Massive blood transfusion (MBT) is a common occurrence in liver transplant (LT) patients. Recipient-related risk factors include cirrhosis, history of multiple surgeries and suboptimal donors. Despite advances in surgical techniques, anesthetic management and graft preservation have decreased the need for transfusions, this complication has not been completely eliminated.
MethodsOne thousand four hundred and sixty-nine LT were performed at our institution between May 2003 and December 2020, and data was available regarding transfusion for 1198 of them. We divided the patients into two groups, with regards to transfusion of 6 or more units of packed red blood cells in the first 24 h posttransplant, and we analyzed the differences between the groups.
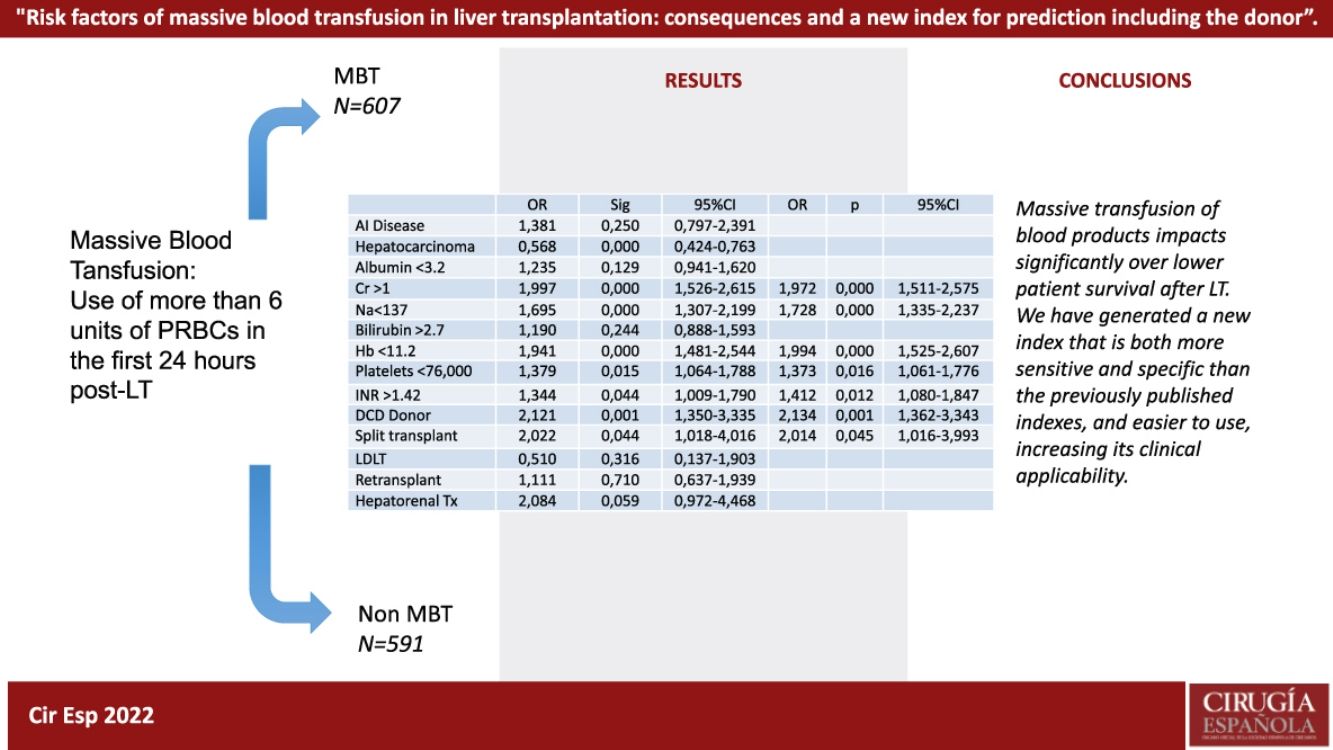
ResultsOut of the 1198 patients, 607 (50.7%) met criteria for MBT. Survival was statistically lower at 1, 3, and 5 years when comparing the groups that had MBT to those that did not (92.6%, 85.2% and 79.7%, respectively, in the non MBT group, vs. 78.1%, 71.6% y 66.8%, respectively, in the MBT group). MBT was associated with a 1.5 mortality risk as opposed to non-MBT patients. Logistical regression analysis of our variables yielded the following results for a new model, including serum creatinine (OR 1.97), sodium (OR 1.73), hemoglobin (OR 1.99), platelets (OR 1.37), INR (OR 1.4), uDCD (OR 2.13) and split liver donation.
ConclusionMassive blood transfusion impacts patient survival in a statistically significant way. The most significant risk factors are preoperative hemoglobin, INR and serum creatinine.
La transfusión masiva de hemoderivados (TMH) es un hecho frecuente en el trasplante hepático (TH). A pesar de los avances en la técnica quirúrgica, manejo anestésico y preservación de órganos, la politransfusión no ha desaparecido.
Métodos1469 TH fueron realizados en nuestro centro entre mayo de 2003 y diciembre de 2020, obteniéndose datos completos de trasfusión de 1198. Dividimos a los pacientes en dos grupos de acuerdo a la necesidad de trasfusión de 6 o más unidades de sangre en las primeras 24 horas después del trasplante, y analizamos las diferencias entre los grupos.
