Immediate breast reconstruction (IBR) after skin-sparing mastectomy in patients undergoing neoadjuvant chemotherapy (NACT) is still controversial. The objective is to determine related factors to the axillary downstaging and complete pathological response (CPR), and how CPR influences the decision of bilateral mastectomy with immediate reduction (IBRBM).
Patients and methodsA retrospective analysis breast cancer patients undergoing NACT and IBRBM between 2000–2018 was performed. We compared two groups;1)CPR and 2) not CPR. Descriptive and comparative statistical analysis.
Results and Discussion69 patients; 26 (37.68%) reached CPR and 43 (62.32%) non-CPR. Median follow-up of 45.3 months (RIQ:23,0–94,0). Age less than 35 (p < 0.001), small size tumor at diagnosis (p = 0.006) and subtype HER2 (p < 0.001) were associated with higher rates of CPR in univariate analysis. Axillary negativization rate was 80% in group 1 and 59.3% in group 2 and lymphadenectomy rates were similar (73.1% and 83.72%).
ConclusionCPR after NACT not conditioned the decision to perform IBRBM.
La reconstrucción mamaria inmediata (RMI)tras mastectomías ahorradoras de piel en pacientes sometidas a quimioterapia neoadyuvante(QTNA) todavía resulta controvertida. El objetivo es determinar factores relacionados con downstaging axilar y respuesta patológica completa (RPC), y como esta, condiciona la decisión de someterse a mastectomía bilateral con reconstrucción inmediata (MBRMI).
Pacientes y MétodosSe realizó un análisis retrospectivo de pacientes con cáncer de mama sometidas a QTNA y MBRMI entre 2000–2018.Comparamos dos grupos; 1)RPC y2) no RPC. Se analizaron datos demográficos, anatomopatológicos y el estadio clínico inicial y final. Análisis estadístico descriptivo y comparativo.
Resultados y Discusión69 pacientes; Grupo1:26 (37,68%) alcanzaron RPC y 43(62,32%) no RPC. Mediana de seguimiento 45,3meses (RIQ:23,0-94,0. En análisis univariante, edad menor a 35 p < 0,001, menor T al diagnósticop = 0,006 y subtipo HER2 p < 0,001 se asociaron significativamente con mayores tasas de RPC. La indicación más frecuente fue la elección de la paciente 31,8%. La tasa de negativización axilar fue del 80% en el grupo 1 y 59.3% en grupo 2 y las tasas de linfadenectomía axilar fueron similares (73.1% y 83.72 %).
ConclusiónLa RPC tras la QTNA no parece ser un factor de peso en la decisión de realizar la MBRMI
Systemic neoadjuvant chemotherapy (NCT) is administered in breast cancer before surgical treatment to achieve a series of clinical objectives.1,2 In large tumors, if an adequate response is achieved, higher rates of breast-conserving surgery are possible by reducing the tumor size, thereby avoiding mastectomy in some patients.3,4
In addition, patients with clinically positive axillae at diagnosis who respond to NCT can benefit from selective sentinel lymph node biopsy after treatment, which avoids axillary dissection if negative.5,6
The response to NCT is also an important prognostic indicator that allows tumor response to be evaluated during treatment.7 This response is greatly influenced by the histological subtype. Non-luminal tumors (with negative hormonal receptors) and tumors that overexpress HER2 have the highest rates of complete pathological response (CPR) at around 33%–45%, while negative luminal and HER2 tumors respond in 15%–23% of cases.8,9
However, despite therapeutic advances including NCT, up to 45% of patients with breast cancer will undergo mastectomy10 and 20%–40% will have some associated reconstructive technique in order to improve quality of life and reduce the socio-psychological impact of mastectomy.11
Although several studies defend that breast reconstruction is safe, viable immediately after skin-preserving mastectomy in patients previously treated with NCT12 some authors still find it controversial.13,14 In addition, the role played by the response to NCT on surgical planning, the decision to perform immediate reconstruction after mastectomy is not known, further studies are needed.
The objectives of this study were to identify tumor and patient characteristics that could be associated with reaching complete pathological response (CPR) and axillary downstaging, and to determine whether CPR after NCT was a factor in the decision to undergo bilateral mastectomy with immediate reconstruction (BMIR).
MethodsA retrospective observational analysis was done to identify patients with breast cancer who had undergone NCT and direct-to-implant breast reconstruction after mastectomy from 2000 to 2018.
