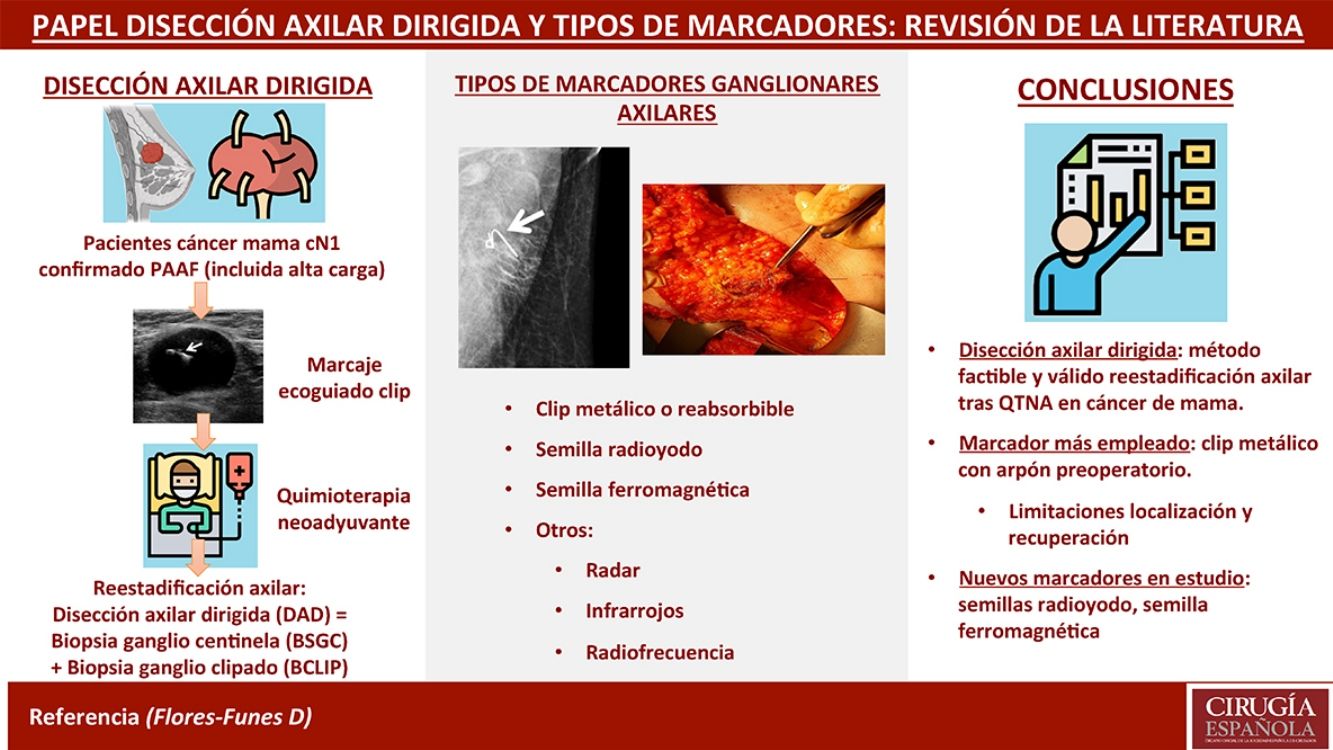
Targeted axillary dissection (TAD) consists of a new axillary staging technique that combines sentinel lymph node biopsy (SLNB) and clipped lymph node biopsy (CLNB) in the same surgery, in order to re-stage patients with breast cancer and positive axillary lymph nodes undergoing neoadjuvant chemotherapy (NACT). Prior to the NACT, the affected lymph node is punctured and a solid marker is left inside echo-guided, in order to biopsy it in the subsequent surgery. There are numerous types of markers: metallic (steel, titanium or polyglycolic acid clips), radioiodine or ferromagnetic seeds, which differ in the method of location (wire, gamma-detection or magnetic probe). The aim of this study is to perform a systematic review about the current status of the TAD, as well as to explain the different techniques and types of axillary marking, based on the current available evidence.
