Renal carcinoma represents 3% of all solid tumors and is associated with renal or inferior caval vein (IVC) thrombosis between 2% and 10% of patients, extending to right atrial in 1% of cases.
MethodsThis is a retrospective study that comprises 5 patients who underwent nephrectomy and thrombectomy by laparotomy because of renal tumor with IVC thrombosis level III.
ResultsFour patients were males and one was female, and the mean age was 57.2 years (range: 32–72). Most important clinical findings were hematuria, weight loss, weakness, anorexia, and pulmonary embolism. Diagnostic confirmation was performed by CT scanner. Metastatic disease was diagnosed before surgery in 3 patients. Suprahepatic caval vein and hepatic hilium (Pringle's maneouver) were clamped in 4 patients, and ligation of infrarrenal caval vein was carry out in one patient.
Five patients developed mild complications (Clavien I/II). No patient died and the mean hospital stay was 8.6 days. All patients were treated with chemotherapy, and 3 died because distant metastasis, but 2 are alive, without recurrence, at 5 and 60 months, respectively.
ConclusionsNephrectomy and thrombectomy in renal tumors with caval thrombosis can be curative in absence of metastasis or, at less, can increase survival or quality of live. Then these patients must be treated in liver transplant units because major surgical and anesthesiologic expertise. Adjuvant treatment with tyrosin kinase inhibitors must be validate in the future with wider experiences.
El carcinoma renal representa el 3% de los tumores sólidos y se asocia a trombosis de la vena renal o vena cava inferior (VCI) en el 2-10% de los pacientes; se extiende hasta la aurícula derecha en el 1% de los casos.
MétodosEstudio retrospectivo de una serie de 5 enfermos intervenidos por tumor renal con trombosis tumoral de VCI de nivel iii, tratados con nefrectomía y trombectomía por laparotomía.
ResultadosCuatro de los pacientes eran hombres y uno era mujer, con una edad media de 57,2 años (rango: 32-72). Como clínica predominó la hematuria, síndrome constitucional y tromboembolia pulmonar. La confirmación diagnóstica fue por TAC. En 3 pacientes se detectaron metástasis antes de la cirugía. Se realizó Pringle y pinzamiento de vena cava suprahepática en 4 pacientes y ligadura de VCI infrarrenal en uno.
Complicaciones leves (Clavien I/II) se presentaron en 5 pacientes. La mortalidad fue nula y la estancia hospitalaria media fue de 8,6 días. Todos los pacientes se trataron con quimioterapia; fallecieron 3 por metástasis a distancia, permanecen 2 vivos, sin recidiva, a los 5 y 60 meses. Supervivencia media: 26,6 meses.
ConclusionesLa nefrectomía y la trombectomía en tumores renales con trombosis de cava pueden ser curativas en ausencia de metástasis o, al menos, pueden aumentar la supervivencia y mejorar la calidad de vida. Para ello estos pacientes deberían tratarse en unidades de trasplante hepático por su mayor experiencia quirúrgica y anestésica. El tratamiento adyuvante con inhibidores de la tirosina cinasa debe validarse en el futuro con experiencias más amplias.
Renal cell carcinomas represent 3% of solid tumors.1 They cause a thrombus in the renal vein or inferior vena cava (IVC) in 2–10% of patients,2,3 and the thrombus extends to the right atrium in 1% of cases.2 The treatment indicated in these patients involves nephrectomy and thrombectomy of the cava, although this surgery also presents morbidity and mortality. In order to reduce these complications, it is necessary to identify and adequately expose the vena cava, liver and retroperitoneum in conjunction with the Urology Department in order to conduct the nephrectomy. Before surgery, is it essential to define the exact location of the thrombus in the cava to plan the best possible surgical approach. Depending on the location of the thrombus in the IVC, different techniques have been used, such as laparotomy,4–6 assisted laparoscopy,7 venovenous bypass8 or cardiopulmonary bypass with mild hypothermia.9 In this study, we present our experience in the treatment of three tumors by laparotomy.
MethodsWe present a retrospective study of patients with renal tumor and thrombosis of the inferior vena cava that had been treated surgically between 2011 and 2016 by our HBP Surgery and Abdominal Organ Transplantation Units in collaboration with the Urology Department. The objective of this case series is to explain the symptoms, diagnosis and surgical techniques performed to locate the thrombus, as well as to analyze perioperative morbidity and mortality.