The inclusion criteria for this technique were: the tumor-to-breast volume ratio; multicentric and/or multifocal tumors; patients with contraindications for breast-conserving surgery, including inflammatory breast cancer; and patient choice. We excluded from the study patients who had undergone NCT who were candidates for initiation to breast-conserving surgery or who were candidates after a good response to NCT.
The initial clinical stage was determined by physical examination and imaging tests (mammography, ultrasound and/or magnetic resonance imaging) and based on the TNM classification.
Demographic data, pathological data and the final clinical stage were obtained from the Unit database.
CPR was defined as absence of invasive or in situ disease in the breast and armpit; non-CPR was everything that was not included in the previous category, including absence of response or partial response.
Patients were divided into two study groups. Group 1 obtained CPR, and Group 2 did not.
The axillary evaluation was initially clinical, based on physical examination, ultrasound and fine needle puncture, classifying the nodes as negative or positive. After NCT, the nodes were reassessed by physical examination.
Patients with persistent positive nodes underwent axillary lymph node dissection (Berg levels I-II).
Patients with initially positive lymph nodes that turned negative underwent selective biopsy of the sentinel lymph node. If the result was positive, axillary lymph node dissection was performed; if negative, the axilla was left as is.
NCT regimens include anthracycline, taxane or a combination of both. In patients with HER2 overexpression, trastuzumab was added alone or in combination with pertuzumab, depending on when the treatment was administered.
