Adenocarcinoma (ADC) of the anal canal is a rare disease comprising only 5% of all anorectal neoplasias and 1.5% of all gastrointestinal tumors. The World Health Organization classifies anal ADC into 3 types: the first may arise from the mucosa of the transitional zone in the upper canal, the second from the anal glands (ducts) and the last can develop in the environment of a chronic anorectal fistula. Patients with ADC of the anal canal have high rates of pelvic failure, distant metastasis, and lower overall survival than patients with epidermoid carcinoma. Because of limited case reports about this neoplasia, management strategies have not been well established. Most authors of related studies recommend preoperative chemoradiotherapy (CRT) followed by radical surgery. The aim of the present study is to review clinicopathology features and management of anal canal ADC.
El adenocarcinoma (ADC) del canal anal es una entidad rara que representa el 5% de todas las neoplasias anorrectales y un 1,5% de los tumores gastrointestinales. De acuerdo con la Organización Mundial de la Salud se pueden distinguir 3 tipos: el primero tiene su origen en la mucosa de transición del canal superior, el segundo deriva de las glándulas (ductos) anales, y el último deriva de una fístula perianal crónica. Los pacientes con ADC del canal anal presentan mayor porcentaje de enfermedad avanzada, de metástasis a distancia y menor supervivencia global que aquellos con carcinoma escamoso. La escasa casuística publicada sobre esta neoplasia implica que no existe un esquema terapéutico plenamente comprobado. La mayoría de los autores abogan por un tratamiento con quimiorradioterapia (QRT) neoadyuvante seguido de cirugía radical. El objetivo de este artículo es realizar una revisión de la literatura existente sobre las características clinicopatológicas y el manejo del ADC del canal anal.
The anal canal is the terminal part of the large intestine; it is a tubular structure measuring 3–4cm that extends from the perianal skin up to the end of the rectum. The superior portion is covered with rectal mucosa, the middle part (coinciding with the pectineal line) with transitional mucosa and the inferior portion with mucosa with stratified squamous epithelium.1
Carcinomas in this region are classified as anal canal cancer and the patterns of differentiation are, mainly, basaloid (basically squamous in nature, similar to its homonym in the upper respiratory/digestive tract), epidermoid, analogous to skin tumors, and those with a line of differentiation toward adenocarcinoma.1
