundo (11,3%). No se apreciaron diferencias significativas en cuanto a la tasa de cirugía conservadora de esfínteres, estancia hospitalaria, complicaciones posquirúrgicas, número de ganglios afectos/aislados ni márgenes circunferencial y distal afectos.
ConclusiónEs posible realizar el aprendizaje de esta compleja cirugía sin comprometer la seguridad y resultado oncológico del paciente.
Several studies have demonstrated that laparoscopic treatment of colon cancer has oncologic results similar to open surgery, with no increased morbidity or mortality, and offers patients all the advantages of laparoscopic surgery.1–4
Laparoscopic surgery in rectal cancer, however, is more controversial because of its technical complexities due to the anatomical location, need for total mesorectal excision (TME) with adequate margins, continuity with the sphincters and its vicinity to the hypogastric plexus. Nevertheless, there are more and more studies demonstrating that laparoscopic surgery in rectal cancer has oncologic and functional results similar to open surgery.5–9
The learning curve of this procedure is technically more complex than colonic surgery, and the acquisition of advanced laparoscopic surgery skills is still one of the obstacles for the generalized application of colorectal laparoscopic surgery. This surgery requires the identification of tissue planes without injuring the neighboring structures, such as the prostate, vagina and hypogastric plexus, in addition to performing, in most occasions, a colorectal anastomoses, which can be sometimes very complex. An initial training period is necessary, and continuous repetition of the process provides surgeons with the experience necessary to safely perform these complex procedures, without increasing morbidity or mortality or compromising long-term oncologic results. Higher rates of positive circumferential resection margins10 and anastomotic leaks11 have been described when laparoscopic surgery is used for rectal resection.
In this study, we present the short-term results from the learning process of laparoscopic rectal cancer resection and the effects of the surgeons’ learning curves on patient results.
Patients and MethodsWe retrospectively reviewed the first 120 patients who had been treated at our hospital for rectal adenocarcinoma using the laparoscopic approach. The study population was organized chronologically according to the date of surgery, and it was divided into a first group of the initial 40 interventions and a second group that contained the following 80. At the start of the series, we excluded those patients with a body mass index (BMI) higher than 35 and patients with cancer in the lower third of the rectum; as the surgeons gained experience, these patients were later included. All patients were studied with physical examination, rectal exam, total colonoscopy with biopsy, rigid rectoscopy, anorectal ultrasound, thoracic and abdominal computed tomography, nuclear magnetic resonance imaging of the rectum and barium enema in patients without complete colonoscopy. Before surgery, the anesthetist assessed all the patients and determined the American Society of Anesthesiologists (ASA) score.
Patients with adenocarcinoma in stage ii or iii (The International Union Against Cancer/American Joint Committee on Cancer [UICC/AJCC] colorectal cancer staging system) received neoadjuvant treatment with chemoradiotherapy as follows: 3-field pelvic radiotherapy (RT) with 50–54Gy, 5 days per week, 1.8Gy/day, together with oral capecitabine at a dose of 1000–1500mg/m2/day during the whole RT period.
The surgery was performed between 6 and 8 weeks after the end of neoadjuvant treatment. Patients with resectable distant metastasis were treated metachronously after having recovered from the rectal surgery. Patients in stage iii and iv had adjuvant chemotherapy administered.
The interventions were performed by the same surgical team (L.J., H.Q.) with ample experience in open colorectal cancer treatment, in addition to being skilled in advanced laparoscopic surgery. In all cases, antegrade colonic prep was done with polyethylene glycol. Patients were given antithrombotic prophylaxis with low-molecular-weight heparin and antibiotic prophylaxis with amoxicillin–clavulanate or metronidazole plus gentamycin in those with β-lactam allergy.
All patients underwent TME with hypogastric nerve preservation. In cases with tumor infiltration of the levator ani or where it was not possible to obtain a distal margin greater than 1cm, abdominoperineal resection (APR) was carried out. When anterior resection was performed, the anastomoses were mechanical end-to-side colorectal or manual coloanal. A protective ileostomy was constructed with the anterior resections according to the criteria of the surgeon, mainly in cases with neoadjuvant treatment, when the anastomosis was laborious and in all the coloanal anastomoses.