The main variable was to determine the clinical-pathological characteristics of the tumor and patients that could be associated with obtaining CPR and axillary downstaging.
This study was approved by the ethics committee of the hospital and by the Ethics Committee for Clinical Research of Aragon (C.P. - C.I. PI16 / 002).
Statistical analysisFirst, a descriptive analysis was completed of the variables under study. For qualitative variables, specific and absolute frequencies are provided; for quantitative variables, means and standard deviation (SD). The relationship between qualitative variables was assessed using the Chi-squared test. To compare means between two independent groups, the Mann–Whitney U or Student’s t-test were used, according to criteria of normalcy.
The level of statistical significance was established for a P value less than .05. The statistical program SPSS 22.0 for Windows (SPSS Ibérica, Madrid, Spain) was used for the entire analysis.
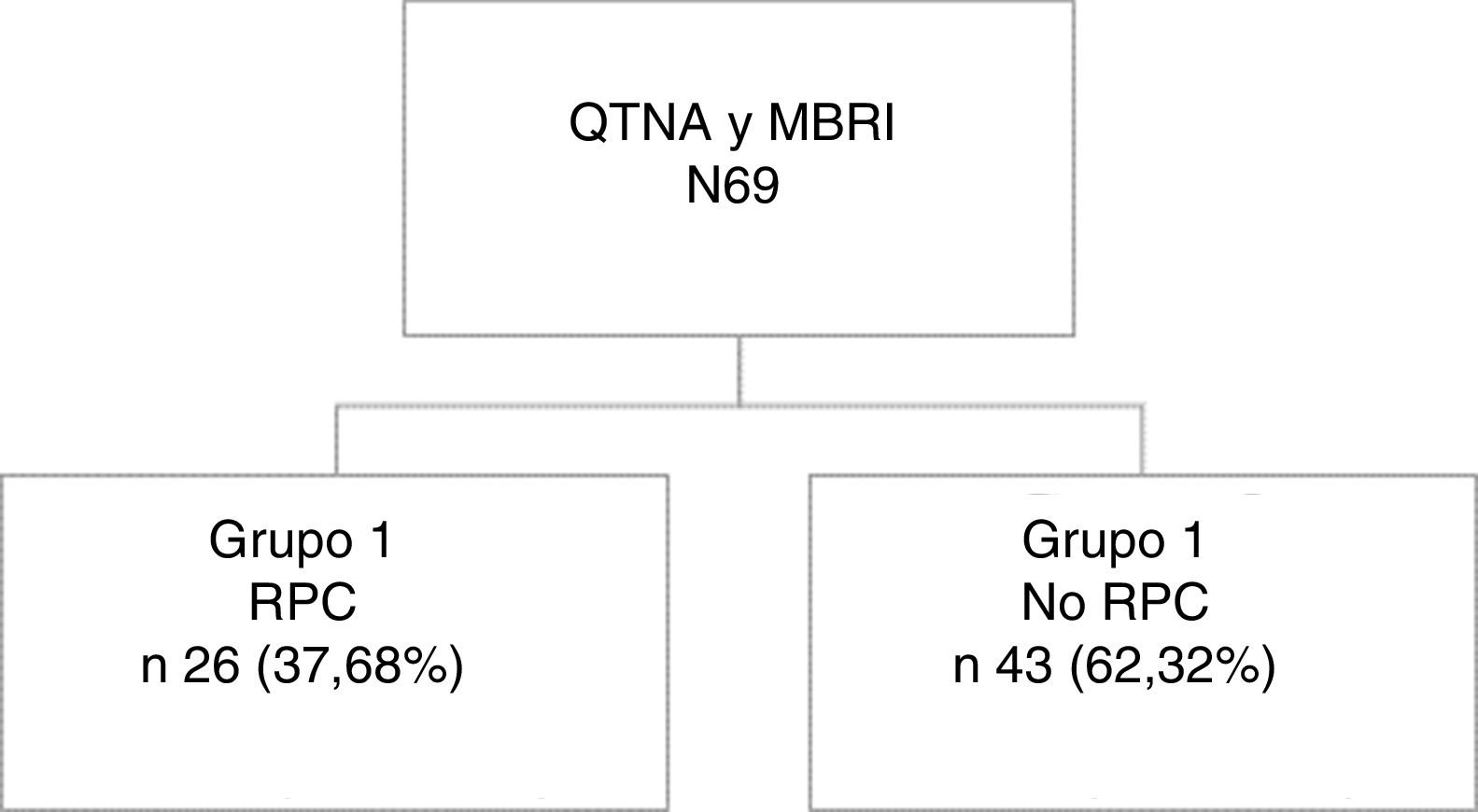
ResultsWe identified 69 breast cancer patients undergoing NCT and subsequent BMIR during the study period: 26 (37.68%) presented CPR and 43 (62.32%) did not present CPR (34 [79%] with partial response and 9 [21%] absence of response). The median patient follow-up was 45.3 (IQR: 23.0–94.0) months (Fig. 1).
- -
General results
- -
Demographic and tumor-related data
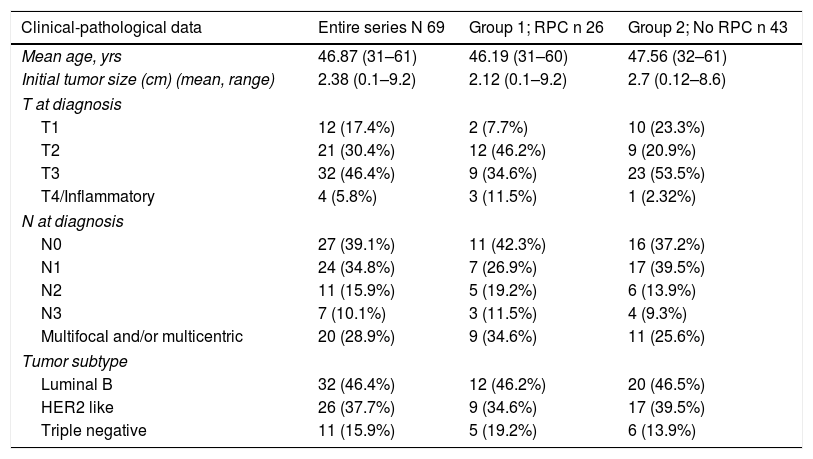
The patients had a mean age of 46.87 years (range 31–61) at the time of surgery. In Group 1, it was 46.19 years (range 31–60) and in Group 2 47.56 years (range 32–61). The clinical-pathological data related to the tumor are shown in Table 1.
Clinical-pathological data and comorbidities.