The objective of this article is to review the literature on the histopathology, symptoms, diagnosis and treatment of adenocarcinoma of the anal canal.
MethodologyWe reviewed the literature published in the MEDLINE/Pubmed and Ovid databases from 1997 to 2012, using the following keywords “anal adenocarcinoma”, “anal neoplasm”, “anal gland carcinoma”, “anal duct carcinoma”, “anal canal”, “immunohistochemistry”, “chemoradiotherapy” and “radical surgery”.
HistopathologyAlthough the anal canal is short in length, it can present a great variety of tumors, which reflects the anatomic, embryologic and histologic complexities of this structure. Tumor localization and the interpretation of morphologic findings are both controversial and, occasionally, very difficult.2
Adenocarcinoma (ADC) of the anal canal is a rare entity. Most cases of ADC have a colorectal phenotype and represent tumors derived from the upper part of the anal canal or cells with glandular characteristics from the transitional zone. Distinguishing between true anal canal ADC and lower rectal ADC with extension to the anal canal can be extremely difficult. According to the World Health Organization (WHO), three types of ADC can be distinguished, mainly determined by their origin: those that originate in the superior part of the anal canal, those that are derived from anal ducts or glands and those associated with chronic anorectal fistulae2,3:
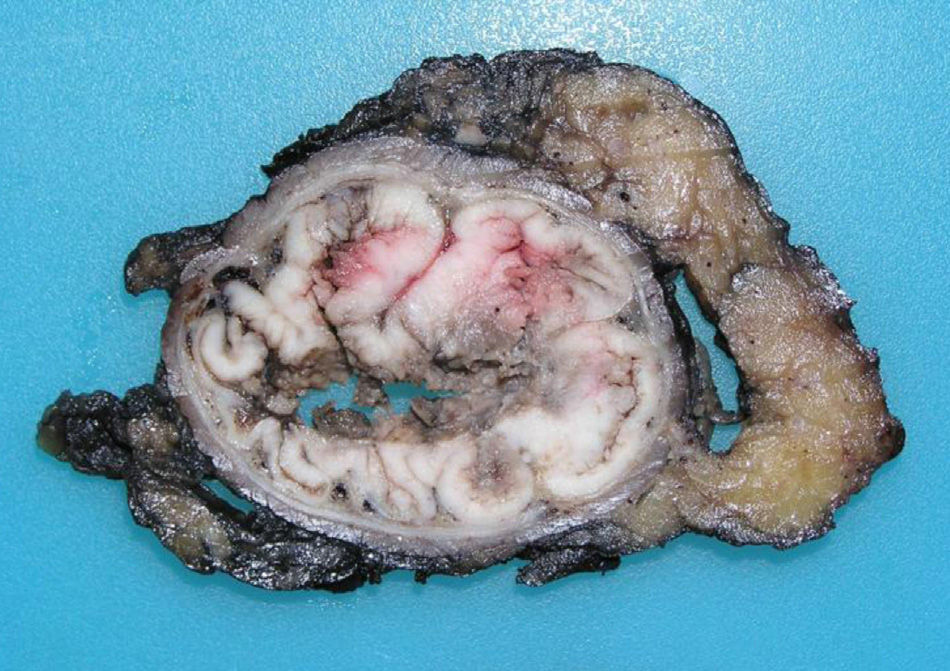
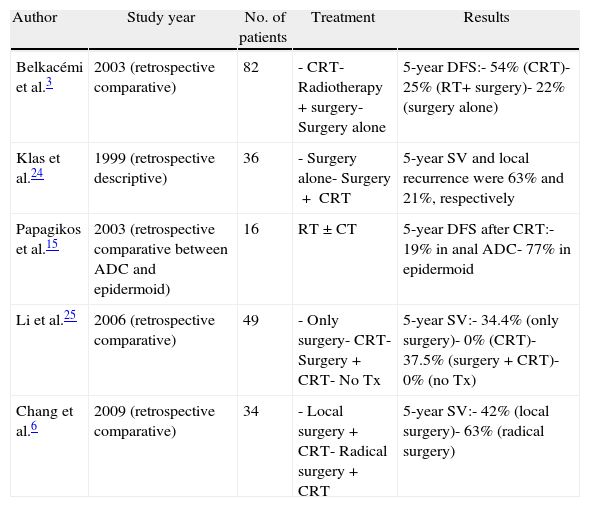
Tumors that originate in the mucosa of the superior portion of the anal canal are the most common (Fig. 1) and present with a colorectal phenotype. In evolved stages, it is extremely difficult to differentiate them from distal rectal ADC. Their main clinical implication is based on their capacity for local extension, owing to the double lymphatic drainage toward the inguinal and femoral chains. The usual immunohistochemical phenotype coincides with the immunohistochemical profile of ADC of the lower rectal segment, consisting in CK20+, CK7− and CDX2+. CK7 is occasionally positive, as can also occur exceptionally in rectal ADC, but it would similarly and characteristically co-express CK20.2,4,5
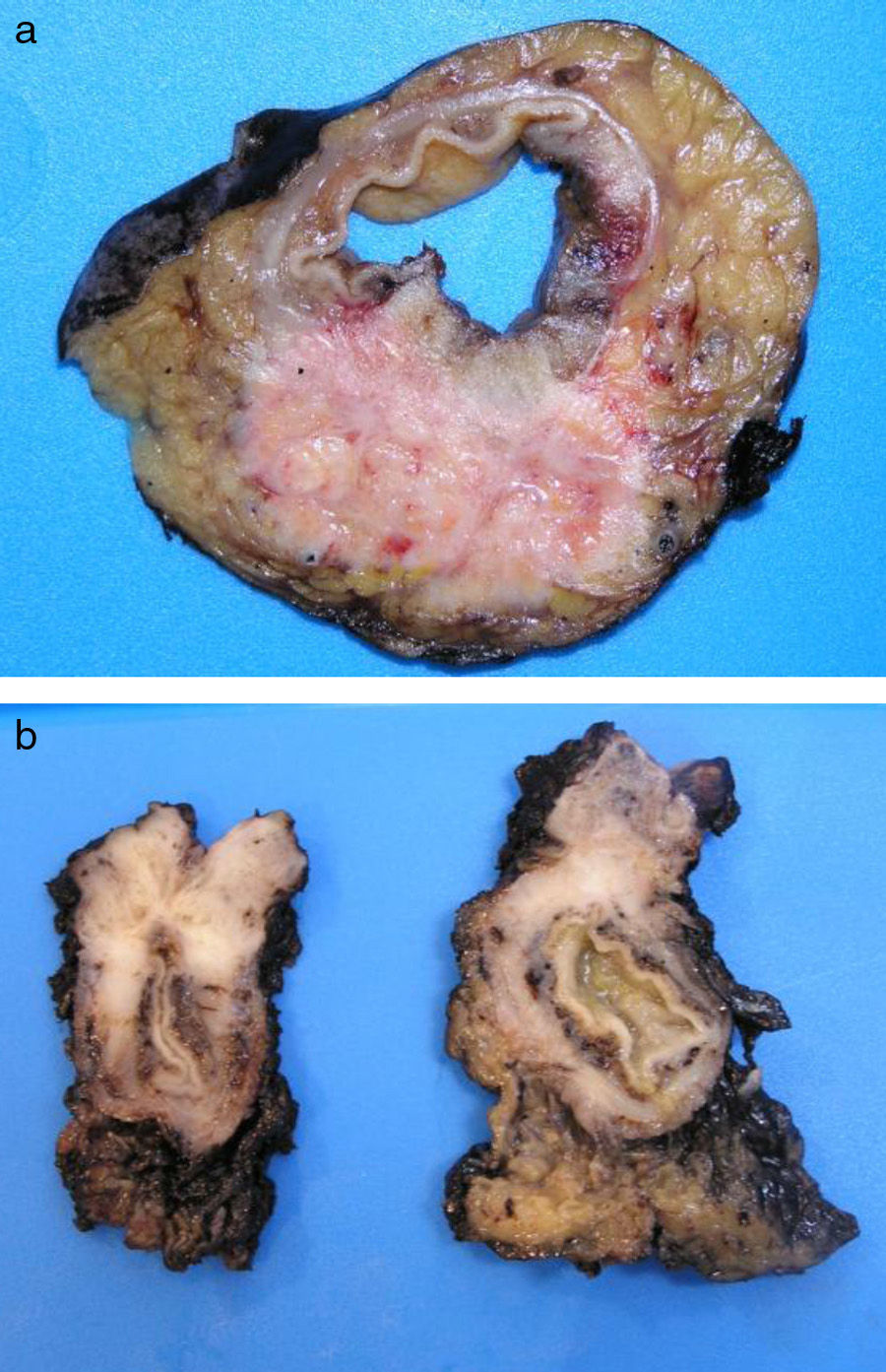
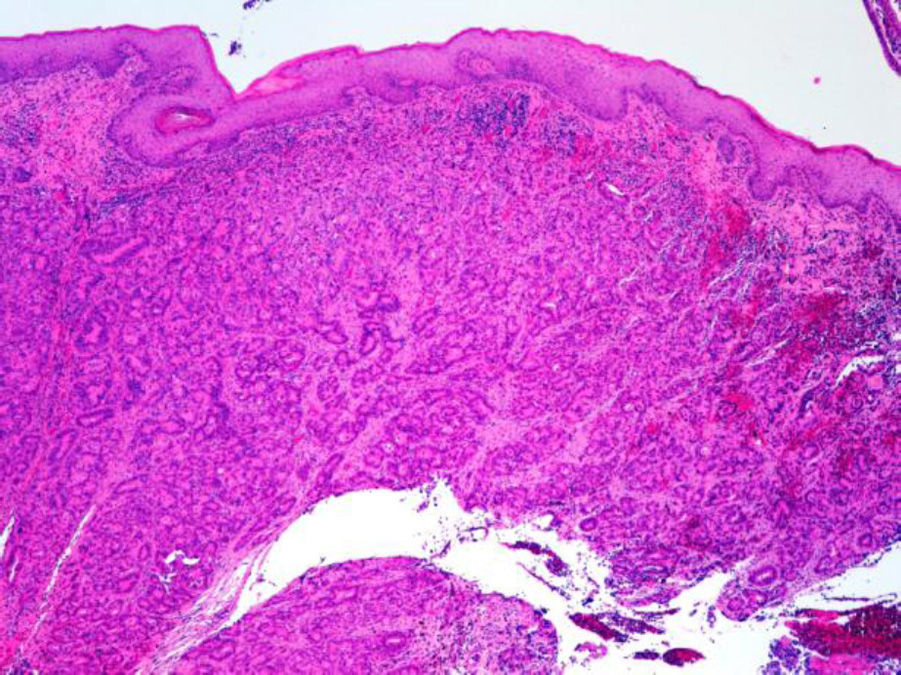
