Immediate reconstruction (IBR) after mastectomy in patients who have received neoadjuvant chemotherapy (NACT) remains controversial. The aim of this study is to analyze and compare oncological results as well as complication and reoperation rates in patients undergoing NACT and a control group.
MethodsRetrospective observational case-control study of patients with breast cancer who underwent bilateral mastectomy and direct-to-implant IBR (BMIBR) from 2000-2016. The group that received NACT was matched 1:5 to patients without NACT (Control group).
We evaluated differences between groups using the Chi-squared or Fisher test (qualitative variables), Mann-Whitney U or Student’s t-test (quantitative variables). The survival analysis was performed using Kaplan-Meier curves and log-rank test (SPSS 22.0).
ResultsThe study included a total of 171 patients with BMIBR: 62 patients (36.3%) after NACT and 109 patients (63.7%) in the control group without NACT. Median follow-up was 52.0 (IQR: 23.0–94.0) months.
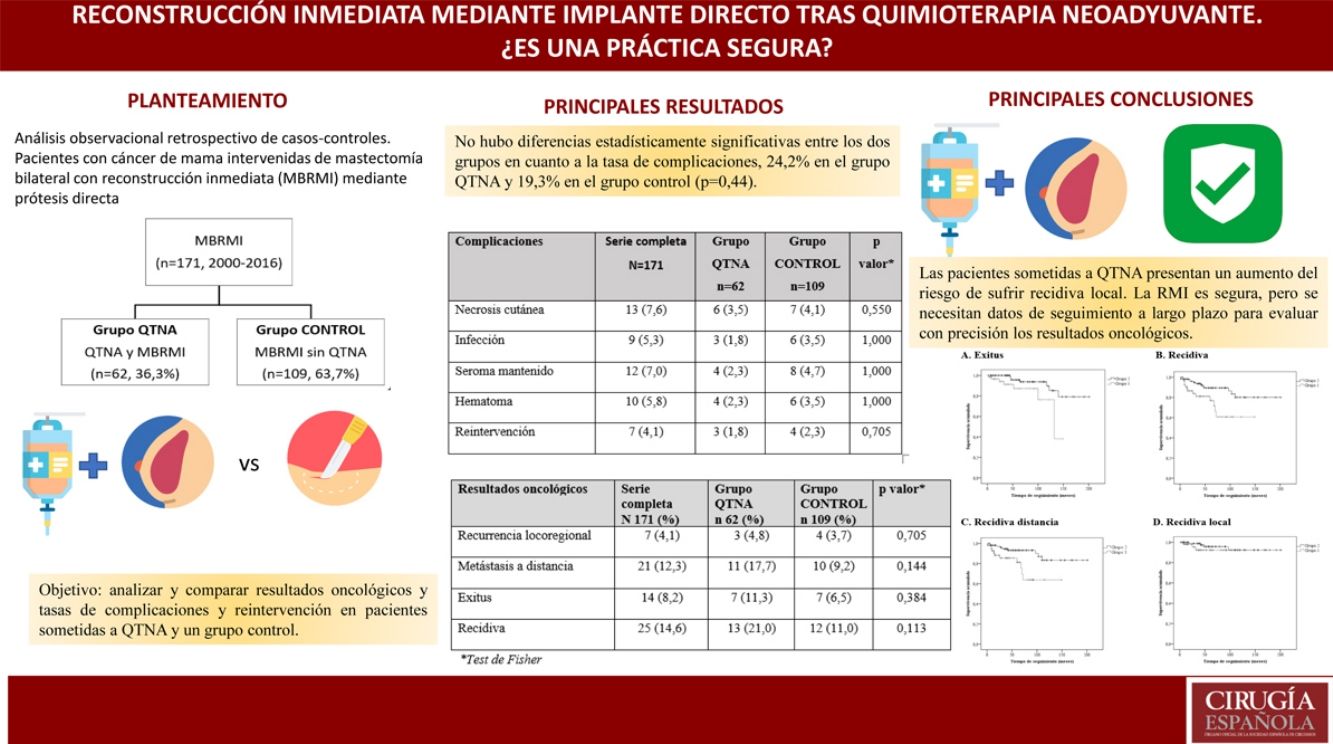
In both groups, the indication for BMIBR was patient choice (32.7%). There were no statistically significant differences between groups in terms of complication rate (24.2% in the NACT group and 19.3% in the control group [P = .44]), but differences in oncological results were found.