ResultadosDe los 1198 pacientes, 607 (50.7%) cumplieron criterios de TMH· La supervivencia fue estadísticamente inferior a 1, 3, y 5 años cuando comparamos los grupos en función de TMH o no (92·6%, 85·2% y 79·7%, respectivamente, en el no TMH, vs. 78·1%, 71·6% y 66·8%, respectivamente, en el grupo de TMH). Respecto al análisis de supervivencia, la TMH se asoció a un riesgo 1.5 veces mayor de mortalidad en contra de los pacientes sin TMH· El análisis de regresión logística nos permitió la creación de un nuevo modelo incluyendo creatinina sérica (OR 1.97), sodio (OR 1.73), hemoglobina (OR 1.99), plaquetas (OR 1.37), INR (OR 1.4), uDCD (OR 2.13) y trasplante procedente de split.
ConclusiónLa transfusión masiva de hemoderivados impacta en la supervivencia del paciente de forma estadísticamente significative. Los factores de riesgo preoperatorios más significativos han sido la hemoglobina, el INR y la creatinine.
Massive blood transfusion (MBT) is common in patients undergoing liver transplantation (LT).1 The risks of MBT associated with a LT for cirrhosis, prior multiple abdominal surgeries or use of suboptimal donors, have been reduced but not completely eliminated.2,3 Advances in surgical technique, anesthetic management and graft preservation are insufficient to avoid this complication entirely.4,5
There is no universal definition for MBT. McCluskey et al.6 initially defined MBT as transfusion of more than six packed red blood cells (PRBC) units during the first 24 h after LT, but this description was later changed to administering more than eight intraoperative units of PRBCs, which roughly correspond to the standard circulating blood volume.7 This was then modified again to the use of more than ten intraoperative units of PRBCs,8 and has since then been modified on multiple occasions.9 Not only is there no consensus on the definition of massive transfusion, but there is no standardized time frame, be it intraoperative or postoperative, for said definition. Further complicating the matter is the need to factor in a center's transplant volume, inter-surgeon differences, differences in surgical techniques and patient differences, which explain the difficulty in reaching a universal consensus. Regardless of this lack of consensus on the number or PRBCs or time frame, MBT in liver transplant patients is described in as many as 10–41,9% in the literature.3,5–7,10,11
There are multiple previous models that have shown subpar results in predicting the need for blood transfusion during or after LT. They share many common variables including international normalized ratio (INR), preoperative hemoglobin concentration, number of platelets and serum creatinine,6,8,10,11 but there are studies that have highlighted other less analyzed factors, such as liver enzymes.9 In general, these indexes have been based mostly on the recipient's degree of liver dysfunction, especially with regards to pre-LT kidney function, hemoglobin and platelet levels, with the McCluskey index being the most used to date due to its ease of calculation.12
The clinical implications of polytransfusion have been studied in the short term and up to 90 days post-LT,3,9–11 however there are no long-term data on the influence of polytransfusion on patient and graft survival.
Furthermore, most of the published literature to date has only considered variables related to the recipient, disregarding the influence that the donor may have on the transfusion of blood products during implantation, which is especially important in donors with greater ischemic stress. So far, there is only limited information on risk of transfusion and subsequent survival in selected donor types, such as uncontrolled donors after circulatory death (uDCD),13 split liver,14 or living donor grafts.15 Our study aims to identify a simple and clinically useful index to predict massive transfusion of blood products during the first 24 h after LT, which incorporates donor variables in the analysis, and compare the resulting index to existing scores. Furthermore, we will explore the long-term effects of MBT on patient survival, in one of the largest series, with over 20 years of follow-up.
MethodsStudy population and designWe performed a total of 1469 L T at our institution from May 2003 to December 2020. We had complete data regarding blood product transfusion for 1198 patients, which were included in our study. We excluded patients under 18 years of age, multivisceral or cardiohepatic transplants, classic technique (cava vein resection), and patients requiring venovenous bypass from this study. We defined MBT as the use of more than 6 units of PRBCs in the first 24 h post-LT (in accordance with the definition by McCluskey et al.6), and we divided patients in two groups according to whether or not they met this transfusion criterion: MBT group (n = 607) and non-MBT group (n = 591). A retrospective study was performed to compare the characteristics of both patient group. Patient follow-up was completed until March 1, 2022, or until the date of death. This study was approved by our institutional review board and was registered in the Research Registry (nº22/52). This study was conducted and reported according to the declaration of Helsinki. All data generated or analyzed during this study are available upon request.
Transplant methodology and transfusionAll patients underwent a LT using the vena cava preservation technique (piggy-back) under general anesthesia, and monitored using an arterial line and a Shaldon vascath. Noradrenaline and phenylephrine were used to increase vascular tone as needed. Patients had a full blood panel, including arterial blood gasses (ABG), at the beginning of the anhepatic phase, prior to reperfusion and again post-reperfusion. Plasma and blood transfusion therapy was performed according to a standardized algorithm based on the results of laboratory coagulation status and thromboelastometry (ROTEM®, Pentapharm GMBH, Munich, Germany).