La disección axilar dirigida (DAD) consiste en una nueva técnica de estadificación axilar que combina la biopsia selectiva del ganglio centinela (BSGC) y la biopsia del ganglio marcado con clip (BCLIP) en la misma cirugía, para reestadificar a las pacientes con cáncer de mama con ganglios axilares positivos tratadas mediante quimioterapia neoadyuvante (QTNA). Para su realización, previo a la QTNA, se punciona el ganglio metastásico de manera ecoguiada y se deja un marcador en su interior, para biopsiarlo de manera dirigida en la cirugía posterior. Existen numerosos marcadores: desde clips de acero, titanio o ácido poliglicólico hasta semillas de radioyodo o ferromagnéticas, que difieren en su método de localización y recuperación (arpón, sonda de detección gamma, o sonda magnética). El objetivo de este trabajo es realizar una revisión sistemática del estado actual de la TAD, así como explicar las diferentes técnicas y tipos de marcaje axilar, con base en la evidencia disponible.
Targeted axillary dissection (TAD) is a new axillary staging technique that combines selective sentinel lymph node biopsy (SLNB) and clipped lymph node biopsy (CLNB) in the same surgical procedure. The technique arose from the need to re-stage patients with breast cancer and positive axillary lymph nodes at diagnosis who had been treated with neoadjuvant chemotherapy (NACT), after observing in the ACOSOG Z10711 study that SLNB alone was not sufficient due to its high false negative rate (FNR) (12.6%). In 2016, this same working group, led by Boughey, tested the usefulness of lymph node marking in a subgroup of 107 patients, in whom metastatic axillary lymph nodes were marked. Iodized seeds were placed prior to surgery, and the nodes were later removed in the operating room using targeted dissection, which obtained an FNR of 6.8% when using axillary lymph node dissection (ALND) as a reference.2 These results were corroborated that same year by Caudle et al.,3 who observed an FNR of 4.2% in a series of 191 patients treated by ALND with previous SLNB and CLNB. This group coined the name “targeted axillary dissection”.
To perform TAD, and prior to the initiation of NACT, a solid marker is implanted in the metastatic ganglion under ultrasound guidance for later removal in a targeted manner during the subsequent surgery. There are several types of solid markers that can be used: from steel, titanium or polyglycolic acid clips, to radioiodine or ferromagnetic seeds, which differ in their location and retrieval methods (wire, gamma detection probe, or magnetic probe). The role of TAD is not yet clear, nor is it clear which type of marker is most suitable based on accessibility, price, safety, and ease of implantation and recovery. Therefore, the objective of this study is to review the current status of TAD, as well as to discuss the different techniques and types of axillary marking based on the current evidence available.
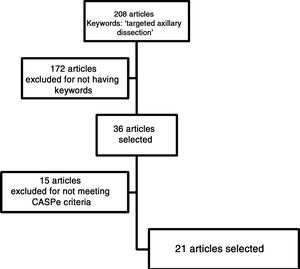
MethodsWe conducted a systematic review of the articles available on TAD, focusing on the different types of axillary markers used and under study, their advantages and disadvantages. To this end, a bibliographic search was carried out using the MEDLINE (via Pubmed), Google Scholar, ISI Web of Science and SCOPUS databases. The term used in the different search engines was targeted axillary dissection, and studies were selected from the last 10 years. For the choice of articles to be analyzed, we completely read the abstract of all the articles whose titles had at least one of the following terms: clip markers, clipped lymph node, wire localization, intraoperative ultrasound, iodine seed, magnetic seed and breast cancer. As it is a relatively new topic, and no high-quality studies have been published (randomized clinical trials and meta-analyses), all types of studies were included in the review.
The selected articles were read critically in order to discard articles that did not meet at least 70% of the criteria proposed by the CASPe4 network depending on the type of study selected (reviews, cohort studies, cases and controls, clinical trials). We reviewed those papers whose references contained information relevant to the topic. Finally, the conclusions of each of the articles were analyzed, and all the references were prepared with the Mendeley Desktop© program from Elsevier® for inclusion in this article.