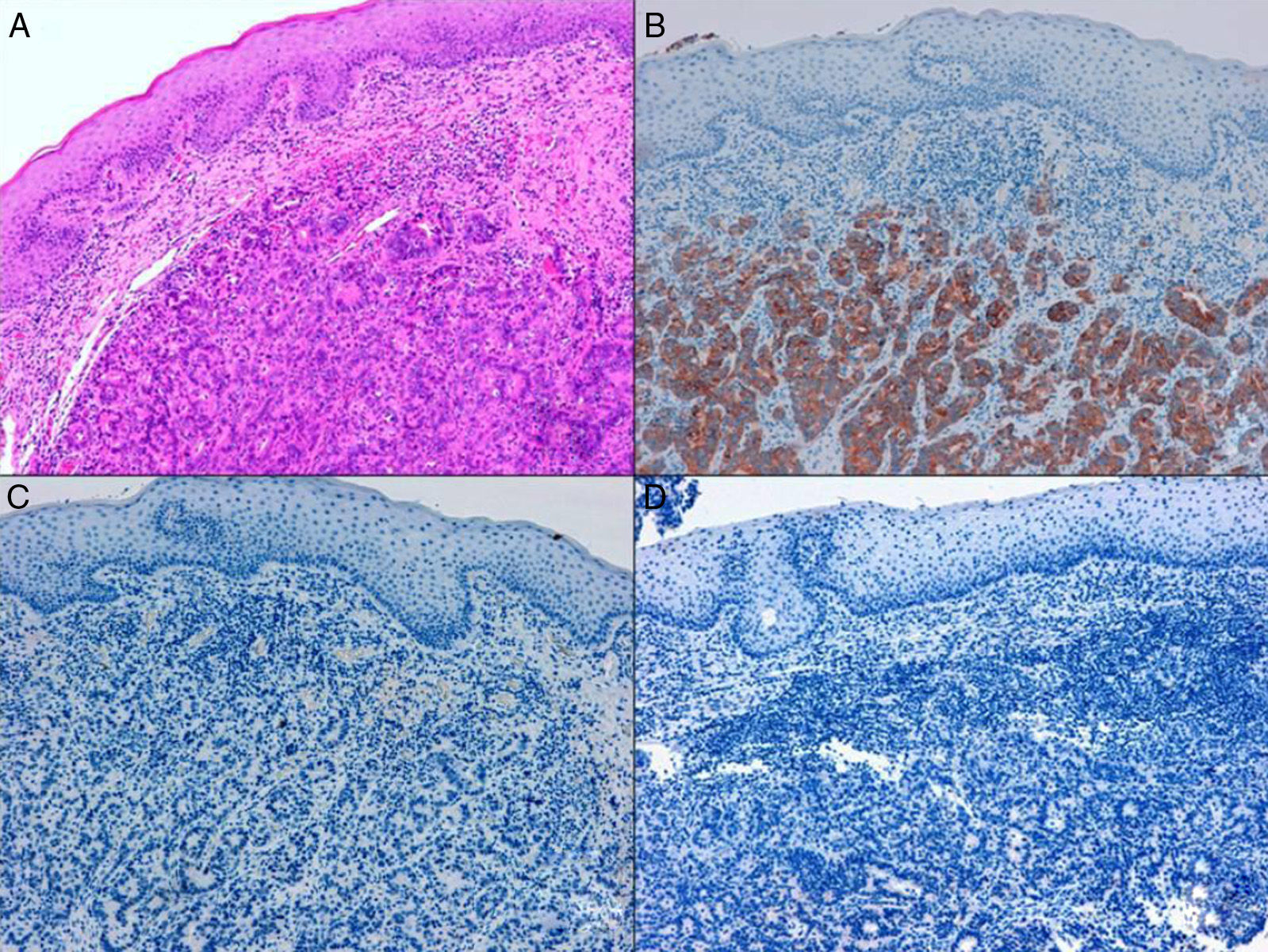
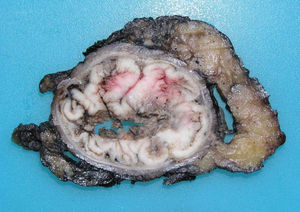
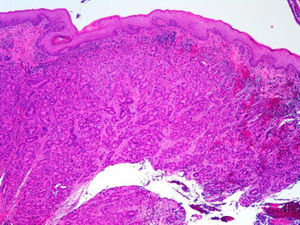
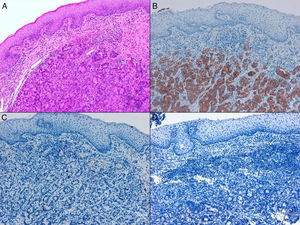
ADC that develops in the duct or glands of the anal canal (anal ductal ADC or anal gland ADC) is extremely rare. It corresponds with a specific intramural subtype of anal canal ADC (Fig. 2A and B). Its diagnosis is sometimes done by exclusion, since histologically detecting normal gland elements and associated ducts, or extension of ADC, generally occurs in very early stages. The recent definition of anal gland ADC by Hobbs et al.4 does not require demonstrating this continuity with the anal canal glands. In addition to the morphological characteristics of the neoplasia, what has acquired greater protagonism in the diagnosis is that no intraluminal growth is observed (the normal glands of the anal canal are distributed in the submucosal layer, penetrating the sphincter musculature and even reaching the perianal fat). Furthermore, this tumor subtype is not associated with dysplasia of the mucosal surface (Fig. 3) and is not generally related to pre-existing fistulae. These tumors may produce small quantities of mucin and their immunochemical profile is CK20−, CK7+ and CDX2− (Fig. 4), comparable to the immunohistochemical profile of normal glands of the anal canal and similar to the profile of the transitional epithelium of the anal canal.2,4–8
(A) Submucosal neoplastic gland component in ADC of the anal glands (hematoxylin and eosin ×20); (B) ADC of the anal glands with positive immunoexpression in the glands for CK7 (cytokeratin 7×10); (C) ADC of the anal glands negative for CK20 (cytokeratin 20×10); (D) ADC of the anal glands negative for CDX2 (CDX 2×10).
Finally, ADC associated with chronic colorectal fistulae (congenital or acquired, with a long-term evolution of 10–20 years) is usually secondary to chronic inflammatory processes such as Crohn's disease or other evolved benign perianal diseases. Sometimes, fistulae are secondary to dilatations of the anal canal glands or ducts, and the morphology and immunohistochemistry of the tumor would be identical to that of anal gland/duct ADC. Jones and Morson9 have suggested that some of these carcinomas associated with fistulae originate in congenital duplications in the distal end of the hindgut. Generally, they adopt a well-differentiated mucinous ADC pattern, but the exact histogenetic