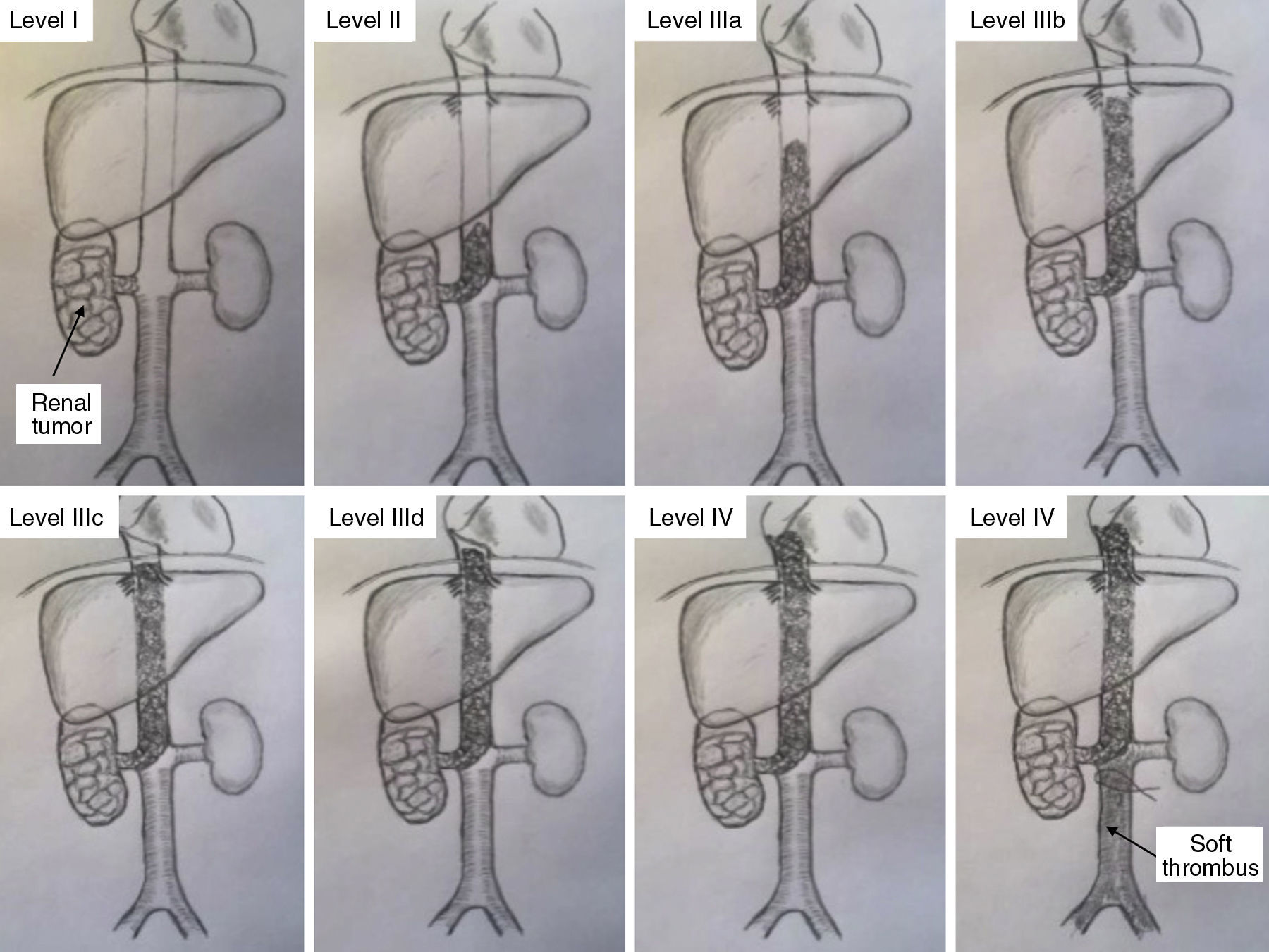
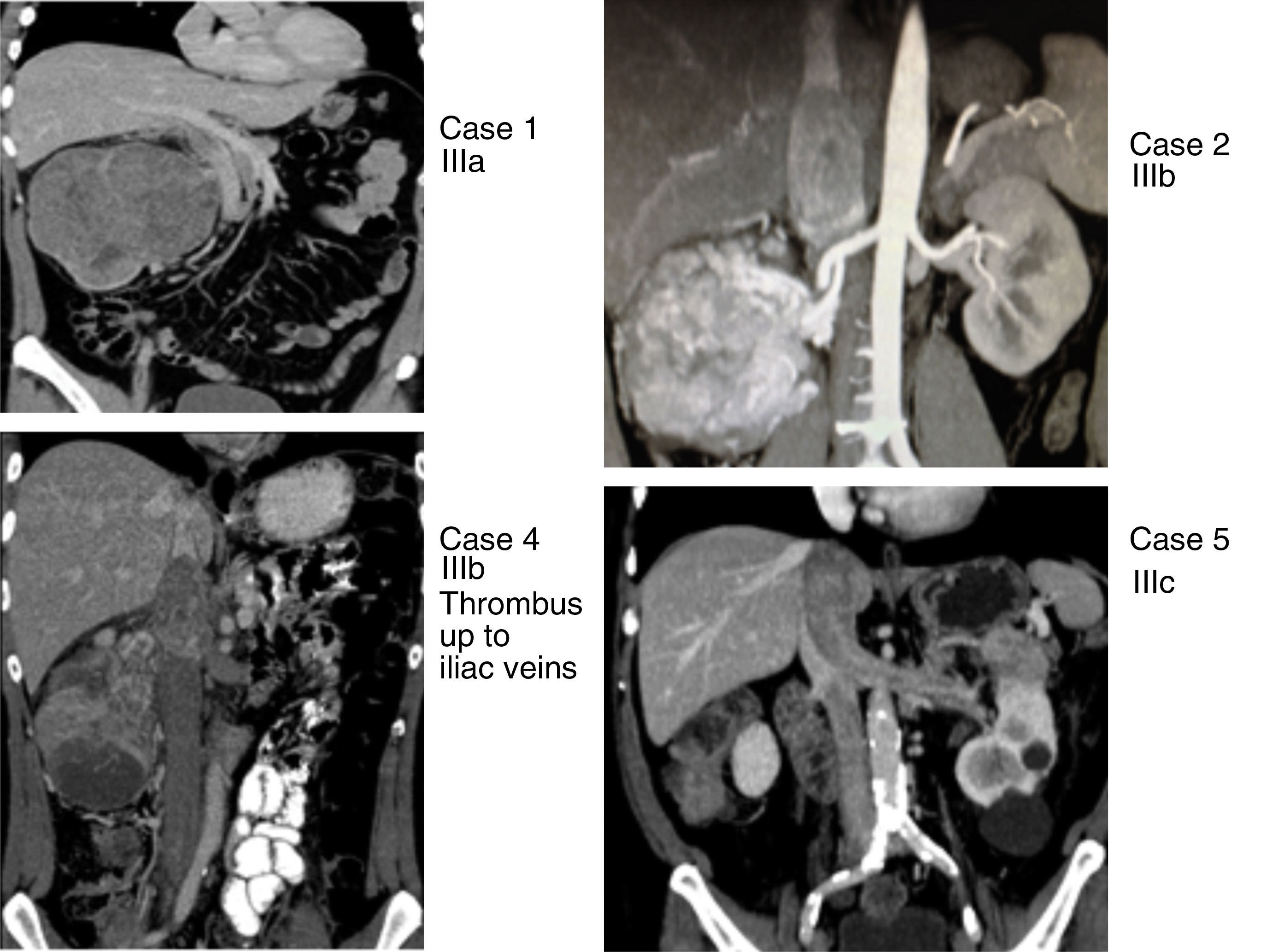
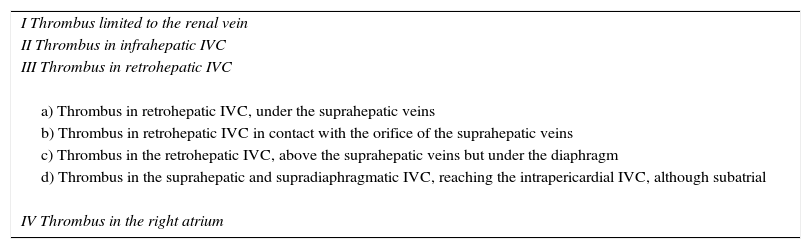
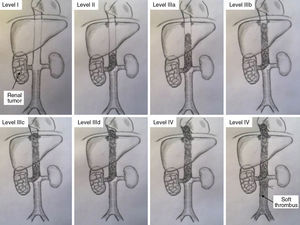
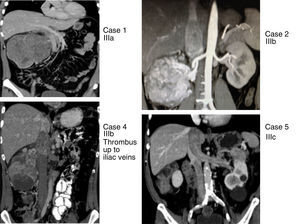
This series includes 5 patients, for whom demographic, preoperative, intraoperative and postoperative variables (Clavien et al. classification10), histological characteristics and survival were collected and analyzed. The classification adopted was the Ciancio et al. classification,11 which is based on the extension or invasion of the thrombus in the IVC (Table 1, Fig. 1). Out of the 5 cases, one corresponded with level IIIa, 3 IIIb and one IIIc. The radiological diagnosis was reached with ultrasound and CT in all cases, which demonstrated the level of invasion of the thrombus in the IVC (Fig. 2).
Classification of IVC Thrombosis Levels.
| I Thrombus limited to the renal vein |
| II Thrombus in infrahepatic IVC |
| III Thrombus in retrohepatic IVC |
| a) Thrombus in retrohepatic IVC, under the suprahepatic veins |
| b) Thrombus in retrohepatic IVC in contact with the orifice of the suprahepatic veins |
| c) Thrombus in the retrohepatic IVC, above the suprahepatic veins but under the diaphragm |
| d) Thrombus in the suprahepatic and supradiaphragmatic IVC, reaching the intrapericardial IVC, although subatrial |