Patients in the NACT Group had three times more risk of recurrence at a given time than patients in the control group (3.009 [1.349–6.713]) according to the univariate analysis.
ConclusionDirect-to-implant IBR after skin-sparing mastectomy is a viable option for breast cancer patients undergoing neoadjuvant chemotherapy.
La reconstrucción inmediata (RMI) tras mastectomía en pacientes que han recibido quimioterapia neoadyuvante (QTNA) sigue siendo controvertida. El objetivo de este estudio es analizar y comparar resultados oncológicos y las tasas de complicaciones y reintervención en pacientes sometidas a QTNA y un grupo control.
MétodosAnálisis observacional retrospectivo de casos-controles. Pacientes con cáncer de mama intervenidas de MBRMI mediante prótesis directa durante el periodo 2000-2016. Grupo que recibió QTNA emparejadas máximo 1:5 a las pacientes sin QTNA (Grupo control).
Evaluamos diferencias entre grupos mediante test Chi 2 o Fisher (variables cualitativas), U de Mann Whitney o T Student (variables cuantitativas).Análisis de supervivencia mediante curvas de Kaplan–Meier y test de log-rank.SPSS 22.0.
ResultadosUn total de 171 pacientes con MBRMI, 62 pacientes (363%) tras QTNA y 109 pacientes (63.7%) en grupo control sin QTNA. Mediana de seguimiento de 52,0 (RIQ:23,0-94,0)meses.
La indicación para practicar una MBRMI más frecuente en ambos grupos es la elección de la paciente (32.7%).No hubo diferencias estadísticamente significativas entre los grupos en cuanto a tasa de complicaciones [24.2% en el grupo QTNA y 19.3% en grupo control (P = 0,44)].
Sí existen diferencias en resultados oncológicos. Las pacientes del Grupo QTNA tienen tres veces más riesgo que las pacientes del grupo control de presentar recidiva en un momento determinado del tiempo 3,009 (1,349-6,713) según análisis univariante.
ConclusionesLa RMI mediante prótesis directa tras mastectomías ahorradoras de piel es una opción viable de tratamiento para pacientes con cáncer de mama sometidas a quimioterapia neoadyuvante.
The gold standard surgical treatment for breast cancer is breast-conserving surgery followed by radiation therapy. However, despite therapeutic advances, up to 45% of patients will undergo mastectomy,1 and some 20%–40% will have an associated reconstructive technique in order to improve quality of life and diminish the socio-psychological impact of mastectomy.2,3
In the last decade, the use of bilateral mastectomy has been on the rise, as are breast reconstruction rates,4,5 which can be deferred or immediate (IBR), using autologous or heterologous reconstruction and direct implants.
Although several studies argue that IBR is feasible after skin-saving mastectomies in patients who have previously received neoadjuvant chemotherapy (NACT),6 this is still controversial.7,8
The objective of this retrospective case-control study is to analyze and compare complications, sequelae and re-operation rates, as well as oncological results, between patients who have undergone NACT and bilateral mastectomy with immediate reconstruction (BMIBR) and those who did not.
To date, no prospective, matched, case-control studies have been published about patients with breast reconstruction after neoadjuvant treatment. Therefore, this present study, despite having the limitations of being a retrospective study, is able to provide useful information that increases the number of indications for IBR after NACT, which is proven to be safe.
MethodsA retrospective observational analysis was performed to identify patients who had undergone bilateral mastectomy as a treatment for breast cancer with direct prosthetic IBR at our hospital from 2000-2016. From this group, patients with BMIBR after NACT (the NACT group) were selected, which were matched at a maximum ratio of 1:5 versus patients who had not received NACT (control group).