The criteria for administering blood components were as follows: 1) PRBCs were transfused when the hemoglobin levels were below 8 g/dL; 2) Fresh frozen plasma (FFP) was given if the International Normalized Ratio (INR) was greater than 1.2 or if the prothrombin time (in seconds) as measured by the extrinsic system test (EXTEM) was greater than 80 s; 3) Platelets were administered to maintain a platelet count above 50 × 109/L or a “maximum clot firmness” greater than 35 mm according to EXTEM; 4) Exogenous fibrinogen was given if plasma levels were less than 150 mg/dL or if maximum clot firmness was less than 8 mm in the fibrinogen coagulation test.16,17 Tranexamic acid (10 mg/kg bolus followed by a 1 mg/kg/h infusion) was indicated if there was diffuse bleeding of nonsurgical origin or significant hyperfibrinolysis assessed by thromboelastometry (Lysis at 30 min; CLI30 EXTEM CA5; 50%).
Recipient variablesWe analyzed the following patient characteristics in both groups: age, sex, body mass index, LT indication, type of LT (use of liver grafts from living-donor liver transplantation [LDLT], split-liver transplantation [SLT] and uncontrolled donor circulatory death [uDCD]), the recipient’s pre-LT laboratory values, MELD and MELD-Na scores, McCluskey index, blood product transfusion, ICU and ward stay, 1-, 3-, and 5-year patient and graft survival.
Laboratory values were collected from blood samples drawn at admission on the day of the LT. Likewise, the number of uPRBC transfused were collected from anesthesiology and intensive care unit (ICU) records, which are the two locations where patients remain during the first 48 h post-LT.
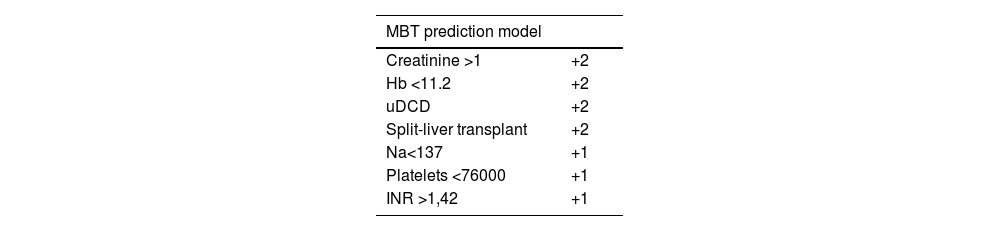
Statistical analysisQualitative variables are expressed as absolute numbers and relative frequencies are expressed as percentages. Associations were analyzed using Chi squared or Fischer test, as needed. Most of the quantitative variables did not have a normal distribution according to the Kolmogorov–Smirnov test, therefore all the quantitative variables were expressed as medians and percentiles expressed as 0 and 100. The relationship between quantitative variables was analyzed using the Mann–Whitney U test. We converted all the statistically significant variables in the group analysis into dichotomous variables, based on the median value of the MBT group. We subsequently used binary logistic regression to calculate the MBT odds ratio, generating a model from the final group of significant variables. Our prediction model added two points to the variables with an OR of MBT greater than 1.9 and added a single point to those with OR < 1.9. Survival analysis was performed using the Kaplan-Meier estimator, using the Log-rank test. A p value <0.05 was considered statistically significant.
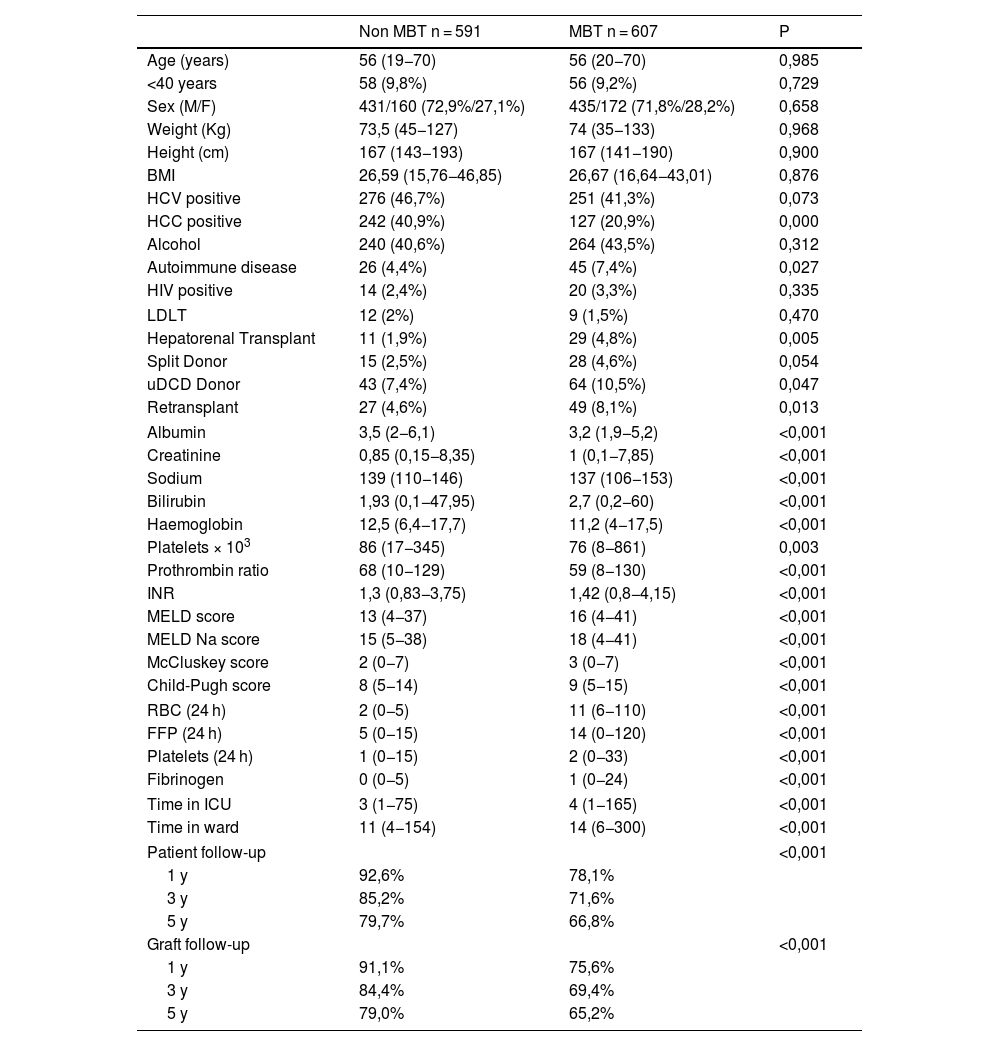
ResultsOf the 1198 patients studied, 607 (50.7%) patients fit criteria for MBT. Of the studied characteristics, the groups showed statistically significant differences regarding the presence of hepatocarcinoma, autoimmune diseases, hepatorenal transplant, use of uncontrolled donor circulatory death (uDCD), retransplantation, serum albumin values, creatinine, sodium, bilirubin, pre-LT hemoglobin values, platelet count, INR, and MELD, McCluskey and Child scores.