| IV Thrombus in the right atrium |
Source: from Ciancio et al.11
Classification of thrombosis of the inferior vena cava (according to Ciancio et al.11).
Renal tumors with thrombosis of the inferior vena cava (cases 1, 2, 4 and 5) with levels IIIa, IIIb and IIIc, according to the Ciancio et al. classification.11
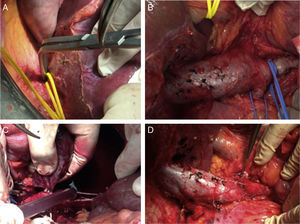
As for the surgical technique (except for level IIIa), in cases where the thrombus was proximal to the suprahepatic veins, intraoperative transesophageal echocardiogram was used to evaluate the mobility of the thrombus during surgery. We used an inverted T incision in 3 patients and a Mercedes in the remaining 2, which are standard incisions in liver transplantation. In all cases, we used a Stieber rib grip to widely separate the rib edge upwards, which allowed us to comfortably carry out the dissection of the liver and IVC. Renal tumors, especially large ones, present infiltration, firm adhesions and displacement of other organs (duodenum-pancreas, colon-hepatic angle and hepatic hilum in case 1, colon-hepatic angle in case 2 and tail of the pancreas in case 5). Initially, we freed the posterolateral side of the kidney using a posterior approach to identify, ligate and divide the renal artery, reducing collateral circulation and facilitating renal dissection.