| Clinical-pathological data | Entire series N 69 | Group 1; RPC n 26 | Group 2; No RPC n 43 |
|---|---|---|---|
| Mean age, yrs | 46.87 (31–61) | 46.19 (31–60) | 47.56 (32–61) |
| Initial tumor size (cm) (mean, range) | 2.38 (0.1–9.2) | 2.12 (0.1–9.2) | 2.7 (0.12–8.6) |
| T at diagnosis | |||
| T1 | 12 (17.4%) | 2 (7.7%) | 10 (23.3%) |
| T2 | 21 (30.4%) | 12 (46.2%) | 9 (20.9%) |
| T3 | 32 (46.4%) | 9 (34.6%) | 23 (53.5%) |
| T4/Inflammatory | 4 (5.8%) | 3 (11.5%) | 1 (2.32%) |
| N at diagnosis | |||
| N0 | 27 (39.1%) | 11 (42.3%) | 16 (37.2%) |
| N1 | 24 (34.8%) | 7 (26.9%) | 17 (39.5%) |
| N2 | 11 (15.9%) | 5 (19.2%) | 6 (13.9%) |
| N3 | 7 (10.1%) | 3 (11.5%) | 4 (9.3%) |
| Multifocal and/or multicentric | 20 (28.9%) | 9 (34.6%) | 11 (25.6%) |
| Tumor subtype | |||
| Luminal B | 32 (46.4%) | 12 (46.2%) | 20 (46.5%) |
| HER2 like | 26 (37.7%) | 9 (34.6%) | 17 (39.5%) |
| Triple negative | 11 (15.9%) | 5 (19.2%) | 6 (13.9%) |
Likewise, three tumor subtypes are classified: luminal B (ER/PR +, HER2 −, KI67> 20%) Her2 like (ER −, PR −, HER2 +) and triple negative (ER −, PR −, HER2 −).
The distribution of these factors among the study groups was homogeneous (P = .005).
In the bivariate analysis, age under 35 yrs (P < .001), lower T at diagnosis (P = .006) and the HER 2 subtype (P < .001) significantly correlated with higher rates of CPR.
- -
Indications
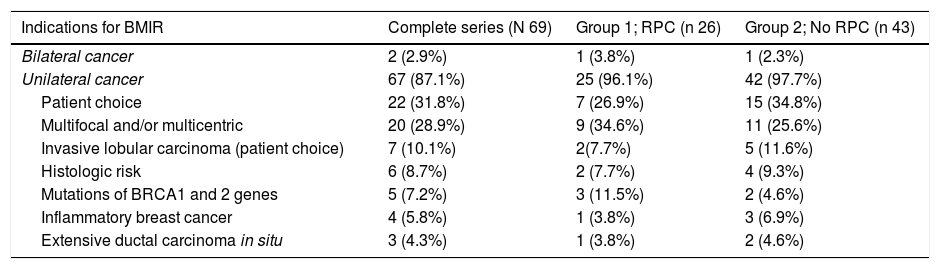
The most frequent indication for BMIR was patient choice 22/69 (31.8%) followed by multicentric and/or multifocal tumors (MC and/or MF) 20/69 (28.9%) for the entire series, and MC and/or MF in Group 1 9/26 (34.6%) and patient choice 13/43 (30.2%) in Group 2, as shown in Table 2.
- -
Response to NCT
Indications for bilateral mastectomy with immediate reconstruction after NCT.
| Indications for BMIR | Complete series (N 69) | Group 1; RPC (n 26) | Group 2; No RPC (n 43) |
|---|---|---|---|
| Bilateral cancer | 2 (2.9%) | 1 (3.8%) | 1 (2.3%) |
| Unilateral cancer | 67 (87.1%) | 25 (96.1%) | 42 (97.7%) |
| Patient choice | 22 (31.8%) | 7 (26.9%) | 15 (34.8%) |
| Multifocal and/or multicentric | 20 (28.9%) | 9 (34.6%) | 11 (25.6%) |
| Invasive lobular carcinoma (patient choice) | 7 (10.1%) | 2(7.7%) | 5 (11.6%) |
| Histologic risk | 6 (8.7%) | 2 (7.7%) | 4 (9.3%) |
| Mutations of BRCA1 and 2 genes | 5 (7.2%) | 3 (11.5%) | 2 (4.6%) |
| Inflammatory breast cancer | 4 (5.8%) | 1 (3.8%) | 3 (6.9%) |
| Extensive ductal carcinoma in situ | 3 (4.3%) | 1 (3.8%) | 2 (4.6%) |
From the entire series, 26 patients (37.68%) (Group 1) obtained CPR, while 43 (62.32%) did not (Group 2).
Patients in Group 1 had a smaller tumor size (T) at diagnosis (P = .006) and were more frequently subtype HER 2 (P < .001), but no statistically significant differences were found in terms of lymph node status (N) at diagnosis (P = .177).
- -
Axillary downstaging
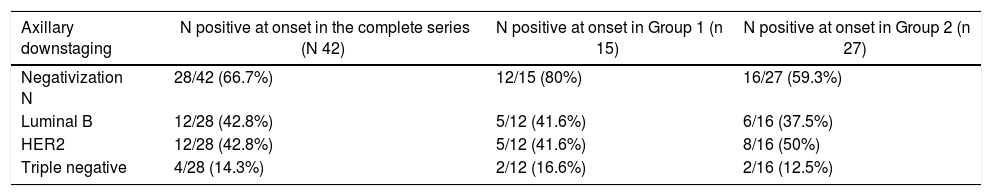
Out of the 69 patients, 42 (60.8%) had axillary involvement at diagnosis; 28 of these (66.8%) were negative after NCT. Out of the 42 patients with positive N, 15 corresponded to Group 1 with CPR and 27 to Group 2.
