Enhanced recovery after surgery (ERAS) constitutes the application of a series of perioperative measures based on the evidence, in order to achieve a better recovery of the patient and a decrease of the complications and the mortality. These ERAS programs initially proved their advantages in the field of colorectal surgery being progressively adopted by other surgical areas within the general surgery and other surgical specialties. The main excluding factor for the application of such programs has been the urgent clinical presentation, which has caused that despite the large volume of existing literature on ERAS in elective surgery, there are few studies that have investigated the effectiveness of these programs in surgical patients in emergencies. The aim of this article is to show ERAS measures currently available according to the existing evidence for emergency surgery.
La rehabilitación multimodal quirúrgica (ERAS) constituye la aplicación de una serie de medidas perioperatorias basadas en la evidencia, con el fin de lograr una mejor recuperación del paciente y una disminución de las complicaciones y la mortalidad. Estos programas de rehabilitación multimodal inicialmente demostraron sus ventajas en el ámbito de la cirugía colorrectal siendo adoptados progresivamente por otras áreas quirúrgicas dentro de la cirugía general y por otras especialidades quirúrgicas. El factor excluyente principal para la aplicación de este tipo de programas ha sido la presentación clínica urgente, lo que ha provocado que a pesar del gran volumen de literatura existente sobre ERAS en cirugía electiva, existan pocos estudios que hayan investigado la efectividad de estos programas en pacientes quirúrgicos en urgencias. El objetivo de este artículo es mostrar las medidas de recuperación intensificada de que disponemos en la actualidad según la evidencia existente para cirugía urgente.
Multimodal postoperative rehabilitation or enhanced recovery after surgery (ERAS) programs apply a series of perioperative strategies aimed at patients who are going to undergo a surgical procedure in order to reduce the stress caused by the surgical procedure, thereby achieving better patient recovery, fewer complications and less mortality.1
Multimodal rehabilitation programs began to show their advantages in the field of colorectal surgery (Kehlet and Wilmore2), with decreased morbidity and improved efficiency. Subsequently, these programs have been adopted in other areas, both in our specialty and in other surgical specialties.3
This article is a narrative review of the main publications in the currently available literature regarding multimodal rehabilitation in emergency surgery, based on a bibliographic search of the Cochrane Plus (Cochrane Library), Medline, EMBASE and Scopus databases from 1995 to 2019.
Below, we describe a series of common pre-, intra- and postoperative ERAS measures in urgent surgery as well as a series of factors particular to the management of the selected pathologies.
Common measuresAlthough there are probably certain things that can be extrapolated from elective surgery, the protocolization of multimodal rehabilitation measures for urgent surgery has a series of peculiarities of its own. Therefore, modified ERAS programs are used, with a preoperative phase that has little margin for optimization. This makes it necessary to have a greater impact on the intra- and postoperative phases.
Preoperative measuresA high preoperative HbA1c level or preoperative hyperglycemia is clearly associated with increased morbidity. Since the determination of HbA1c in emergency departments is quite difficult, glycemic control is recommended throughout the perioperative period, with a target of 140–180mg/dL.4
Given the urgent nature of the pathology, patient acceptance and satisfaction are other concerns to be studied. These issues are combated with proper protocolization, extensive preoperative counseling, and information on the benefits of this type of treatment and early discharge.
Intraoperative measuresThere are different factors over which strict control must be maintained, such as intraoperative fluid therapy, hypothermia prevention, analgesia, hemodynamic changes, antiemetic prophylaxis, etc.