Data were collected for the following variable types: demographic (age; comorbidities such as obesity, HTN, DM; and active smoking); clinical/pathological (indication, clinical stage, etc.); and surgical (type of intervention, reconstructive techniques used, and postoperative morbidity). Postoperative complications (those that appeared within 30 days after the intervention) and sequelae (after 30 days) were evaluated. Likewise, the oncological results were evaluated.
This study adheres to the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of our hospital and by the Clinical Research Ethics Committee of Aragon (CEICA, Comité Ético de Investigación Clínica de Aragón) with registration number C.P.-C.I. PI16/002.
Patient SelectionThe inclusion criteria for BMIBR were:
- none–
Multicentric or multifocal carcinoma not treatable with conservative surgery. Multifocality: 2 or more tumor foci in the same quadrant and less than 5 cm from the primary focus; and multicentricity: 2 or more tumor foci in different quadrants of the same breast or more than 5 cm from the primary focus.
- none–
Large in situ component of the infiltrating tumor.
- none–
High risk due to family history (no known mutation): defined by 2 or more family members (at least one a close relative with breast or ovarian cancer at an early age, before the age of 50).
- none–
Known mutation in BRCA1 and BRCA2 genes.
Patients with inflammatory carcinoma, as well as BMIBR with no present or past cancer (pure prophylactic mastectomy), were excluded.
The criteria we used for the administration of NACT (regimens and durations) were the updated guidelines and recommendations that were current at the time of treatment, which varied over the course of the study.
Patients with distant metastases at the time of diagnosis and those with progression of the disease during NACT were excluded from the study because they did not undergo IBR after mastectomy.
Patients with tumors larger than 5 cm in diameter and/or 4 or more positive axillary lymph nodes also received adjuvant radiotherapy.
Surgical TechniqueThe surgical technique involved resection of the breast tissue, leaving thin skin flaps, with variations in the skin incision and either complete preservation or a free nipple-areola complex (NAC) graft. The incisions varied according to the size and configuration of the breast affected and the contralateral breast, the size and location of the tumor, previous scars and the surgeon’s preference:
- none–
Subcutaneous mastectomy through external lateral incision.
- none–
Modified Spira technique: implant with a double shell, using a de-epithelialized flap attached to the pectoralis major muscle and free NAC graft after negative intraoperative biopsy of the base of the nipple.
- none–
Skin-saving mastectomy following the short Wise pattern: periareolar incision with vertical extension towards the inframammary fold and lateral and medial extension along the fold.
- none–
Skin-saving, nipple-saving mastectomy through an external radial incision.
IBR was performed using a silicone implant with an anatomical design.
Pathological Evaluation and Oncological ResultsThe clinical stage was established according to the classification of the seventh edition of the AJCC.9
A complete clinical response was defined as the absence of palpable and/or visible tumor on MRI after NACT. Partial response was defined as tumor reduction. Any increase in tumor size was considered progression of the disease.
The locoregional recurrence was defined as the appearance of a new tumor in the ipsilateral chest wall (skin, subcutaneous tissue or pectoral muscle) or recurrence in the ipsilateral axilla, supraclavicular lymph nodes, or internal or subclavicular mammary chains.
Distant metastasis was defined as any recurrence in all other areas not included in the locoregional recurrence.
Statistical AnalysisTo evaluate the differences between the groups under study, the Chi-squared or Fisher’s tests were used in the case of qualitative variables, and the Mann-Whitney U test or Student’s t test were used for quantitative variables according to normal criteria.
To assess overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS) and locoregional recurrence-free survival (LRFS), Kaplan-Meier curves and log-rank tests were used.
The primary point of the study was DFS; different Cox regression models were adjusted to evaluate the time until the event. A P value of .05 was considered statistically significant. The SPSS 22.0 program was used for the entire study.
ResultsDuring the study period, 171 BMIBR interventions were performed to treat breast cancer at our hospital: 62 patients (36.3%) had received NACT, and 109 patients (63.7%) received no neoadjuvant treatment.