In the CPR group, the rate of negativization was 80% (12 of the 15 patients with positive N); 41.6% were luminal tumors B (5/12), 41.6% HER 2 (5/12) and 16.6% triple negative (2/12).
In Group 2, without CPR, the axillary negativization rate was 59.3% (16 out of 27 patients with positive N): 37.5% luminal tumors B (6/16), 37.5% to HER2 (6/16) and 12.5% triple negative (2/16). These results are shown in Table 3.
Axillary downstaging.
| Axillary downstaging | N positive at onset in the complete series (N 42) | N positive at onset in Group 1 (n 15) | N positive at onset in Group 2 (n 27) |
|---|---|---|---|
| Negativization N | 28/42 (66.7%) | 12/15 (80%) | 16/27 (59.3%) |
| Luminal B | 12/28 (42.8%) | 5/12 (41.6%) | 6/16 (37.5%) |
| HER2 | 12/28 (42.8%) | 5/12 (41.6%) | 8/16 (50%) |
| Triple negative | 4/28 (14.3%) | 2/12 (16.6%) | 2/16 (12.5%) |
The axillary lymphadenectomy rates were similar (73.1% [19/26] in Group 1 and 83.72% [36/43] in Group 2). However, the rate of positive sentinel lymph node biopsy requiring axillary lymphadenectomy were different in the two groups (0% [0/19] in Group 1 and 16.27% [7/36] in Group 2), and no statistically significant differences were found depending on whether they had reached CPR or not (P = .12).
DiscussionWith this study, our intention was to determine factors related to axillary downstaging and CPR, and how these influence the decision to undergo BMIR.
The overall rate of CPR in our study was 37.68%. However, as observed in other studies, the axillary response rate was higher.
Thus, we found significant axillary downstaging: 66.8% of patients (26/42) who had affected nodes at diagnosis converted to negative nodes after treatment. The negativization rate was higher in the CPR group (80%) compared to the group without CPR (59.3%).
As described, earlier stages (smaller tumor size) and the younger age of patients are factors that are also associated with obtaining CPR in this study.15
However, the analysis performed does not correspond with the therapeutic approach carried out in the axilla. During the first years of the study (2000–2015), axillary lymphadenectomy was performed in all patients with positive axilla at diagnosis, regardless of the response to NCT. However, in recent years we have witnessed a paradigm shift, with BSGC being performed after NCT (even marking positive ganglia with different techniques before treatment to be able to later identify them in case of complete responses).16
In general, the patients in this study treated with bilateral mastectomy instead of breast-conserving surgery were not candidates given the characteristics of the tumors themselves. However, the decision to perform bilateral mastectomy in patients with a unilateral tumor (i.e. contralateral prophylactic mastectomy) was in a large percentage of cases (31.8%) the choice of the patients themselves.
CPR was not associated with the decision to undergo BMIR. Among the patients who chose to undergo this procedure without having another clinical indication of those listed Table 2 (22 out of 69 patients [31.8%]), only 31.8% (7/22) presented CPR; the remaining 68.1% (15/22) were patients without CPR.
The indications in patients with CPR (Group 1) were similar to those of patients without CPR (Group 2). Patient choice was followed by multifocal and/or multicentric presentations and inflammatory carcinoma (see Table 2) without finding statistically significant differences (P = .07).
The present study has limitations due to its retrospective nature and the small sample size as the cases are from a single institution. Thus, assessing the impact of the response to NCT on surgical treatment is complicated and the results cannot be generalized.
In conclusion, the complete pathological response after neoadjuvant treatment does not seem to be a significant factor in the decision to perform BMIR.
Conflict of interestsThe authors have no conflict of interests to declare
Please cite this article as: Allué Cabañuz M., del Amo M.D.A., Guemes Sanchez A.T. Factores relacionados con la obtención de respuesta patológica completa tras quimioterapia neoadyuvante en cáncer de mama y su efecto sobre la reconstrucción tras mastectomía ahorradora de piel. Cir Esp. 2020;98:149–153.