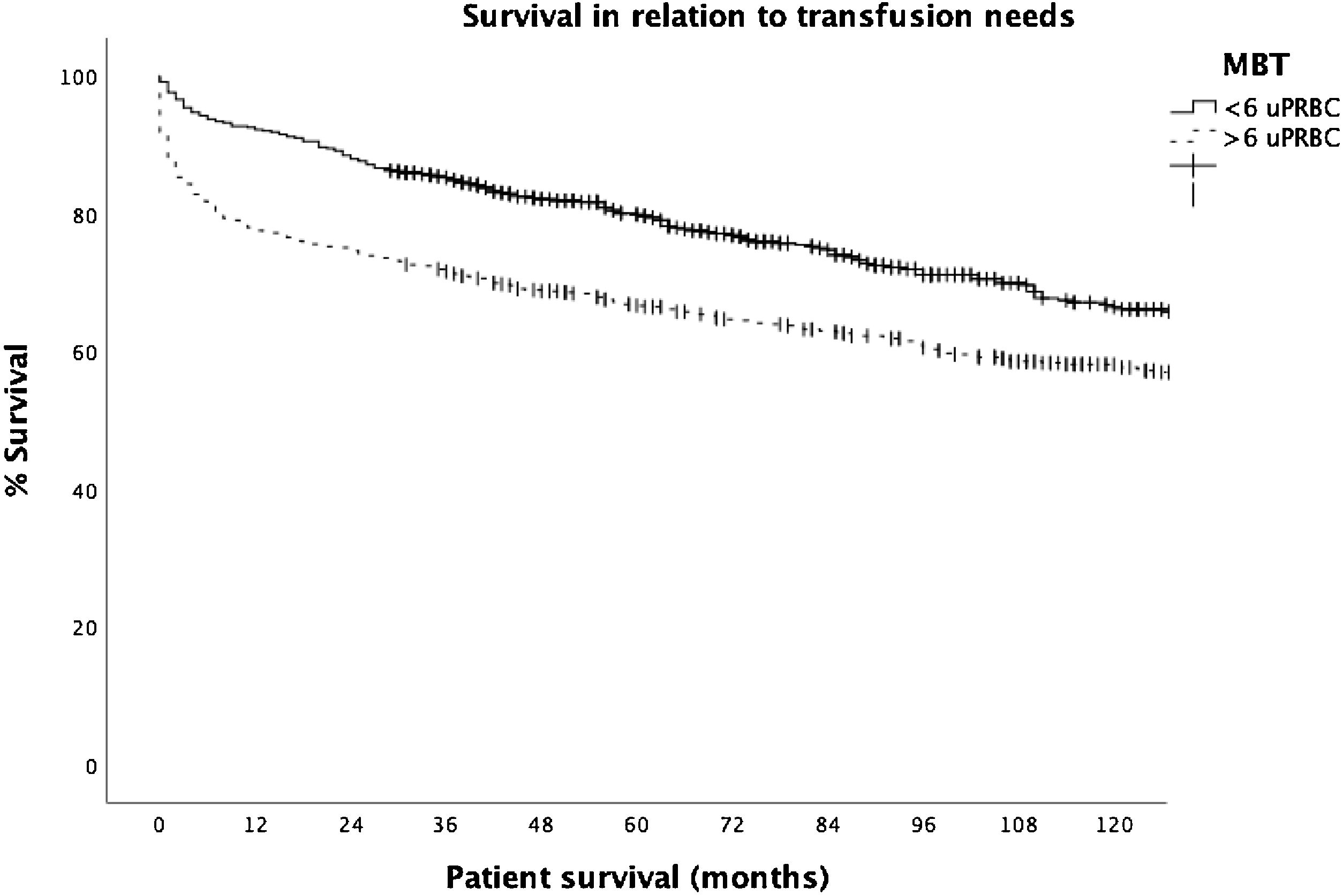
The number of transfused blood products other than blood (FFP, platelets, and fibrinogen) was significantly higher in patients in the MBT group. In addition, the length of ICU and hospital stay were significantly more prolonged in patients in the MBT group. This is also true for survival, where patient survival at 1-, 3-, and 5-years were significantly lower in patients in the MBT group (78.1%, 71.6%, and 66.8%, respectively) as compared to the non-MBT group (92.6%, 85.2%, and 79.7%, respectively); (P ≤ 0.001) (Table 1; Fig. 1).