Surgical TechniquePneumoperitoneum was created with a pressure of 12–15mmHg, and the following working ports were put in place: one 11mm supraumbilical, 2 in the right iliac fossa with diameters of 5 and 12mm and another 5mm port in the left iliac fossa. When necessary, a fifth 10mm port was placed in the suprapubic region to provide separation in the area of the recto-uterine recess, and occasionally an accessory port was used in the upper right abdomen to mobilize the splenic flexure. In patients with anterior resection and mechanical anastomosis, a mini-laparotomy was done, usually horizontally, for the extraction of the surgical specimen and the placement of the anvil of the stapler; anastomoses were done intracorporeally. In abdominoperineal resection and in the anterior resections with manual coloanal anastomosis, the specimen was extracted through the perineum, requiring no abdominal incision. Conversion to laparotomy was done when it was not possible to complete the total removal of the mesorectum by laparoscopy due to technical problems or incidents during dissection.
The treatment of the perineal wound in all patients who had had an APR included postoperative drain-lavage.12
All the specimens were analyzed by the same expert pathologist, who evaluated the involvement of the circumferential margin (distance≤1mm from the tumor to the mesorectal fascia), involvement of the distal margin (tumor that reaches the distal section) and the number of isolated lymph nodes.
Anastomotic leak symptoms included the detection of dehiscence during digital rectal examination or endoscopy and clinical signs of peritonitis, gas or fecal matter leak through the drain, or pelvic abscess.
Postoperative complications were defined as those that occurred during hospitalization or led to readmission within the first 30 days post-op.
We analyzed sex, age, BMI, ASA score, tumor location, surgical technique, operative time, conversion rate, postoperative complications, hospital stay, the number of lymph nodes and affected margins.
Statistical AnalysisThe data were processed with the SPSS 13.0 statistical package for Windows. The comparisons of 2 means were done with the Student's t test combined with the Behrens–Fisher test, depending on whether there was homogeneity of variance between the 2 samples, or with the non-parametric Mann–Whitney test when the data had a clear non-normal distribution even after the logarithmic transformation (for example, days of hospital stay).
For the study of the relationship between qualitative variables and the comparison of proportions in independent samples, we used an analysis of contingency tables with Pearson's Chi-squared test and the subsequent residual analysis, or with Fisher's exact test.
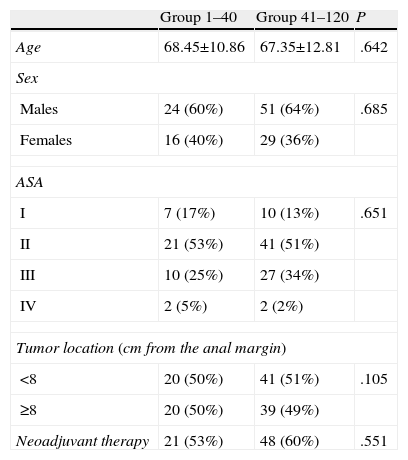
ResultsThe two groups were homogeneous, with no significant differences observed (Table 1).
Characteristics of the 2 Patient Groups.
| Group 1–40 | Group 41–120 | P | |
| Age | 68.45±10.86 | 67.35±12.81 | .642 |
| Sex | |||
| Males | 24 (60%) | 51 (64%) | .685 |
| Females | 16 (40%) | 29 (36%) | |
| ASA | |||
| I | 7 (17%) | 10 (13%) | .651 |
| II | 21 (53%) | 41 (51%) | |
| III | 10 (25%) | 27 (34%) | |
| IV | 2 (5%) | 2 (2%) | |
| Tumor location (cm from the anal margin) | |||
| <8 | 20 (50%) | 41 (51%) | .105 |
| ≥8 | 20 (50%) | 39 (49%) | |
| Neoadjuvant therapy | 21 (53%) | 48 (60%) | .551 |
ASA, American Society of Anesthesiologists.
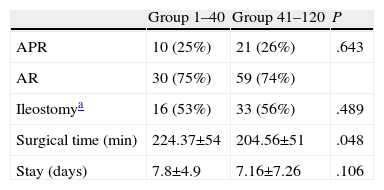
The overall rate of sphincter-conserving surgery was 74%, with no differences between the 2 groups. Protective ileostomy was done in 48% of the patients with sphincter-conserving surgery, again with no differences between the groups. Mean operative time was 224.37min in the 1–40 group and 204.56min in the 41–120 group, with significant differences. The hospital stay was similar in both groups (Table 2).
Results in the 2 Patient Groups.
| Group 1–40 | Group 41–120 | P | |
| APR | 10 (25%) | 21 (26%) | .643 |
| AR | 30 (75%) | 59 (74%) | |
| Ileostomya | 16 (53%) | 33 (56%) | .489 |
| Surgical time (min) | 224.37±54 | 204.56±51 | .048 |
| Stay (days) | 7.8±4.9 | 7.16±7.26 | .106 |
APA, abdominoperineal resection; AR, anterior resection.