This article was prepared in accordance with the guidelines proposed by the Preferred Reporting Items for Systematic Reviews and MetaAnalyses (PRISMA).5
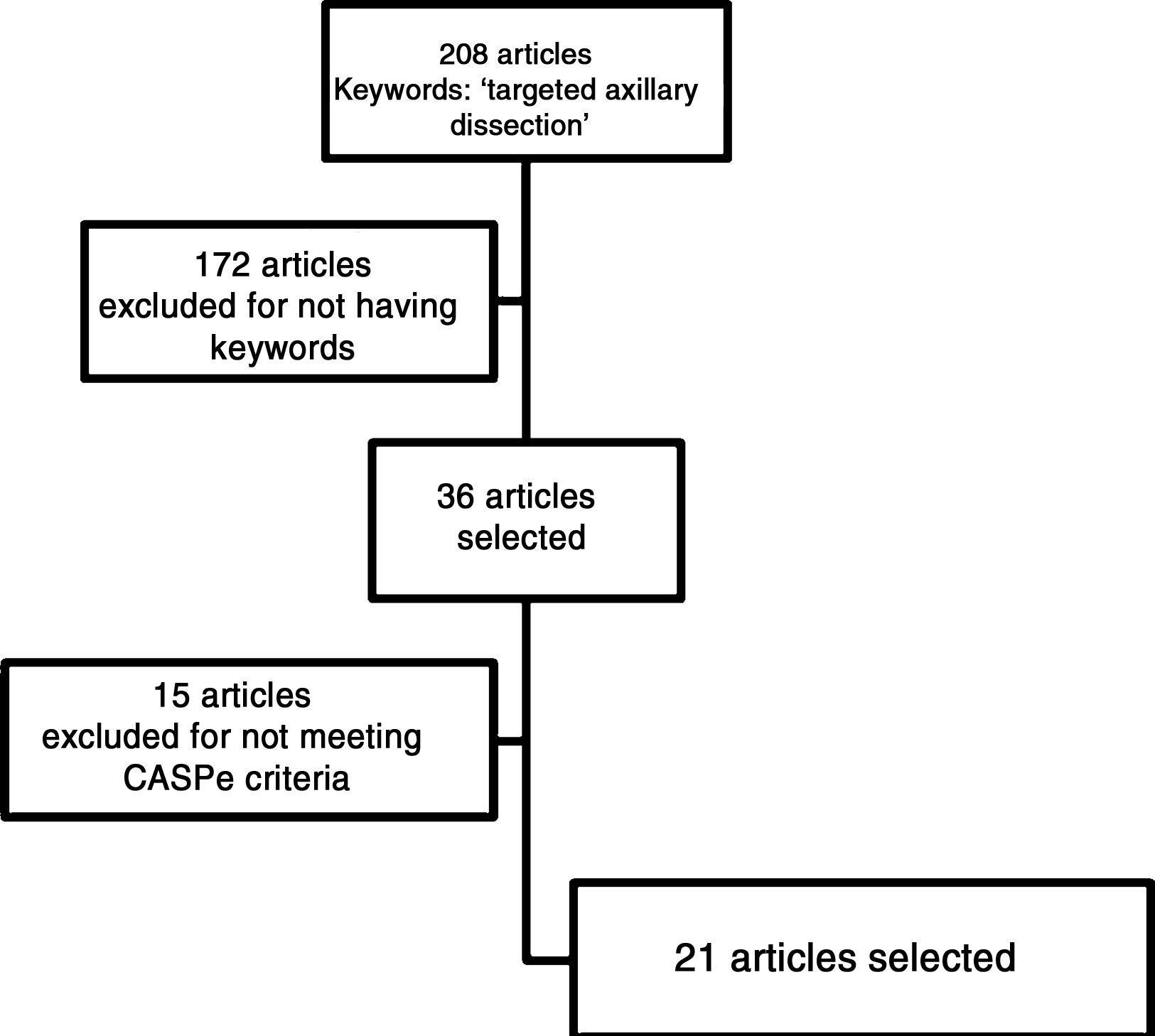
ResultsThe search algorithm found 208 articles related to the term targeted axillary dissection. Thirty-six of these articles were selected because they contained one or more of the keywords mentioned previously. After critical reading, 15 articles were excluded because their quality was not considered adequate, as defined by the CASPe network criteria, leaving a total of 21 articles. In addition, we included a direct reference about the approval and use of Magseed® magnetic seeds (Magseed© by Sysmex®).