origin is often impossible to demonstrate and may belong to any of the anterior subtypes. In addition, they must be differentiated from mucin-producing adenocarcinoma of the lower rectum. In this context, the immunohistochemical study (CK20, CK7 and CDX2) can be variable, and only by combining morphological, immunophenotypic and clinical-evolutive characteristics are we able to propose one origin or another.2,4,5,7–9
EpidemiologyAnal tumors are rare neoplasms of the digestive tube that represent 5% of all anorectal neoplasms and 1.5% of gastrointestinal tumors. The key microscopic aspects in anal canal tumors have progressively changed over the years. The increased experience and support of immunohistochemical techniques and molecular studies have brought about numerous changes in the nomenclature. The most frequent lesion (70%–80% of tumors) in both the anal canal as well as the perianal skin is squamous carcinoma.10–13 Around 10% of all malignant anal lesions are anal ADC (5%–19%), which present a more aggressive natural history than squamous carcinoma, with shorter survival times and higher rates of local as well as distant relapse.2,6,10,13–15
Although good results have been reported in patients with neoplasms detected in early evolutive phases, the clinical similarity with other benign diseases and the lower expression of the tumor component in the mucosa are usually reasons that delay diagnosis and, therefore, make for a poorer prognosis.16 A greater percentage of patients with anal canal ADC present with advanced disease, distant metastasis and, consequently, shorter overall survival compared with squamous carcinoma. Data from the National Cancer Data Base (NCDB) reveal that, at the time of presentation, 9.8% of patients with anal ADC are stage iv, compared with 5% of squamous carcinomas. Likewise, distant lesions occur in 28.1% of patients with ADC compared with 11.8% of squamous cancers. The 5-year survival rate in patients with ADC is poorer in all the stages than in patients with epidermoid carcinoma, with the greatest difference observed in stage iv (13% for patients with ADC and 29% for those with epidermoid cancer).15