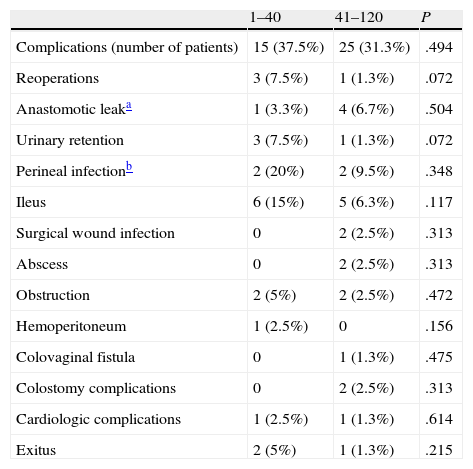
In the 1–40 group, 9 patients (22.5%) had conversion to open surgery, and in the 41–120 group another 9 patients (11.3%) also had conversion. These differences are not significant probably due to a type iistatistical error. There were complications in 15 patients (37.5%) in the 1–40 group and in 25 patients (31.3%) in the 41–120 group, with no differences between groups. Nor were there any differences when we analyzed the complications one by one. There were 2 patient deaths (5%) in the 1–40 group and one (1.3%) in the 41–120 group, with no significant differences (Table 3). The 3 deaths that occurred were due to causes other than surgery: two due to cardiologic problems and one due to respiratory problems.
Postoperative Complications and Distribution.
| 1–40 | 41–120 | P | |
| Complications (number of patients) | 15 (37.5%) | 25 (31.3%) | .494 |
| Reoperations | 3 (7.5%) | 1 (1.3%) | .072 |
| Anastomotic leaka | 1 (3.3%) | 4 (6.7%) | .504 |
| Urinary retention | 3 (7.5%) | 1 (1.3%) | .072 |
| Perineal infectionb | 2 (20%) | 2 (9.5%) | .348 |
| Ileus | 6 (15%) | 5 (6.3%) | .117 |
| Surgical wound infection | 0 | 2 (2.5%) | .313 |
| Abscess | 0 | 2 (2.5%) | .313 |
| Obstruction | 2 (5%) | 2 (2.5%) | .472 |
| Hemoperitoneum | 1 (2.5%) | 0 | .156 |
| Colovaginal fistula | 0 | 1 (1.3%) | .475 |
| Colostomy complications | 0 | 2 (2.5%) | .313 |
| Cardiologic complications | 1 (2.5%) | 1 (1.3%) | .614 |
| Exitus | 2 (5%) | 1 (1.3%) | .215 |
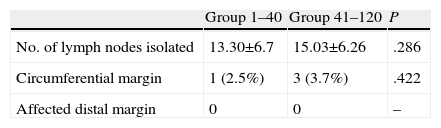
The pathology study of the specimen was similar in the number of isolated lymph nodes and in circumferential and radial margin involvement, with no significant differences (Table 4).
DiscussionColorectal laparoscopic surgery requires a high degree of skill and technical abilities. This is especially true in rectal cancer, since it is technically very demanding. Its complexity involves performing total mesorectal excision, mechanical distal dissection of the lower rectum, coloanal anastomosis or abdominoperineal resection, all while identifying and preserving the autonomic nerves and complying with the oncological principles of adequate margins (circumferential and distal) and proximal ligation of the artery feeding the tumor. This makes it essential for most surgeons to have an initial training period, with continuous repetition of the procedure in order to be technically competent in laparoscopic surgery for rectal cancer. Acquiring the skills necessary to perform this advanced laparoscopic surgery, without compromising patient safety, is one of the most important obstacles of its generalization.
In our unit, we have followed a protocol for learning laparoscopic rectal cancer surgery. The surgeons who perform this surgery should have ample experience in open surgery for colorectal cancer treatment in addition to being skilled in advanced laparoscopic surgery. Other studies have demonstrated that rectal surgery can be learned without having had experience in open colon surgery.13 We believe that it is currently difficult to develop these training programs in a generalized manner since neither laparoscopic colon surgery nor especially laparoscopic rectal surgery is widely used. The surgeons who should be teaching the technique is either still in or have not even begun the training period themselves. Prior to this surgery, we had initiated right and left colon resections with the laparoscopic approach. During the learning period, only 2 surgeons carried out the rectal cancer surgical procedures (L.J. and H.Q.) because, as it is a complex and technically-demanding procedure that requires a high degree of skill and technical abilities, the surgeons work closely together during the surgery and have the opportunity to share their experiences and learn from one another.