The articles selected for this review (Fig. 1) include one systematic review, 2 non-randomized clinical trials, 8 diagnostic test studies and 10 observational comparative studies.
DiscussionTargeted axillary dissectionSolid markers were initially described at the end of the 90 s to mark breast tumor lesions3 and were later adapted for their use in the axillae.
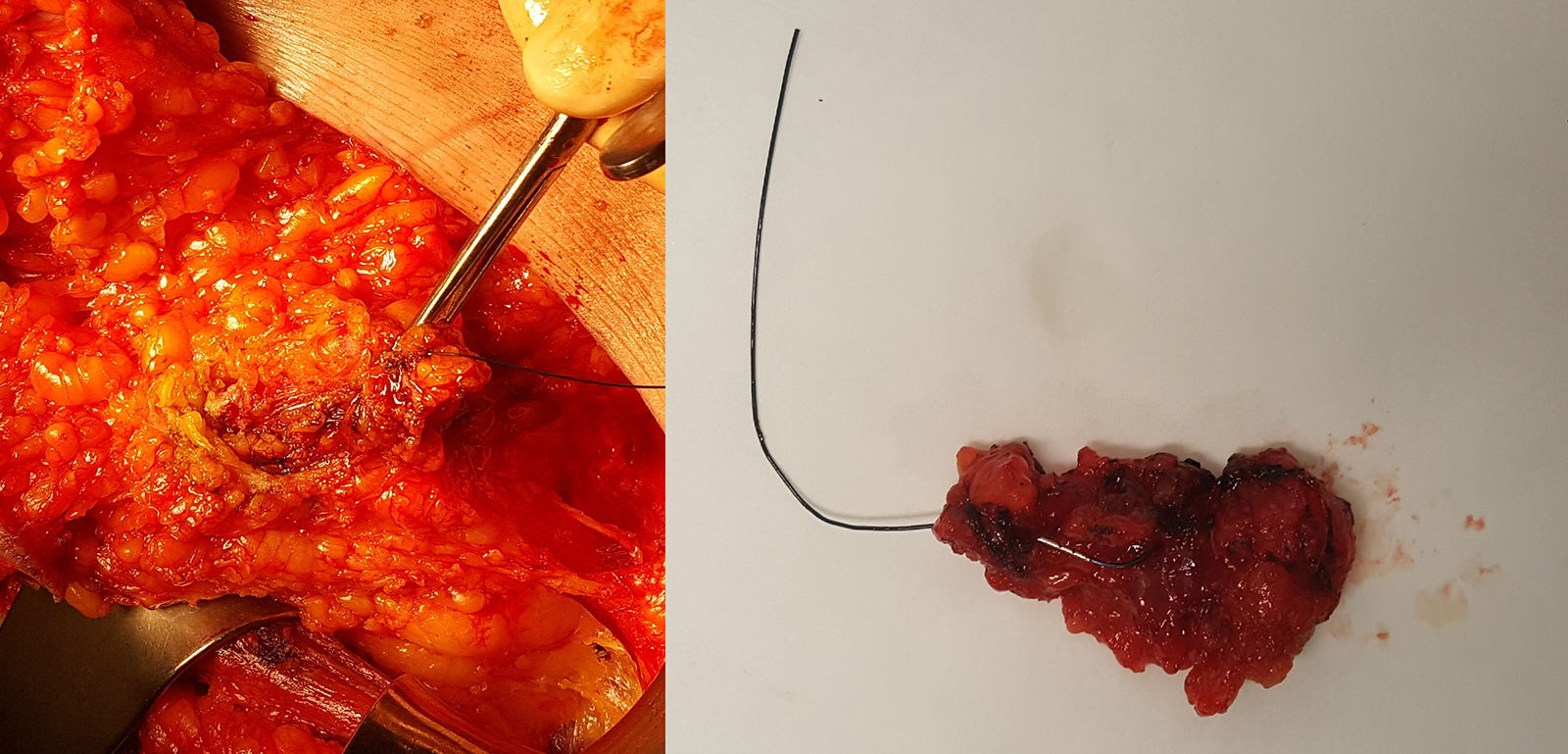
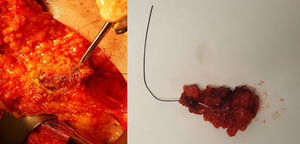
Marking begins at the time of diagnosis: using ultrasound, the radiologist identifies pathological lymphadenopathies confirmed by fine-needle aspiration, which is done jointly with a pathologist who has expertise in breast pathology; the nodes are marked (with 1 or 2 markers) using one of the previously described markers. After administration of NACT for 4–6 months, the patient undergoes another ultrasound evaluation; the clip is located, and the anatomical response of the marked lymph nodes is observed. During surgery, TAD is performed. The surgical technique is simple and similar to that performed in SLNB (Fig. 2): after preoperative identification and injection of the radioactive co0lloid for SLNB, the surgeon makes an incision in the lower part of the axillary cavity, generally along the lower edge of the hairline, or access can be achieved through the mastectomy incision, if applicable. Through the incision, targeted dissection is performed, and the marked lymph node is removed (assisted with an ultrasound-guided wire, intraoperative ultrasound or, in the case of a radioactive seed or a magnetic clip, with the corresponding detector probe). Once the marked lymph node(s) have been extracted, they are scanned with the scintigraphic detector to determine whether they coincide with the sentinel node, and the axillary cavity is also scanned to locate other lymph nodes that capture the radiotracer, which are resected. This part of the intervention is essential, since the value of CLNB lies in the high number of cases in which the marked node does not coincide with the sentinel node (in the study by Caudle et al., 23% of the cases did not coincide). Subsequently, following the protocol of our hospital, we analyzed all the lymph nodes removed with the OSNA method intraoperatively, regardless of the marker used, and the positivity of any of them (including micrometastasis) indicated ALND.