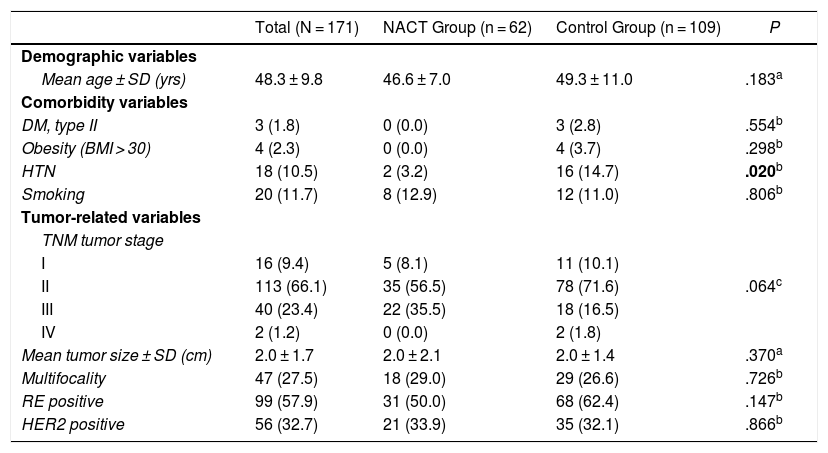
General ResultsThe patients had a mean age of 48.3 ± 9.8 years (range 31–87) at the time of surgery. In the NACT group it was 46.6 ± 7.0 years (range 31–61), and in the control group it was 49.3 ± 11.0 years (range 31–87), P = .183.
Table 1 shows the clinical-pathological data related to the tumor and the comorbidities that could affect the surgical technique as well as the development of complications. The distribution of these factors among the study groups was homogeneous; statistically significant differences were only found for HTN, which was superior in the control group (14.7% vs. 3.2%; P = .020)
Demographic Variables and Comorbidities of Patients and Clinical-pathological Tumor Data.
| Total (N = 171) | NACT Group (n = 62) | Control Group (n = 109) | P | |
|---|---|---|---|---|
| Demographic variables | ||||
| Mean age ± SD (yrs) | 48.3 ± 9.8 | 46.6 ± 7.0 | 49.3 ± 11.0 | .183a |
| Comorbidity variables | ||||
| DM, type II | 3 (1.8) | 0 (0.0) | 3 (2.8) | .554b |
| Obesity (BMI > 30) | 4 (2.3) | 0 (0.0) | 4 (3.7) | .298b |
| HTN | 18 (10.5) | 2 (3.2) | 16 (14.7) | .020b |
| Smoking | 20 (11.7) | 8 (12.9) | 12 (11.0) | .806b |
| Tumor-related variables | ||||
| TNM tumor stage | ||||
| I | 16 (9.4) | 5 (8.1) | 11 (10.1) | |
| II | 113 (66.1) | 35 (56.5) | 78 (71.6) | .064c |
| III | 40 (23.4) | 22 (35.5) | 18 (16.5) | |
| IV | 2 (1.2) | 0 (0.0) | 2 (1.8) | |
| Mean tumor size ± SD (cm) | 2.0 ± 1.7 | 2.0 ± 2.1 | 2.0 ± 1.4 | .370a |
| Multifocality | 47 (27.5) | 18 (29.0) | 29 (26.6) | .726b |
| RE positive | 99 (57.9) | 31 (50.0) | 68 (62.4) | .147b |
| HER2 positive | 56 (32.7) | 21 (33.9) | 35 (32.1) | .866b |
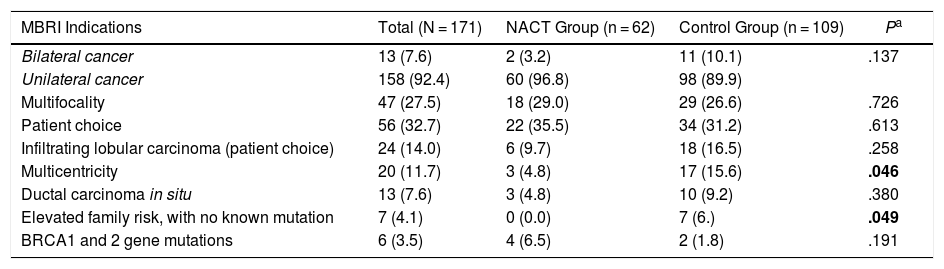
The indications for practicing a more frequent BMIBR in both groups was patient choice (32.7%), followed by multifocal disease (27.5%), as shown in Table 2.