The age of presentation is around the sixth decade of life, with equal distribution between the sexes.3,14 Multiple risk factors have been proposed, such as HPV and HIV, smoking and immunosuppression.10,17 The presence of chronic perianal fistulae, either with or without associated Crohn's disease, represents an important risk factor, especially with a history of 10 or more years of disease.17,18Lymphogranuloma venereum (LGV), as a cause of proctitis, can cause stenosis and perianal fistulae and is also associated with increased risk for rectal ADC.19
Symptoms and diagnosisThe clinical manifestations are usually nonspecific. Patients may present pain, indurations, abscesses, fistulae or palpable masses. Other symptoms include bleeding, pruritus, spotting, prolapse and weight loss.6,13 In the series by Kline et al.,20 15% of the patients were asymptomatic. Typical symptoms include the presence of perianal fistula for more than 10 years or the existence of recurring fistulae, even after surgery.20
Early diagnostic suspicion is crucial in order to avoid any delays in diagnosis or treatment. Although the clinical characteristics can lead us to suspect this type of tumor, the definitive diagnosis can only be established with biopsy and histological studies. In cases of advanced fistulous disease, it is not clear whether the biopsy should be taken from the anal canal close to the internal orifice or by curettage of the external orifice.17
Furthermore, inguinal lymphadenopathies should be explored because, as it has been explained previously, these are aggressive lesions with a high capacity for both local and distant invasion. Local dissemination tends to be greater in those tumors that originate in the glands of the anal canal or fistulous tracts since, as they are located outside the intestinal wall, the dissemination is initiated from a more advanced position.
Other diagnostic methods used to study local and distant extension include endoanal ultrasound, pelvic magnetic resonance and computed tomography.17,18
TreatmentThe limited number of cases published of anal canal ADC does not allow a thoroughly proven therapeutic approach.6 Until the 1990s, most authors recommended radical surgery (abdomino-perineal amputation [APA]) as the treatment of choice for anal canal ADC.21–23
In a retrospective analysis of 192 patients with anal canal cancer treated at the University of Minnesota, Klas et al.24 reported their experience with 36 patients diagnosed with ADC. Twenty-two (61%) were treated with surgery alone (6 APA and 16 local resections) and 14 (39%) with surgery followed by chemoradiotherapy (CRT). 5-year survival and local recurrence were 63% and 21%, respectively. The authors did not compare the two therapeutic methods due to the insufficient number of patients. They attributed the good results to the fact that the majority of the lesions (78%) were smaller than 5cm, and they propose treatment with pre- or postoperative CRT for larger lesions (>5cm).