Biopsy of the clipped lymph node marked with a wire placed under ultrasound guidance; on the left, dissection of the lymph node targeted by the wire through a mastectomy incision; on the right, the surgical piece extracted and sent for pathology study using intraoperative One-Step Nucleic Acid Amplification (OSNA) analysis.
As previously mentioned, the studies by Boughey et al2 and Caudle et al3 were pioneers in the development of this technique (reporting FNR of 6.8% and 4.2%, respectively). After the publication of these results, subsequent studies have been carried out, the majority to validate the technique, describe the advantages and disadvantages of marking, and report the subsequent surgical recovery. Kim et al6 carried out a feasibility study in 20 patients with a total of 24 clipped nodes, 23 of which (95.8%) were able to be recovered during surgery. Another recent validation study,7 probably the largest to date, used TAD in 98 patients and found an FNR of 4.2% if the clip was located in the sentinel node, while the rate rose to 16.7% if the clipped node was not the sentinel (both nodes coincided in 81.4%). One theory that explains this is the distorted axillary lymphatic drainage due to the disease and NACT. This was described by Ahmed and Douek in a note in the British Journal of Surgery,8 in which they summarized the history of TAD and added that this technique ensures with confidence the absence of residual axillary disease after NACT, despite the few studies available. Other studies, such as the Spanish ILINA study,9 or a preliminary validation study of the technique,10 presented similar FNR.
Therefore, the studies published to date have shown that TAD is a feasible and valid technique that enables us to predict the absence of residual axillary disease in more than 95% of cases.