In our protocol, another important factor has been patient selection. In order to obtain good results in learning colorectal surgery, it is essential to select the patients at the start of the learning period, as several authors suggest,14 excluding complex and high-risk cases. When first learning, we should avoid obese patients and those with large tumors, which are factors that will influence the complexity of the surgery and consequently the conversion to open surgery.15,16 With greater experience, the indications for laparoscopic rectal cancer surgery can increase. At the start of our series, we were very selective when choosing patients, and progressively the criteria became less strict, with no increase in complications and fewer conversions.
The incidence of conversion to open surgery varies enormously, ranging between 5% and 20% according to the series published.17–20 Conversion itself is not a complication, although it has been related with a greater number of complications.21,22 This increase in conversion to open surgery may be due to greater complexity of the patients who require conversion, and this does not compromise the oncologic results of the surgery.22 During the learning curve, conversion is related with patient selection as well as the abilities and skills of the surgeon. It cannot be considered a failure of the laparoscopic procedure, but it is instead more of an appeal to the common sense of the surgeon. In fact, the higher the threshold is for conversion, the greater the number of complications. Thus, Bege et al.23 observed no significant variations in the incidence of conversion (16% during learning and 14% once experienced), although the number of complications was 52% in the learning period and 32% once this period had finished, even though the complexity of the cases had increased. In our series, at the beginning the threshold for conversion was very low. When some type of complication arose that could not be easily resolved by laparoscopy, however small it was, or when there were difficulties to advance in the mesorectal excision surgery or the mobilization of the splenic flexure, or it was impossible to insert the endocutter in order to dissect the distal margin or any other incidence that could compromise the safety of the surgery as well as the patient, the procedure was converted to open surgery.
In most of the series, the operative time in laparoscopic surgery is longer than in open surgery, which is a parameter that works against laparoscopic surgery. Many studies define learning colorectal laparoscopic surgery with the operative time, and using this single indicator to judge surgical competence is perhaps not correct.24 When a surgeon starts learning this laparoscopic procedure or any other surgery, he/she should not keep an eye on the clock. The procedure should be free of any pressures from the surgical schedule, anesthesia department or surgical personnel. What is most important is that the procedure is done correctly and that it progresses slowly but carefully. As the experience of the surgical team increases, the surgical time will shorten, with the same patient safety.
In our study, the postoperative rate of complications has been stable, within the limits published in the literature and with no significant differences between the two groups. This has probably been due to the experience in colorectal surgery and advanced laparoscopic surgery of the surgeons as well as the patient selection at the beginning of the series. With growing experience and an increased number of indications, there may be an increase in complications, as has been seen in other series.25 Thus, Ito et al.26 found higher rates of anastomotic leaks and reoperations in the phase where the surgeons had more experience than in the initial phase due to the fact that they included more patients with cancer of the middle and lower thirds of the rectum in the final learning phase.
Some publications have demonstrated that survival is not only linked to lymph node metastasis, but also to the number of isolated lymph nodes.27 Thus, it has been suggested that a minimum number of lymph nodes should be examined to provide the proper cancer stage and to be able to apply the correct adjuvant treatment.28 In some cases, local relapse was greater during the learning period,25 which may be due to lack of skill in the manipulation of the mesorectum with the forceps. This can cause rupture of the mesorectal fascia and perforation of the rectum, with higher rates of local recurrence. There have been no statistically significant differences in the two groups for survival, lymph nodes and number of recurrences, probably due to the previous experience in total mesorectal excision by open surgery. Furthermore, for us, laparoscopic surgery is a means, not an end, and we use a low threshold for conversion to open surgery.
ConclusionIn order to correctly learn laparoscopic surgery for the treatment of rectal cancer, it is necessary to have had adequate training in open mesorectal excision and advanced laparoscopic surgery. When first learning the procedure, proper patient selection is essential and there should be a low threshold for conversion to open surgery. In this manner, it is possible to learn this complex surgical technique without compromising the safety and oncologic results of the patient.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: Luján J, Gonzalez A, Abrisqueta J, Hernandez Q, Valero G, Abellán I, et al. Aprendizaje de la cirugía del cáncer de recto por laparoscopia sin aumento de la morbimortalidad. Cir Esp. 2014;92:485–490.