Patient characteristics, stratified by presence or absence of massive blood transfusion.
| Non MBT n = 591 | MBT n = 607 | P | |
|---|---|---|---|
| Age (years) | 56 (19−70) | 56 (20−70) | 0,985 |
| <40 years | 58 (9,8%) | 56 (9,2%) | 0,729 |
| Sex (M/F) | 431/160 (72,9%/27,1%) | 435/172 (71,8%/28,2%) | 0,658 |
| Weight (Kg) | 73,5 (45−127) | 74 (35−133) | 0,968 |
| Height (cm) | 167 (143−193) | 167 (141−190) | 0,900 |
| BMI | 26,59 (15,76−46,85) | 26,67 (16,64−43,01) | 0,876 |
| HCV positive | 276 (46,7%) | 251 (41,3%) | 0,073 |
| HCC positive | 242 (40,9%) | 127 (20,9%) | 0,000 |
| Alcohol | 240 (40,6%) | 264 (43,5%) | 0,312 |
| Autoimmune disease | 26 (4,4%) | 45 (7,4%) | 0,027 |
| HIV positive | 14 (2,4%) | 20 (3,3%) | 0,335 |
| LDLT | 12 (2%) | 9 (1,5%) | 0,470 |
| Hepatorenal Transplant | 11 (1,9%) | 29 (4,8%) | 0,005 |
| Split Donor | 15 (2,5%) | 28 (4,6%) | 0,054 |
| uDCD Donor | 43 (7,4%) | 64 (10,5%) | 0,047 |
| Retransplant | 27 (4,6%) | 49 (8,1%) | 0,013 |
| Albumin | 3,5 (2−6,1) | 3,2 (1,9−5,2) | <0,001 |
| Creatinine | 0,85 (0,15−8,35) | 1 (0,1−7,85) | <0,001 |
| Sodium | 139 (110−146) | 137 (106−153) | <0,001 |
| Bilirubin | 1,93 (0,1−47,95) | 2,7 (0,2−60) | <0,001 |
| Haemoglobin | 12,5 (6,4−17,7) | 11,2 (4−17,5) | <0,001 |
| Platelets × 103 | 86 (17−345) | 76 (8−861) | 0,003 |
| Prothrombin ratio | 68 (10−129) | 59 (8−130) | <0,001 |
| INR | 1,3 (0,83−3,75) | 1,42 (0,8−4,15) | <0,001 |
| MELD score | 13 (4−37) | 16 (4−41) | <0,001 |
| MELD Na score | 15 (5−38) | 18 (4−41) | <0,001 |
| McCluskey score | 2 (0−7) | 3 (0−7) | <0,001 |
| Child-Pugh score | 8 (5−14) | 9 (5−15) | <0,001 |
| RBC (24 h) | 2 (0−5) | 11 (6−110) | <0,001 |
| FFP (24 h) | 5 (0−15) | 14 (0−120) | <0,001 |
| Platelets (24 h) | 1 (0−15) | 2 (0−33) | <0,001 |
| Fibrinogen | 0 (0−5) | 1 (0−24) | <0,001 |
| Time in ICU | 3 (1−75) | 4 (1−165) | <0,001 |
| Time in ward | 11 (4−154) | 14 (6−300) | <0,001 |
| Patient follow-up | <0,001 | ||
| 1 y | 92,6% | 78,1% | |
| 3 y | 85,2% | 71,6% | |
| 5 y | 79,7% | 66,8% | |
| Graft follow-up | <0,001 | ||
| 1 y | 91,1% | 75,6% | |
| 3 y | 84,4% | 69,4% | |
| 5 y | 79,0% | 65,2% | |
BMI, body mass index; HCV, hepatitis C Virus; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; LDLT, living donor liver transplantation; uDCD, uncontrolled donor after cardiac death; RBC, red blood cells; FFP, fresh frozen plasma; ICU, intensive care unit.