Metal or resorbable clipsClips used as lymph node markers are generally metal (steel or titanium) or partially made of resorbable material (collagen or polyglycolic acid).11 Their purpose is for posterior localization by ultrasound during surgery, either intraoperatively or preoperatively by marking with a wire. The advantages include easy implantation and their affordable price. However, the main disadvantages are their high migration capacity during the NACT period and difficult ultrasound localization. For this reason, some clips have been manufactured with a hydrogel base to facilitate ultrasound visualization.12 However, even with hydrogel clips and new ultrasound probes, localization and retrieval of the clipped lymph node can be problematic in up to 30% of cases.13 The 2 techniques most used for this recovery are:
- •
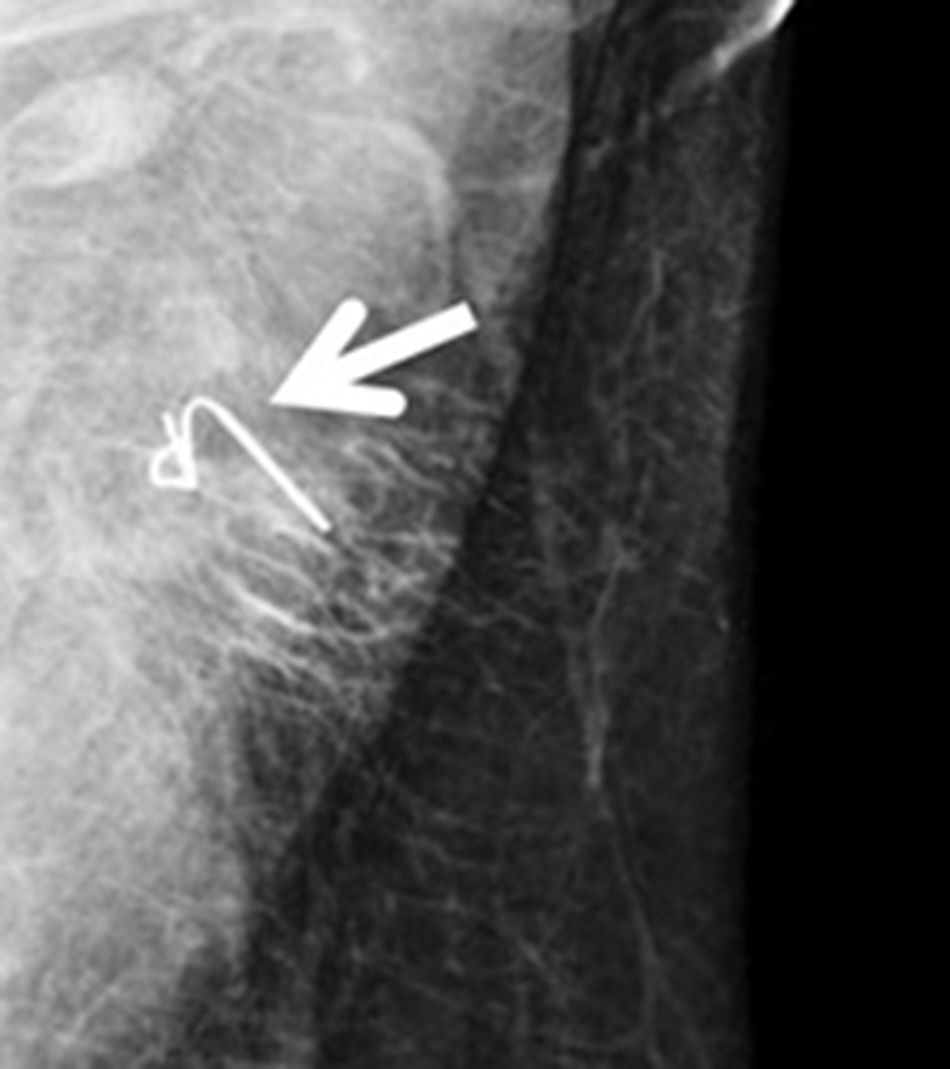
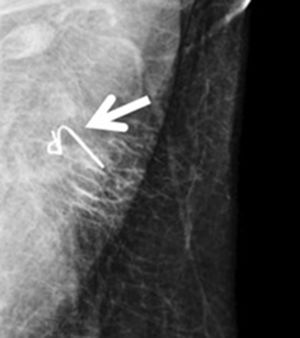
Ultrasound-guided wire (Fig. 3): this involves the ultrasound-guided placement of a wire in the clipped lymph node during the immediate preoperative period, in order to guide the surgeon for later recovery. In 2015, Plecha et al14 described this technique, with a 97.3% success rate in recovering the clipped lymph node during surgery. However, in a later study,15 they found significant limitations of the technique, such as the difficulty to see the clipped node by ultrasound and the high rate of failure to retrieve the clip in their series (30%).