MBRI Indications.
| MBRI Indications | Total (N = 171) | NACT Group (n = 62) | Control Group (n = 109) | Pa |
|---|---|---|---|---|
| Bilateral cancer | 13 (7.6) | 2 (3.2) | 11 (10.1) | .137 |
| Unilateral cancer | 158 (92.4) | 60 (96.8) | 98 (89.9) | |
| Multifocality | 47 (27.5) | 18 (29.0) | 29 (26.6) | .726 |
| Patient choice | 56 (32.7) | 22 (35.5) | 34 (31.2) | .613 |
| Infiltrating lobular carcinoma (patient choice) | 24 (14.0) | 6 (9.7) | 18 (16.5) | .258 |
| Multicentricity | 20 (11.7) | 3 (4.8) | 17 (15.6) | .046 |
| Ductal carcinoma in situ | 13 (7.6) | 3 (4.8) | 10 (9.2) | .380 |
| Elevated family risk, with no known mutation | 7 (4.1) | 0 (0.0) | 7 (6.) | .049 |
| BRCA1 and 2 gene mutations | 6 (3.5) | 4 (6.5) | 2 (1.8) | .191 |
There were patients with more than one indication.
The median follow-up was 52.0 (IQR: 23.0–94.0) months, 34.5 (IQR: 13.0–77.3) months for the NACT group and 62.0 (IQR: 36.5–110.0) for the control group, respectively (P < .001).
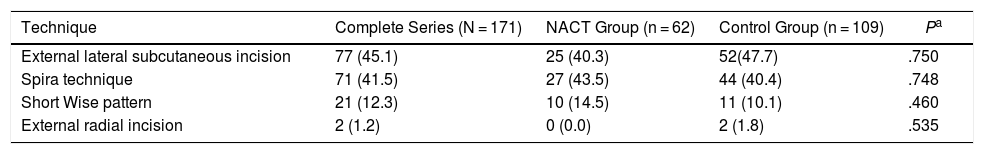
Surgical TechniquesThe distribution of the different types of reconstruction in the two study groups was homogeneous for purely technical reasons. The treatment for each patient was individualized and the most suitable technique chosen. No statistically significant differences were found.
We present the results of the different techniques used in Table 3.
Surgical Technique and Complications During the First Month Post-op.
| Technique | Complete Series (N = 171) | NACT Group (n = 62) | Control Group (n = 109) | Pa |
|---|---|---|---|---|
| External lateral subcutaneous incision | 77 (45.1) | 25 (40.3) | 52(47.7) | .750 |
| Spira technique | 71 (41.5) | 27 (43.5) | 44 (40.4) | .748 |
| Short Wise pattern | 21 (12.3) | 10 (14.5) | 11 (10.1) | .460 |
| External radial incision | 2 (1.2) | 0 (0.0) | 2 (1.8) | .535 |
| Complications | Complete Series (N = 171) | NACT Group (n = 62) | Control Group (n = 109) | Pa |
|---|---|---|---|---|
| Skin necrosis | 13 (7.6) | 6 (3.5) | 7 (4.1) | .550 |
| Infection | 9 (5.3) | 3 (1.8) | 6 (3.5) | 1.000 |
| Maintained seroma | 12 (7.0) | 4 (2.3) | 8 (4.7) | 1.000 |
| Hematoma | 10 (5.8) | 4 (2.3) | 6 (3.5) | 1.000 |
| Re-operation | 7 (4.1) | 3 (1.8) | 4 (2.3) | .705 |
Data are expressed as n (%).