Liver mobilization is performed similarly to liver transplantation: ligature and division of the round ligament; division with electrocoagulation of the falciform ligament, coronary and triangular ligaments, both on the right as well as the left, as well as the hepatorenal ligament. The liver pedicle is surrounded with a vascular loop for a possible Pringle maneuver. Dissection, ligation and division of the retrohepatic veins (piggy-back technique) are completed to expose the IVC and its entire extension, below the suprahepatic veins. The suprahepatic IVC is dissected and surrounded by a vascular loop for potential posterior clamping. This dissection provides for resolution of all cases of levels IIIa and IIIb IVC thrombosis, performing suprahepatic or infrahepatic clamping (below the suprahepatic veins) and clamping below the thrombus, usually at the infrarenal level. If the piggy-back is not complete (without dissection of retrohepatic veins of the caudate lobe), the suprahepatic IVC is clamped at levels IIIa and IIIb for 10–15min along, accompanied by a Pringle maneuver of equal duration. At level IIIc, we performed the same dissection, but “milked” the thrombus caudally before clamping the suprahepatic IVC.
Although we do not present any cases, at levels IIId (intrapericardial IVC thrombus) or IV (as long as the thrombus is not adhered to the atrium), the thrombus can be removed abdominally, as in previous levels, by infrahepatic cavotomy with previous diaphragmatic incision, intrapericardial dissection of the IVC, and milking the thrombus with the fingers until it surpasses the infrahepatic IVC, at which time it is clamped at this level above the thrombus.
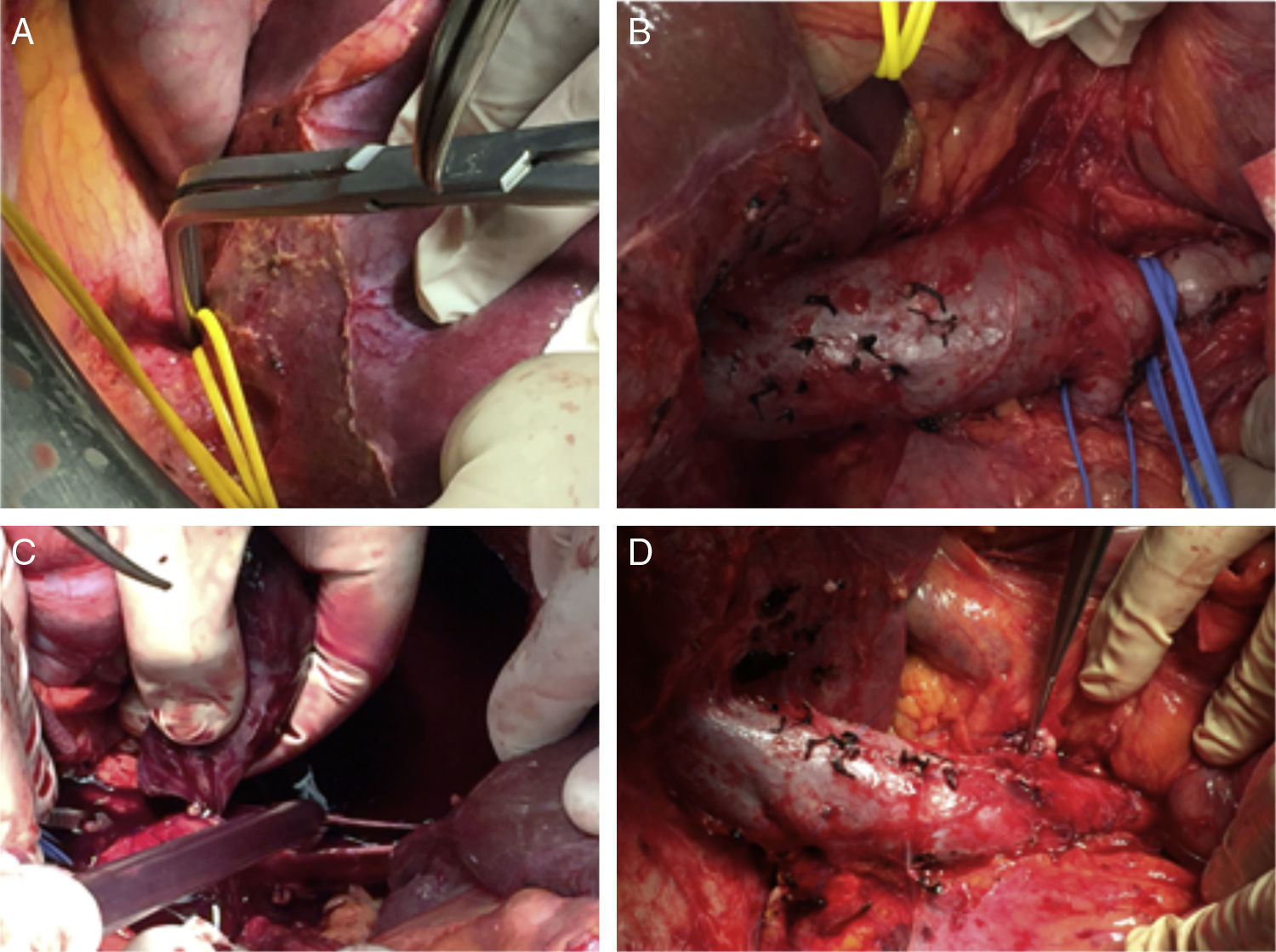
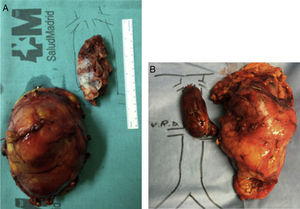
Once this dissection is performed, we place the patient in Trendelenburg position, clamp the infrarenal IVC (below the thrombus) and the renal vein affected by the thrombus, the contralateral renal vein and the IVC above the thrombus of the cava (at the infrahepatic level, if we have performed a complete piggy-back, or at the suprahepatic level supplemented with a Pringle maneuver if it is incomplete). At this time, we perform a longitudinal cavotomy measuring 3–5cm and extract the thrombus with the index finger, washing afterwards with heparinized saline. A longitudinal continuous suture of the IVC is then carried out with 4-5/0 polypropylene. Before making the last stitch, we release the infrarenal IVC and the contralateral renal vein to eliminate thrombi and air, and then fill with blood the lumen of the IVC previously occupied by the thrombus. Simultaneously, the Pringle and IVC clamps are removed above the thrombus. The renal vein affected by the tumor thrombosis is divided at its union with the IVC, thereby impeding a residual tumor thrombus from remaining in the stump, and the nephrectomy is then completed (Figs. 3 and 4).