- •
Intraoperative ultrasound: this involves performing ultrasound-guided surgery, locating the clipped node directly in the operating room with an intraoperative ultrasound probe. This technique was mostly described by the only Spanish group that has published about TAD to date, as part of the ILINA9 study. In this study, also for validation in 46 patients (they also performed later ALND), the authors were able to locate and remove the lymph node by intraoperative ultrasound in 95.7% of cases, with an FNR of 4.1%. They concluded that this technique is valid and feasible in experienced hands, with less discomfort for the patient.
These are titanium seeds marked with 125I, which are implanted before initiating NACT. During surgery, the node is located with a hand-held gamma detection probe for resection. The main study that describes the technique and demonstrates its usefulness is the Marking axillary lymph nodes with radioactive iodine seeds (MARI) study,16 in which, after a mean of 17 weeks from its implantation, TAD was performed in 100 patients, with a localization rate of 97% and an FNR of 7%. The later study by Caudle et al.3 also used iodine seeds as a locator but implanted one week prior to the intervention. The main advantage of the technique is that it is easy to locate the seed intraoperatively with the gamma probe. Its main limitations are: the radioactivity generated (although the radiation emitted is so low [0.04−0.19 mCi] that it can be kept in place throughout the neoadjuvant treatment), and the need for a specialist in nuclear medicine together with infrastructure to handle and dispose of this type of seeds, which is not available at all hospitals. Due to its high success rate in intraoperative localization, the use of radioiodine seeds could be proposed as a valid alternative to classical clipping.
Magnetic seedsThese low-nickel iron oxide seeds can be implanted at a depth of up to 3–4 cm and remain in the tissue with no time restriction.17 The seed is located by a probe that generates an alternating magnetic field, providing its intraoperative localization with a numerical count and audio tone demonstrating the strength of the magnetic field and distance of the seed. It is a safe and reliable method for conservative breast surgery.18 The first feasibility studies of the technique in axillary lymph nodes have recently been published, with a detection rate of 97%,19 although there are no studies on its specific use in TAD. As there are very few studies on the subject, and there are no comparative studies with other markers, the limitations of the technique are not yet known. However, feasibility studies show that magnetic seeds are another option to classic marking with clips or iodine seeds.
Other markers being developed- •
Infrared light and radar technology20,21: this consists of the implantation of a radar or infrared reflector in the area to later locate the reflection of the radar or infrared waves by means of an intraoperative probe. It is being tested in breast surgery, although it is not yet being applied in the axilla due to the large size of the reflector (1.2 cm).
- •
Radiofrequency identifiers: recently approved by the Food and Drug Administration for use in humans, it consists of the placement of an encapsulated microchip that emits a radiofrequency signal that can be detected by an intraoperative probe. First described in 2008,22 it has only been used in non-palpable breast lesions.23
There is increasing evidence that TAD is a feasible and valid method to adequately restage patients with breast cancer and pathological axillary lymphadenopathies treated by NACT, suggesting that ALND could be safely omitted in patients with complete response to chemotherapy. The most commonly used axillary markers are metal clips, although they have limitations, especially their localization and subsequent surgical recovery. New methods, such as the use of iodine or magnetic seeds (already in use in some hospitals) or the identification by radar, infrared or radiofrequency, seem promising to improve posterior localization. However, studies are necessary, preferably randomized comparative clinical trials, to determine their use in clinical practice.
FundingThis study has received no specific funding from public, commercial or non-profit organizations.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Flores-Funes D, Aguilar-Jiménez J, Martínez-Gálvez M, Ibáñez-Ibáñez MJ, Carrasco-González L, Gil-Izquierdo JI, et al. El problema de la estadificación axilar en el cáncer de mama tras quimioterapia neoadyuvante. Papel de la disección axilar dirigida y tipos de marcadores ganglionares. Cir Esp. 2020. https://doi.org/10.1016/j.ciresp.2020.03.012