The overall complication rate was 29.8% for the entire group, and there were no statistically significant differences between the two groups in terms of the complication rate: 32.2% in the NACT group and 28.4% in the control group (P = .44).
The most frequent postoperative complication was skin necrosis (7.6%), followed by maintained seroma (7.0%) and hematoma (5.8%), as shown in Table 3. Skin necrosis was the most frequent in the NACT group, and maintained seroma was the most frequent in the control group.
The reoperation rate in the early postoperative period was 1.8 % for the NACT group and 2.3 % for the control group (P = .705).
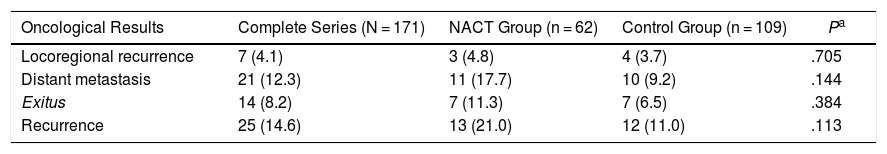
Oncological Results8.2% of the patients died due to breast cancer during the study: 7 from the NACT group (11.3%) and 7 from the control group (6.5%) (Table 4).
Oncological Results.
| Oncological Results | Complete Series (N = 171) | NACT Group (n = 62) | Control Group (n = 109) | Pa |
|---|---|---|---|---|
| Locoregional recurrence | 7 (4.1) | 3 (4.8) | 4 (3.7) | .705 |
| Distant metastasis | 21 (12.3) | 11 (17.7) | 10 (9.2) | .144 |
| Exitus | 14 (8.2) | 7 (11.3) | 7 (6.5) | .384 |