In our case 4 with level IIIb thrombosis, a soft thrombus was also detected, which began 3cm below the renal veins and reached the iliac veins. In these cases, important chronic collateral circulation develops, in such a way that the gonadal vein and the lumbar branches connect with the azygos and hemiazygos system to reach the right atrium. Thus, after the aforementioned cava thrombectomy, we double-ligated the infrarenal IVC with silk 1-0 above the soft thrombus to prevent its migration toward the atrium. The mentioned collateral veins should not be ligated so as not to compromise the venous return toward the atrium. Proper anesthetic management of these patients is also very important by anesthesiologists with experience in liver transplantation.
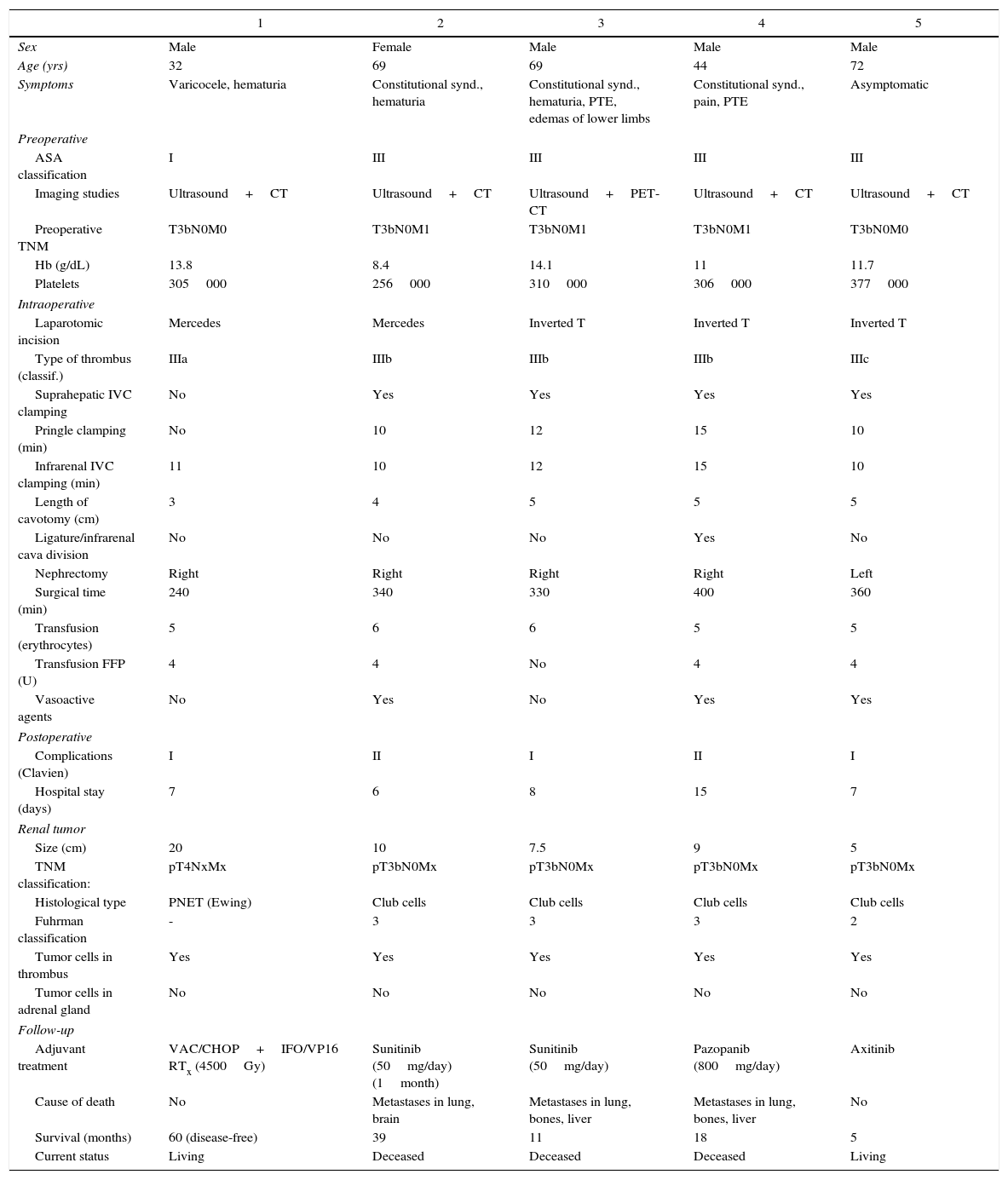
ResultsOut of this series of 5 patients, 4 were men and one was a woman, with a mean age of 57.2 years (range: 32–72). Predominant symptoms included hematuria, weight loss and pulmonary thromboembolism (PTE). In all cases, ultrasound was performed as the first diagnostic test, followed by CT scan for confirmation (PET/CT in one patient). In 3 patients, pulmonary, hepatic, bone or cerebral metastases were detected before surgery.