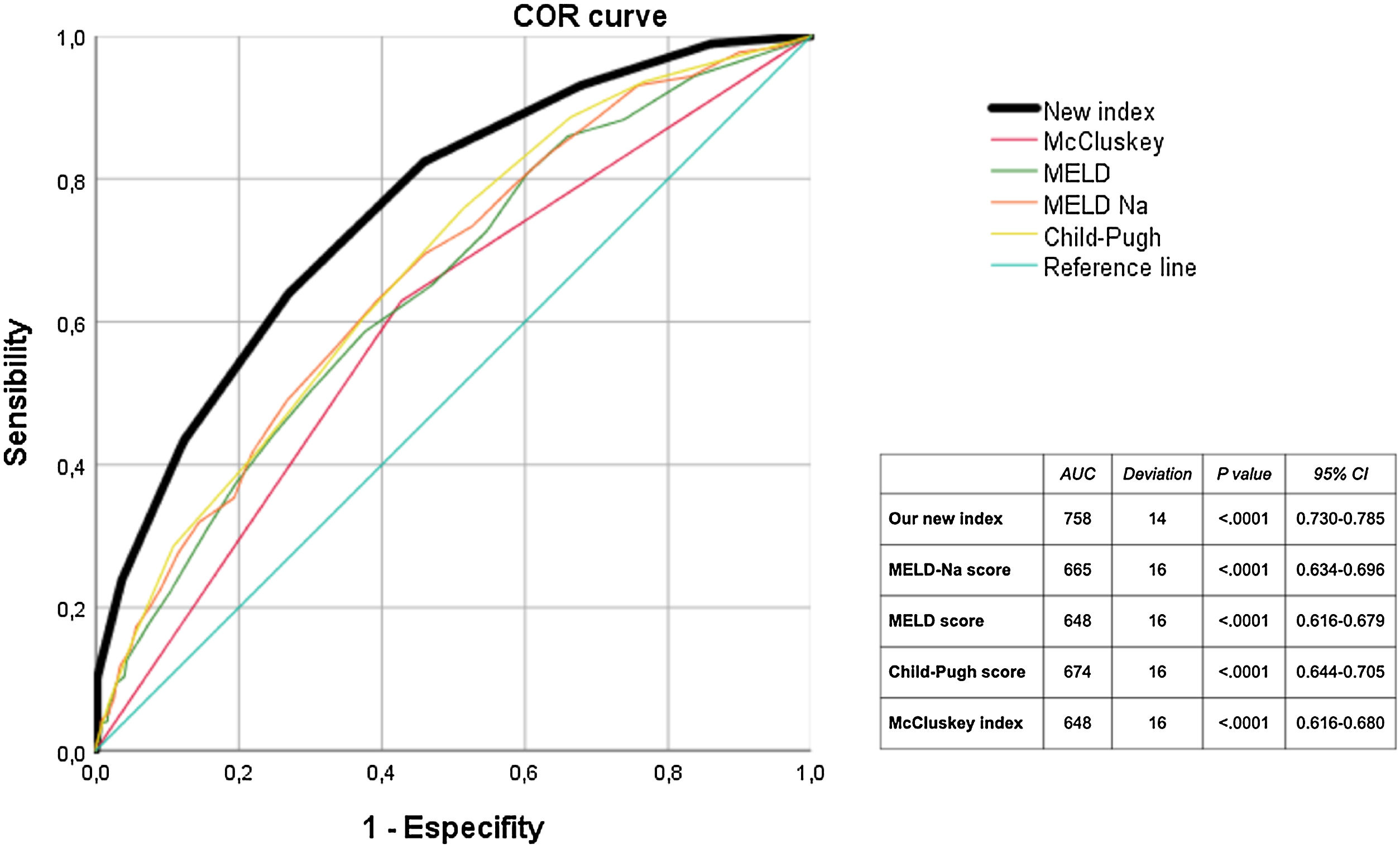
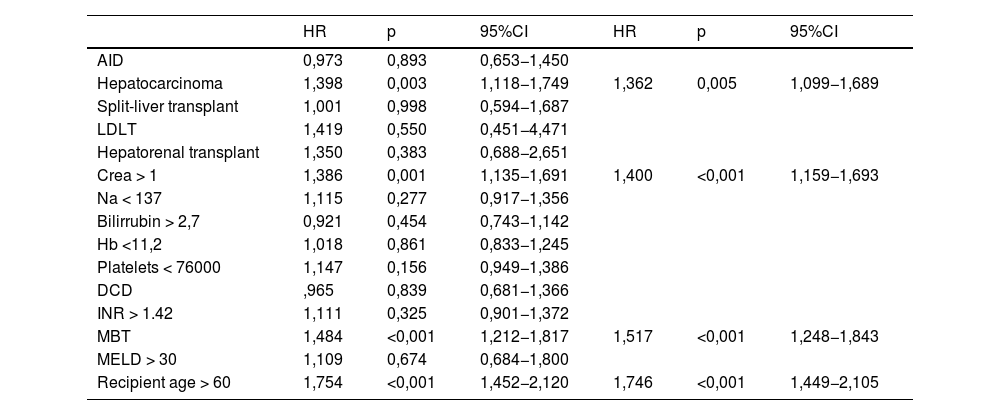
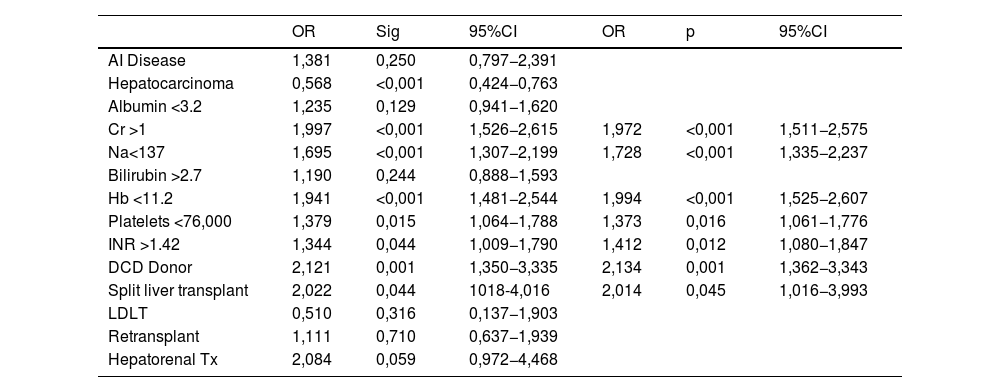
In the Cox regression analysis, we demonstrated that several factors, such as the presence of hepatocarcinoma (HR, 1.362), creatinine level (HR 1.4), recipient age >60 yr (HR 1.746), and MBT (HR 1.517) influenced patient survival (Table 2). Through logistic regression, we selected the variables for a new model that included serum creatinine and sodium, hemoglobin, platelets, international normalized ratio (INR), uDCD donor, and split liver donor (Table 3). We created a new model based on the OR of the statistically significant variables (Table 4). Patient survival in MBT group was lower than in non–MBT group along the follow-up. This model had an area under the curve (AUC) of 0.758 for predicting MBT, which is much higher than that for MELD and Child scores or McCluskey index (Fig. 2).