A multicenter retrospective study that included 82 patients diagnosed with anal ADC treated in different European centers3 recommended combined CRT as the best treatment, while reserving radical surgery (APA) only for rescue therapy. In this study, the patients were managed with combined CRT, radiotherapy (RT) plus surgery or surgery alone. Overall survival and disease-free interval were higher in those patients with CRT, compared with those with RT plus surgery or surgery alone. The multivariate analysis showed that the T and N stages, histologic grade and therapeutic method were independent prognostic factors for survival. Nevertheless, this study has several shortcomings: neoadjuvant therapy was not used in any of the groups, adjuvant chemotherapy was not used after surgery, the patients who were included in the RT plus surgery group were significantly older than the patients in the combined CRT group, and in 75% of the surgical patients local resection was performed. These factors could be responsible for the high level of local and distant recurrence of the group with RT plus surgery.
Papagikos et al.15 studied 16 patients with anal ADC who were treated with RT either with or without chemotherapy with curative intent. The results of this treatment were compared with a group of patients with epidermoid carcinoma who were treated with CRT. Mean follow-up was 45 months for patients with ADC and 44 for those with epidermoid cancer. Although the patients with epidermoid carcinoma presented more advanced primary tumors, the local and distant recurrences were significantly higher in patients with ADC. Moreover, 5-year disease-free survival after CRT was 19% in patients with ADC compared with 77% of those with epidermoid carcinoma. This study concluded that treatment with definitive CRT, which has been demonstrated to be useful in epidermoid tumors, presents poor local control and a high level of distant recurrences in patients with ADC. They recommend preoperative CRT followed by APA to maximize the pelvic control of the disease. Adjuvant chemotherapy should be considered for micrometastatic disease.