| Recurrence | 25 (14.6) | 13 (21.0) | 12 (11.0) | .113 |
Data are expressed as n (%).
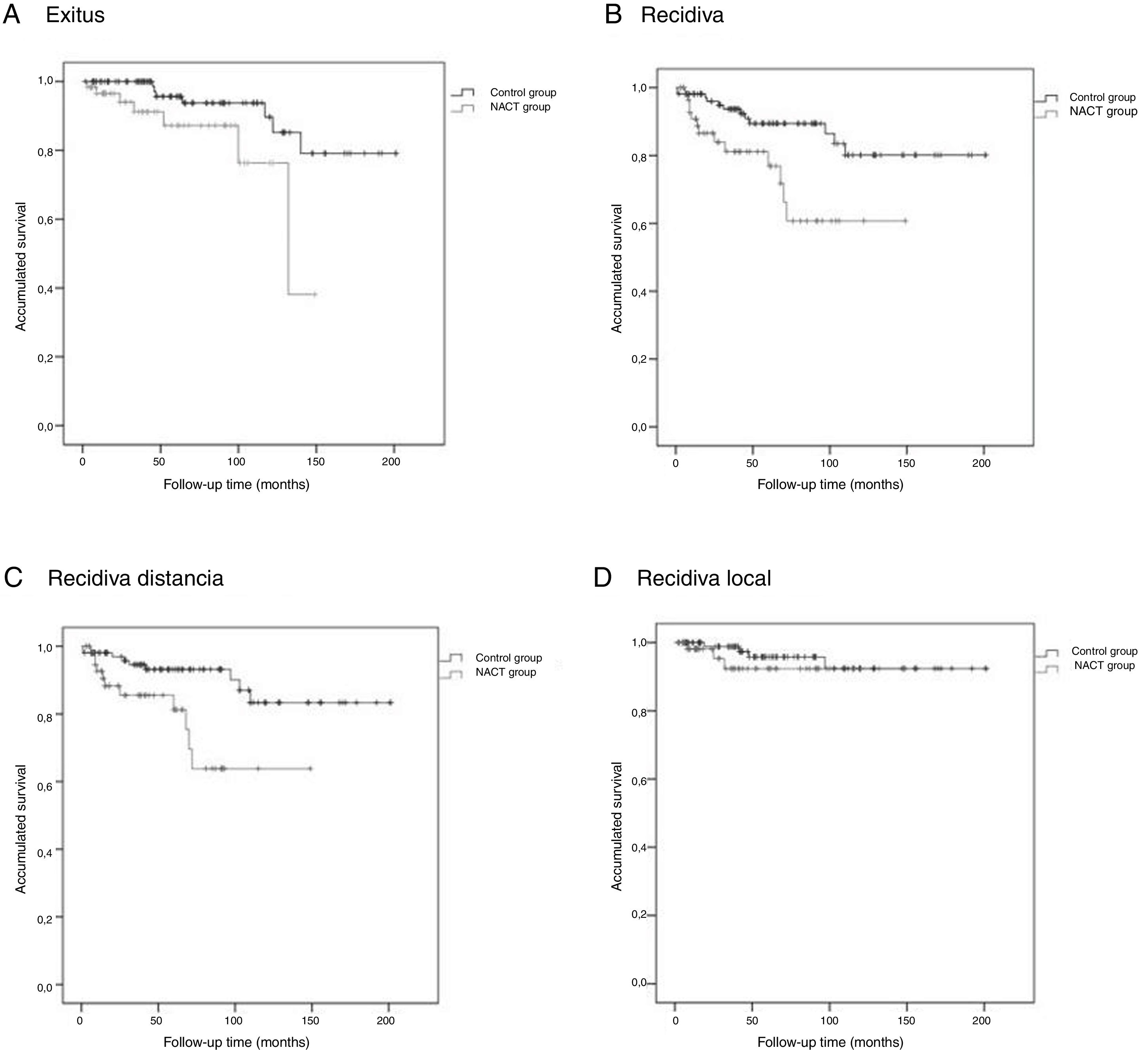
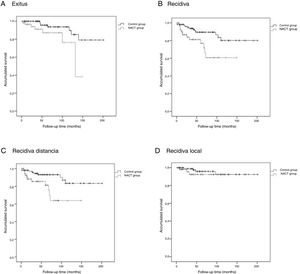
The Kaplan-Meier survival curves for OS (log-rank test, P = .016), DFS (log-rank test, P = .005), DMFS (log-rank test, P = .004) and LRFS (log-rank test, P = .368) are shown in Fig. 1.
The average OS was 46.1 (95% CI: 36.2–55.9) months, the average DFS was 40.6 months (95% CI: 31.7–49.5), the average DMFS was 39.8 months (95% CI: 31.2–48.5) and the average LRFS was 43.0 months (95% CI: 33.5–52.5).
Control GroupThe average OS was 75.9 (95% CI: 65.8–86.1) months, the average DFS was 72.5 months (95% CI: 62.4–82.6), the average time of DMFS was 70.8 (95% CI: 60.7–80.9) months and the average time of LRFS was 71.3 (95% CI: 61.4–81.2) months.
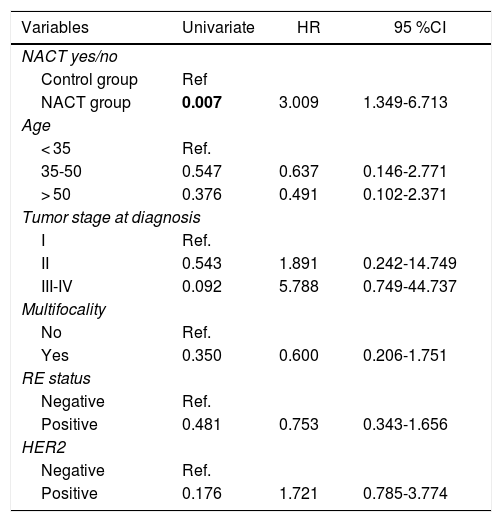
There were statistically significant differences between the groups for all variables except local recurrence. The survival curves (Fig. 1) and univariate analysis demonstrated that patients in the NACT group had 3 times more risk than patients in the control group for having recurrence at a certain moment in time (3.009; 1.349–6.713) (Table 5).