Cox regression analysis over survival.
| HR | p | 95%CI | HR | p | 95%CI | |
|---|---|---|---|---|---|---|
| AID | 0,973 | 0,893 | 0,653−1,450 | |||
| Hepatocarcinoma | 1,398 | 0,003 | 1,118−1,749 | 1,362 | 0,005 | 1,099−1,689 |
| Split-liver transplant | 1,001 | 0,998 | 0,594−1,687 | |||
| LDLT | 1,419 | 0,550 | 0,451−4,471 | |||
| Hepatorenal transplant | 1,350 | 0,383 | 0,688−2,651 | |||
| Crea > 1 | 1,386 | 0,001 | 1,135−1,691 | 1,400 | <0,001 | 1,159−1,693 |
| Na < 137 | 1,115 | 0,277 | 0,917−1,356 | |||
| Bilirrubin > 2,7 | 0,921 | 0,454 | 0,743−1,142 | |||
| Hb <11,2 | 1,018 | 0,861 | 0,833−1,245 | |||
| Platelets < 76000 | 1,147 | 0,156 | 0,949−1,386 | |||
| DCD | ,965 | 0,839 | 0,681−1,366 | |||
| INR > 1.42 | 1,111 | 0,325 | 0,901−1,372 | |||
| MBT | 1,484 | <0,001 | 1,212−1,817 | 1,517 | <0,001 | 1,248−1,843 |
| MELD > 30 | 1,109 | 0,674 | 0,684−1,800 | |||
| Recipient age > 60 | 1,754 | <0,001 | 1,452−2,120 | 1,746 | <0,001 | 1,449−2,105 |
AID, autoimmune disease; DCD, donor from cardiac death; LDLT, living donor liver transplantation; INR, international normalized ratio; MELD, model for end stage liver disease; MBT, massive blood transfusion.
Logistic binary regression for MBT.
| OR | Sig | 95%CI | OR | p | 95%CI | |
|---|---|---|---|---|---|---|
| AI Disease | 1,381 | 0,250 | 0,797−2,391 | |||
| Hepatocarcinoma | 0,568 | <0,001 | 0,424−0,763 | |||
| Albumin <3.2 | 1,235 | 0,129 | 0,941−1,620 | |||
| Cr >1 | 1,997 | <0,001 | 1,526−2,615 | 1,972 | <0,001 | 1,511−2,575 |
| Na<137 | 1,695 | <0,001 | 1,307−2,199 | 1,728 | <0,001 | 1,335−2,237 |
| Bilirubin >2.7 | 1,190 | 0,244 | 0,888−1,593 | |||
| Hb <11.2 | 1,941 | <0,001 | 1,481−2,544 | 1,994 | <0,001 | 1,525−2,607 |
| Platelets <76,000 | 1,379 | 0,015 | 1,064−1,788 | 1,373 | 0,016 | 1,061−1,776 |
| INR >1.42 | 1,344 | 0,044 | 1,009−1,790 | 1,412 | 0,012 | 1,080−1,847 |
| DCD Donor | 2,121 | 0,001 | 1,350−3,335 | 2,134 | 0,001 | 1,362−3,343 |
| Split liver transplant | 2,022 | 0,044 | 1018-4,016 | 2,014 | 0,045 | 1,016−3,993 |
| LDLT | 0,510 | 0,316 | 0,137−1,903 | |||
| Retransplant | 1,111 | 0,710 | 0,637−1,939 | |||
| Hepatorenal Tx | 2,084 | 0,059 | 0,972−4,468 |
Although there is no homogeneous definition in the literature, authors agree that mass transfusion of blood products is associated with worse patient and graft prognosis, as well as an increase in length of hospital stay and ICU admission.3–6,9,10,18 Traditionally, massive bleeding has been correlated with a worse hepatic functional reserve in the recipient, and is associated with more complex surgeries.3,11 In recent years, the improvement of surgical and anesthetic techniques has resulted in a reduction in transfusion rates in most series. This is especially true with better recipient selection and with an increased use of tromboelastometry to guide transfusion.1,19
The effect of sex and body mass index on MBT has not been proven in studies to date. This is significant, considering obesity may be a risk factor for an increased difficulty in surgical dissection. Although certain studies had identified age below 40 as a risk factor for transfusion,6,9 there are several studies, such as ours, where a significant relationship is not established.5,10
We did not find a relationship between the underlying cause for LT and massive transfusion, which mirrors the results of most studies. This is probably due to the fact that transfusion needs likely depend more on the time at which the disease is transplanted and the degree of hepatocellular dysfunction that it conditions, rather than the etiology itself. This would also justify the fact that most of the patients transplanted for hepatocarcinoma, who have a better functional status, have a lower risk of MBT, but a lower long-term survival, as in most of the published series.8,9,20 This is also likely associated, at least in part, with the broadening of LT criteria and the use of suboptimal donors.21,22
In our study, there is a statistically significant difference in transfusion of blood products with different types of donors, as well as for cases of retransplantation. The fact that these grafts present greater ischemic stress,23–25 as well as a more technically challenging hepatectomy in retransplantation cases, would explain the greater need for blood product transfusion.5,26 This is perfectly described in Azuley's recent definition of surgical difficulty in LT.25 A higher rate of polytransfusion-related biliary complication has also been noted in split liver transplantations,14 and uDCD donors.27
In our analysis, as in most of the reported series, there is a significant correlation between renal function and blood product transfusion rates. Poor renal function pre-transplant has been linked to short-term LT outcomes and increased transplant costs,28 and it appears that the presence of chronic kidney disease may aggravate liver dysfunction.29
The variables related to the hematological state prior to LT largely condition transfusion needs; hence hemoglobin, platelets or INR appear almost universally in most predictive indexes6,9,10,30 although not all studies have managed to identify them as statistically significant.31 Nonetheless, it seems logical and biologically plausible that low pre-LT hemoglobin levels are inversely related to transfusion needs, as well as a direct relationship with coagulopathy.