Clamping of the suprahepatic IVC and Pringle maneuver (10–15min) was performed in 4 patients, while in the first case (level IIIa) clamping was done under the suprahepatic veins. The infrarenal IVC was clamped (below the thrombus) in all cases for 10–15min. Definitive silk ligation of the infrarenal IVC above the soft thrombus, after thrombectomy of the main thrombus, was performed in one patient. Mean surgical time for nephrectomy and thrombectomy was 334min (range: 240–400), and mean transfusion of packed red blood cells was 6.4 units.
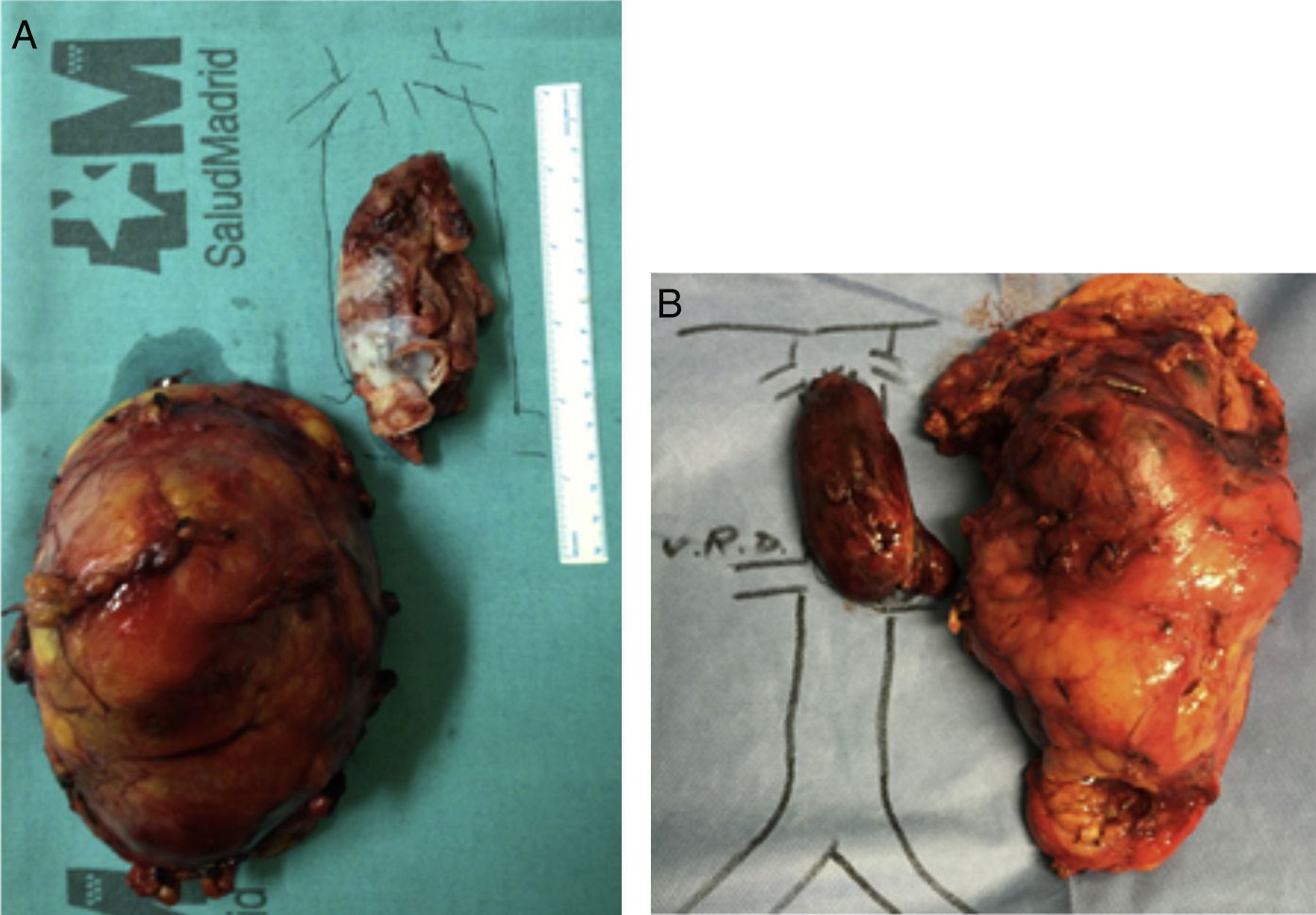
The primitive tumor was located in the right kidney in 4 cases and in the left in one, with a size of 5–20cm. In 4 patients, the tumor was comprised of club cells, while the other was a Ewing tumor. Tumor cells were isolated in the cava thrombus in all patients, whereas the adrenal gland showed no tumor invasion in any of the cases.