A retrospective study by Li et al.25 that included 49 patients with anal canal ADC concluded that APA together with CRT is the recommended treatment. Five-year survival in patients with APA alone, CRT, APA plus CRT, and with no treatment was 34.4%, 0%, 37.5% and 0%, respectively.
Chang et al.6 published a series with more than 20 years of experience including 34 patients. The patients, who were considered potentially curable, were treated with local surgery followed by RT or CRT, or radical surgical treatment (APA) plus pre- or postoperative CRT. The disease-free survival was 13 months after local resection and 32 months after radical surgery, and overall 5-year survival was 43% for local treatment and 63% for patients treated with radical surgery (Table 1).
Studies About the Treatment of Anal ADC.
| Author | Study year | No. of patients | Treatment | Results |
| Belkacémi et al.3 | 2003 (retrospective comparative) | 82 | - CRT- Radiotherapy+surgery- Surgery alone | 5-year DFS:- 54% (CRT)- 25% (RT+ surgery)- 22% (surgery alone) |
| Klas et al.24 | 1999 (retrospective descriptive) | 36 | - Surgery alone- Surgery+ CRT | 5-year SV and local recurrence were 63% and 21%, respectively |
| Papagikos et al.15 | 2003 (retrospective comparative between ADC and epidermoid) | 16 | RT±CT | 5-year DFS after CRT:- 19% in anal ADC- 77% in epidermoid |
| Li et al.25 | 2006 (retrospective comparative) | 49 | - Only surgery- CRT- Surgery+CRT- No Tx | 5-year SV:- 34.4% (only surgery)- 0% (CRT)- 37.5% (surgery+CRT)- 0% (no Tx) |
| Chang et al.6 | 2009 (retrospective comparative) | 34 | - Local surgery+CRT- Radical surgery+CRT | 5-year SV:- 42% (local surgery)- 63% (radical surgery) |
CRT, chemoradiotherapy; DFS, disease-free survival; RT, radiotherapy; SV, survival; Tx, treatment.
Patients with inguinal lymphadenopathies at disease presentation have poorer prognoses due to the higher percentage of distant disease.15,18 According to some authors,3,6,26 the inguinal dose of RT should vary in correlation with the existence of inguinal affectation and the therapeutic modality. Therefore, it is recommended to use doses above 54–55Gy when initial CRT is used, while, in cases where only RT was used, the corresponding dose should be increased to 60–66Gy. In patients with no known inguinal node invasion who receive treatment with CRT either with or without associated surgery, the recommended prophylactic dose is 45Gy. Due to the poor prognosis of patients who present with inguinal lymphadenopathies, Papagikos et al.15 prioritize the use of initial CRT (with doses above 55Gy) and additional systemic chemotherapy, with surgical resection used selectively or for isolated local relapse.
Currently, most authors advocate maximizing the local control of the disease, avoiding transanal resection (which has had a negative impact on survival in some series),6,27 and diminishing the risk of metastasis with the use of intensive chemotherapy.
Given the fact that the anal glands are histologically and embryologically different from the anal squamous epithelium, the recommended chemotherapy regime does not usually include mitomycin. What is generally used are classic rectal AC regimes based on 5-fluorouracil, either with or without associated oxaliplatin.6,7,15
ConclusionsAnal canal adenocarcinoma is a rare entity that is occasionally difficult to distinguish from adenocarcinoma of the lower rectum with extension to the anal canal. Due to its aggressive behavior, early suspicion is crucial to avoid delayed diagnosis and treatment. Although there is no standardized protocol for the treatment of anal canal ADC, the current recommended approach is preoperative CRT followed by radical surgery (APA), with subsequent adjuvant therapy for the prevention of micrometastasis. CRT used alone should be reserved for those patients who would not tolerate radical surgery and, according to some authors, when there are proven inguinal lymph node metastases.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ferrer Márquez M, Velasco Albendea FJ, Belda Lozano R, Berenguel Ibáñez MM, Reina Duarte Á. Adenocarcinoma del canal anal. Revisión de conjunto. Cir Esp. 2013;91:281–286.