In our sample, the MELD score was the most sensible score to predict the need for MBT. Previous studies had already mentioned the accuracy of this score,20,32,33 in particular when combining it with preoperative hemoglobin levels.30 Unlike the McCluskey study, our study did not correlate retransplantation or patient age with increased transfusion in the multivariate analysis.
In our study, the type of donor significantly affects the likelihood of MBT. It must be noted, however, that our series has a significant local bias, since most centers do not use uDCD donors, and our analysis showed this type of donor is strongly associated with MBT. This difference highlights the many difficulties in finding a universal index, due to the large local biases and intrinsic differences in practices between centers. The importance of having an accurate index that predicts a high likelyhood of MBT lies in allowing the transplant center to prepare for polytransfusion, alerting the regional or national bloodbanks, which would be especially important for less frequent blood groups, such as B or AB groups.
Most of the studies so far have enjoyed great internal validity. However, this is expected as the samples on which the statistical analyses are carried out and tested are the same. The only index that has been externally validated by other centers is the McCluskey index,32,34 however the area under the curve (AUC) was less than 0.7. This lack of external validation is surprising, as most of the indexes share many of the same variables, and there is an obvious need for this type of validation.
On the other hand, there are indexes that have better performance on ROC curves than the McCluskey index, but at the expense of being far more complex, making them difficult to adopt universally or become clinically applicable at the bedside.9 Hence, there is a need to have both simple parameters and formulas, to achieve a more widespread use. The new index generated by our sample meets this, as it has a great AUC and a very good internal validity. Nevertheless, it is possible that its external validity may be limited in centers with a greater experience than ours in split liver transplantation, since we perform only one or two cases a year, which may justify our local higher transfusion rate. A very interesting consideration is that in our sample, the more complex indexes do not show a greater sensitivity in predicting MBT. In fact, the best AUC for MBT is achieved with the MELD-Na score, higher than the McCluskey index even.
Our series is also important because of the prolonged follow-up period, and the fact that it is the first to definitively show the association between massive transfusion and a decrease in long-term survival, which had only been tangentially explored in two other reports.10,11 In fact, the impact on survival is so significant, that massive transfusion poses the same survival risks as that of having a recipient above the age of 60 years. This fact may be due both to MBT being a surrogate marker for a more technically complex transplant, as well as to the biological stress generated by polytransfusion, and its long-term effects. In facto polytransfusion is associated with worse survival and higher recurrence rates in major liver surgery for malignancy.35,36
It is possible that, in the future, donor factors may contribute less significantly to the risk of transfusion, as perfusion machines may optimize grafts and may have an associated decrease in ischemic stress.37,38
Our study has the limitations typically associated with a retrospective study, as well as the fact that we have included patients from a long time ago. This is, however, counteracted by including one of the largest sample sizes in published works on the topic.
In conclusion, massive transfusion of blood products significantly decreases patient survival after LT. We have generated a new index that is both more sensitive and specific than the previously published indexes, and easier to use, increasing its clinical applicability.
Author contributionsAll authors approved the submitted manuscript and have made important contributions to this research. Iago Justo, Carlos Jiménez-Romero, and Cristina Rivas: writing the article; Iago Justo, Carlos Jiménez-Romero, Cristina Rivas, Oscar Caso, Alberto Marcacuzco: study design, interpretation, drafting, and critical review of the article; Iago Justo, Carlos Jiménez-Romero, Adolfo García, Alberto Marcacuzco, Alejandro Manrique, and Alvaro García-Sesma: data collection, and data analysis.
Ethical approvalThe approval of the Ethics Committee of the” Doce de Octubre” University Hospital was waived due to the retrospective nature of the study.
Conflict of interestThe authors declare no conflict of interest.
The data that support the findings of this study are available from the corresponding author upon reasonable request
The authors acknowledge the medical students Alcoba L, Alvarez P, Otero B for their contribution to collect the data.