Postoperative complications were minor in all patients (Clavien I in 3 and Clavien II in 2), with an average hospital stay of 8.6 days. Out of the 5 patients, 4 were treated with chemotherapy (one currently in treatment), and radiotherapy was added to the patient with renal Ewing's sarcoma. Two patients survived to 60 months, and the last patient treated to 5 months; the remaining 3 died of metastatic disease at 11, 18 and 39 months, with a mean survival of 26.6 months (Table 2).
Renal Tumors With Vena Cava Thrombosis.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Male |
| Age (yrs) | 32 | 69 | 69 | 44 | 72 |
| Symptoms | Varicocele, hematuria | Constitutional synd., hematuria | Constitutional synd., hematuria, PTE, edemas of lower limbs | Constitutional synd., pain, PTE | Asymptomatic |
| Preoperative | |||||
| ASA classification | I | III | III | III | III |
| Imaging studies | Ultrasound+CT | Ultrasound+CT | Ultrasound+PET-CT | Ultrasound+CT | Ultrasound+CT |
| Preoperative TNM | T3bN0M0 | T3bN0M1 | T3bN0M1 | T3bN0M1 | T3bN0M0 |
| Hb (g/dL) | 13.8 | 8.4 | 14.1 | 11 | 11.7 |
| Platelets | 305000 | 256000 | 310000 | 306000 | 377000 |
| Intraoperative | |||||
| Laparotomic incision | Mercedes | Mercedes | Inverted T | Inverted T | Inverted T |
| Type of thrombus (classif.) | IIIa | IIIb | IIIb | IIIb | IIIc |
| Suprahepatic IVC clamping | No | Yes | Yes | Yes | Yes |
| Pringle clamping (min) | No | 10 | 12 | 15 | 10 |
| Infrarenal IVC clamping (min) | 11 | 10 | 12 | 15 | 10 |
| Length of cavotomy (cm) | 3 | 4 | 5 | 5 | 5 |
| Ligature/infrarenal cava division | No | No | No | Yes | No |
| Nephrectomy | Right | Right | Right | Right | Left |
| Surgical time (min) | 240 | 340 | 330 | 400 | 360 |
| Transfusion (erythrocytes) | 5 | 6 | 6 | 5 | 5 |
| Transfusion FFP (U) | 4 | 4 | No | 4 | 4 |
| Vasoactive agents | No | Yes | No | Yes | Yes |
| Postoperative | |||||
| Complications (Clavien) | I | II | I | II | I |
| Hospital stay (days) | 7 | 6 | 8 | 15 | 7 |
| Renal tumor | |||||
| Size (cm) | 20 | 10 | 7.5 | 9 | 5 |
| TNM classification: | pT4NxMx | pT3bN0Mx | pT3bN0Mx | pT3bN0Mx | pT3bN0Mx |
| Histological type | PNET (Ewing) | Club cells | Club cells | Club cells | Club cells |
| Fuhrman classification | - | 3 | 3 | 3 | 2 |
| Tumor cells in thrombus | Yes | Yes | Yes | Yes | Yes |
| Tumor cells in adrenal gland | No | No | No | No | No |
| Follow-up | |||||
| Adjuvant treatment | VAC/CHOP+IFO/VP16 RTx (4500Gy) | Sunitinib (50mg/day) (1month) | Sunitinib (50mg/day) | Pazopanib (800mg/day) | Axitinib |
| Cause of death | No | Metastases in lung, brain | Metastases in lung, bones, liver | Metastases in lung, bones, liver | No |
| Survival (months) | 60 (disease-free) | 39 | 11 | 18 | 5 |
| Current status | Living | Deceased | Deceased | Deceased | Living |
FFP: fresh frozen plasma; RTx: radiotherapy; PTE: pulmonary thromboembolism; PNET: primitive neuroectodermal tumor; TNM: tumor node metastasis; IVC: inferior vena cava.
In the Memorial series,3 82% of patients with renal tumor and IVC thrombosis were symptomatic at the time of diagnosis, and 19% had distant metastases. On the other hand, in the Mayo Clinic series,12 about 40% of these patients with renal tumor and IVC thrombosis presented metastases at the time of surgery: 28.5% at a distance and 11.7% of the regional lymph nodes.
In our short series, taking into account that all patients presented level III thrombosis, 80% showed symptoms (mainly constitutional syndrome, hematuria and PTE), and 60% had distant metastases at the time of diagnosis.
The diagnosis of an IVC thrombus is confirmed with magnetic resonance imaging, which is especially useful for detecting a soft thrombus in the infrarenal IVC,13 or by multi-slice CT scan.14
Level I or II IVC thrombi are treated by dissection of the IVC, without the need for a complete piggy-back, with clamping of the contralateral renal vein and the IVC above and below the thrombus, followed by cavotomy and thrombectomy.