Uni- and Multivariate Analysis for Recurrence.
| Variables | Univariate | HR | 95 %CI |
|---|---|---|---|
| NACT yes/no | |||
| Control group | Ref | ||
| NACT group | 0.007 | 3.009 | 1.349-6.713 |
| Age | |||
| < 35 | Ref. | ||
| 35-50 | 0.547 | 0.637 | 0.146-2.771 |
| > 50 | 0.376 | 0.491 | 0.102-2.371 |
| Tumor stage at diagnosis | |||
| I | Ref. | ||
| II | 0.543 | 1.891 | 0.242-14.749 |
| III-IV | 0.092 | 5.788 | 0.749-44.737 |
| Multifocality | |||
| No | Ref. | ||
| Yes | 0.350 | 0.600 | 0.206-1.751 |
| RE status | |||
| Negative | Ref. | ||
| Positive | 0.481 | 0.753 | 0.343-1.656 |
| HER2 | |||
| Negative | Ref. | ||
| Positive | 0.176 | 1.721 | 0.785-3.774 |
As a result of recent advances in chemotherapy treatments and agents targeting HER2, the use of NACT has increased,10 and the number of patients undergoing IBR after NACT has likewise grown.
The oncological safety of IBR skin-saving or skin-and-nipple-saving mastectomy conducted in patients with advanced stage breast cancer is still controversial, especially when NACT is administered.
In our study, we found differences in OS, DFS, DMFS and LRFS between the 2 groups after being matched by age and clinical stage.
Many surgeons hesitate to perform IBR after NACT as it could increase the rate of complications, which would result in the delayed administration of adjuvant treatments, such as radiotherapy or trastuzumab.11,12
Aurilio et al.13 reported significantly high local recurrence rates compared to the simple mastectomy group without reconstruction in estrogen-receptor negative patients.
However, many other studies have not shown a significant increase in complications in patients who underwent IBR after NACT.14–17 Song et al.18 reported that a meta-analysis including 11 studies showed that NACT does not increase the complication rate in patients with IBR.
In a prospective study published by Donker et al.19 not only was no increase in short-term complications shown, but these were significantly lower (15% vs. 29%; P = .042) in patients with IBR after NACT compared to those who received adjuvant CTx after IBR.
The results of our study are consistent with these data since we have found no statistically significant differences in terms of complication rates, sequelae and/or need for re-operation between the study groups.
Yes, some differences were observed, such as the increased risk of recurrence in patients of the NACT group, which leads us to think that patients who receive NACT, despite presenting a more unfavorable tumor stage, are slightly younger and have fewer comorbidities, which could affect the development of complications.
Although a prospective randomized controlled trial would be ideal, there are many difficulties for performing IBR, not only of a technical nature established by the surgeon but also in terms of patient preferences. Furthermore, it is difficult to accurately predict the clinical response to NACT, which makes surgical planning difficult.
There have been few matched case-control studies similar to ours. Therefore, studies of this type provide useful information, allowing for the number of IBR indications after NACT to be increased, as it is proven to be safe.
However, there are several limitations in this study that do not allow the results to be generalized. First of all, it is a retrospective study, with a relatively short follow-up time considering the current breast cancer survival times. Secondly, since it is the experience of a single institution, the sample size is small to establish an accurate analysis of the oncological results. In addition, the difference in follow-up in both groups is also a limitation to generalize the results. It would be necessary to carry out multicenter studies with a longer follow-up times to be able to generalize them.
ConclusionsIBR using direct prosthesis after skin-saving mastectomy may be a viable treatment option for patients with breast cancer receiving NACT.
These patients have an increased risk of local recurrence, but long-term follow-up data is needed to accurately assess cancer outcomes.
FundingThis study has received no funding.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank the Breast Unit at the Hospital Clínico Universitario Lozano Blesa in Zaragoza (Spain), including surgeons, nursing staff and patients.
Please cite this article as: Allué Cabañuz M, Arribas del Amo MD, Gil Romea I, Val-Carreres Rivera MP, Sousa Domínguez R, Güemes Sánchez AT. Reconstrucción inmediata mediante implante directo tras quimioterapia neoadyuvante. ¿Es una práctica segura? Cir Esp. 2019;97:575–581.