It has been demonstrated that, in cases of level III IVC thrombi, thrombectomy can be performed without using bypass techniques, thereby basically avoiding neurological, pulmonary, or coagulopathy complications.3,4,10 In level IV thrombosis, venovenous or cardiopulmonary bypass can be avoided if the thrombus is free (not adhered to the atrium) using the intraabdominal approach, followed by division of the diaphragm. Thus, the intrapericardial IVC and right atrium are controlled, and the thrombus is “milked” with the fingers toward the IVC below the suprahepatic veins.15
If ligation and division of all retrohepatic veins or the piggy-back technique are used as in liver transplantation, it is not necessary to clamp the hepatic hilum in level III thrombosis prior to thrombectomy.15 Alternatively, in our experience, in levels I, II and III it is not necessary for the piggy-back to be complete if we clamp the hepatic hilum (Pringle maneuver) and the suprahepatic IVC for 10–15min, which is sufficient time to perform the 3–5cm cavotomy, thrombectomy and suture of the vena cava.
In the rarest case of thrombosis with complete IVC obstruction associated with an infrarenal soft thrombus reaching up to the iliac veins, collateral circulation formed through the lumbar veins that drain the system of the azygos and hemiazygos veins. Here, thrombectomy of the formed thrombus should be done with ligation or division with a stapler of the IVC above the soft thrombus, which is left intact to prevent its mobilization toward the atrium.16
It has been observed that the higher the level of the thrombus, the greater the need for blood transfusion during surgery.3,15 Two American series report between 4.3 and 4.7 units of transfused packed red blood cells,3,15 while the mean number of units transfused in our patients was 5.4. The reported average surgical time has been between 250 and 467min,3,12,15 while the mean was 334min in our patients.
In our series, we have observed the presence of tumor cells in all the cava thrombi but the absence of these cells in the adrenal gland excised together with the renal tumor.
In the series of 77 patients at Memorial,3 which considered all levels of thrombosis, the incidence of major complications was 36% and minor complications 18%. Hemorrhage and PTE are serious complications associated with the higher level of cranial extension of the thrombus.17 Our patients have only presented minor complications (Clavien I or II). According to different series,3,6,12 perioperative mortality was 3.5–6%, which was null in our experience. Mean reported hospital stay was between 7 and 9 days12,15 and 8.6 days in our series.
The higher the level of the thrombus, the more advanced the tumor stage,3,15 and median survival decreases.18,19 Independent factors of poor prognosis include the presence of metastasis at the time of diagnosis and non-club cell renal tumors.6 There is controversy regarding radical surgery in cases of renal tumors with distant metastases and cava thrombosis: some authors state that the possibility of increased survival is not justified by the high morbidity and mortality associated with surgery.20 However, recently, other authors have demonstrated the absence of differences in morbidity and mortality rates, whether or not there is distant metastasis.21
After surgery, 5-year survival is 15–20% with regional or distant metastases and only 4% with both types of metastases. In cases with no metastasis, survival reaches 60%.12 However, even in patients with distant metastases, surgery is still indicated as it increases survival and improves quality of life.15,22–24 In patients with distant metastases, mean survival rates of 13 months and 2-year survivals of 26% have been reported.25 In general, nephrectomy and thrombectomy are associated with greater survival than conservative treatment in cases of renal tumors with IVC thrombosis (19.8 months versus 6.9 months).18 Another series confirms that the mean survival of patients who are not treated surgically is only 5 months.26 Surgical treatment of metastatic lesions can be performed simultaneously with the renal surgery or at a later stage, depending on the patient's condition. Thus, surgical rescue of a metachronous lesion is considered when it is a single lesion in a patient in good general condition.27
Recently, several series have reported patients treated by laparoscopy28 or robotic surgery,29,30 with results similar to those obtained by laparotomy.
Several cases of neoadjuvant therapy with sunitinib have been reported, in which level IV thrombi have been reduced in size.31 Initial treatment with tyrosine-kinase inhibitors (sunitinib, pazopanib, axitinib) should be considered for its potential in both infra-staging as well as the reduction of postoperative morbidity.32
We conclude that nephrectomy and thrombectomy in renal tumors with cava thrombosis may be a curative technique in the absence of metastasis or may at least increase survival and improve quality of life. These patients should be treated in liver transplantation units due to their greater surgical and anesthetic experience. In the future, adjuvant treatment with tyrosine-kinase inhibitors should be validated with more extensive studies.
Authorship/Collaborators- Carlos Jiménez-Romero: study design; composition of the article.
- María Conde: data collection; composition of the article.
- Federico de la Rosa: data collection; analysis and interpretation of the results.
- Alejandro Manrique: analysis and interpretation of the results; critical review and approval of the final version.
- Jorge Calvo: data collection; analysis and interpretation of the results.
- Óscar Caso: data collection; analysis and interpretation of the results.
- Carlos Muñoz: data collection; analysis and interpretation of the results.
- Alberto Marcacuzco: data collection; critical review and approval of the final version.
- Iago Justo: critical review and approval of the final version; study design.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Jiménez-Romero C, Conde M, de la Rosa F, Manrique A, Calvo J, Caso Ó, et al. Tratamiento de la trombosis de vena cava inferior asociada a los tumores renales. Cir Esp. 2017;95:152–159.