It is a real challenge to establish restrictive fluid therapy/goal-directed fluid therapy in the emergency setting because it is a break from the traditional teaching applied in critically ill patients, which almost requires large volumes of fluid without restrictions to combat hypotension, vasodilation and the consequent capillary leakage of fluid. The consistency of the results obtained in elective surgery in terms of restrictive fluid therapy makes it necessary to consider whether excess fluid can, in fact, create or perpetuate something that we want to avoid.5
The use of balanced electrolyte solutions (lactated Ringer's, plasma-lyte) is recommended over saline or colloids (grade of recommendation: GR-IIC), which is especially important in the management of unstable patients.6
It is essential to keep the room warm and avoid patient heat loss as much as possible. More than 50% of patients in the emergency room have hypothermia. The most important consequences of hypothermia are increased perioperative blood loss/coagulopathy, heart problems (myocardial ischemia, arrhythmias) and increased wall infections.7 Preoperative active warming is indicated in high-risk patients (over 60 years) (GR-IC), and it is recommended in all emergency surgery patients.8
Regarding pain management, the best therapeutic option is balanced or multimodal analgesia. This involves combining different drugs or anesthetic techniques with different mechanisms of action (regional TAP block [transversus abdominis plane] in cases of laparotomy, etc.) and in doses lower than those used in monotherapy. The aim is to achieve greater analgesic potency with fewer adverse effects. There are many papers that support this concept of analgesia.9
Likewise, the risk of postoperative nausea and vomiting in all patients should be stratified using the Apfel scale, and prophylactic measures should be based on this.1
Postoperative measuresAs in any ERAS protocol, early introduction of the oral diet and ambulation is recommended (as well as the early removal of catheters and drains, if any), while taking into account that the urgent nature of the pathology will require a cadence that is different from elective surgeries.
A novel trend in postoperative patient care is the idea of the ‘handoff’, or transfer, of patients to the reanimation area, avoiding noise or stress in order to achieve a more placid post-anesthetic recovery and avoid problems associated with an inadequate transfer (greater initial postoperative pain, anxiety, etc.).10
Specific measures by pathologiesAcute appendicitisThe literature shows that the mean postoperative stay for acute appendicitis is 1.8–2.2 days, which is similar for open or laparoscopic surgery.
An ERAS protocol would allow for earlier return to home, school or work and would reduce postoperative discomfort, costs, and even the possibility of day surgery, which would provide individual, family, health and social benefits, reducing hospital costs and loss of productivity.
Preoperative measuresAntibiotic prophylaxis has been shown to be effective in preventing superficial surgical site infections and intra-abdominal abscesses in patients with uncomplicated appendicitis. However, there is no evidence to support the routine administration of postoperative antibiotics. Therefore, in uncomplicated acute appendicitis (in the absence of gangrene or perforation), only one preoperative dose is recommended.11
Likewise, preoperative voluntary urination is recommended to avoid catheterization.
Intraoperative measuresRegarding the access route, laparoscopic appendectomy should be the first option if the surgical team has been trained. The laparoscopic procedure offers clear advantages in terms of less pain, lower incidence of surgical site infection, shorter hospital stay, earlier return to work and decrease in general costs (GR-IA).12
Nasogastric and drain tubes should be avoided in uncomplicated acute appendicitis. The routine use of surgical drains does not reduce the incidence of intra-abdominal abscess.13
Although minimally invasive, laparoscopic appendectomy in uncomplicated acute appendicitis continues to produce considerable postoperative pain, hospitalizations of 1–2 days, and 1–3 weeks missed work or school. Recommendations include pre-incisional port infiltration (local anesthetic and epinephrine), opioid-sparing pre- and postoperative multimodal analgesia, along with single-dose parenteral NSAIDs at the end of the procedure. In 2017, Hamill et al. conducted a non-systematic review of evidence-based measures to optimize recovery after laparoscopic appendectomy.13 Some of their notable conclusions were: the protocolized approach has not yet been studied in randomized clinical trials; neither minilaparoscopy nor SILS (Single-incision Laparoscopic Surgery) improved recovery; TAP block did not reduce postoperative pain14,15; on the other hand, local intraperitoneal anesthesia showed benefits in adults.16,17 No trials were found about NOTES (natural orifice transluminal endoscopic surgery) appendectomy or about the use of drain tubes.
Intraoperative fluid therapy, prevention of hypothermia, analgesia and hemodynamic changes should be strictly monitored to reduce metabolic stress, along with antiemetic prophylaxis (with dexamethasone and ondansetron).
Postoperative measuresIt is mandatory to underline the importance of insisting on the early initiation of oral diet and ambulation.
Lefrancois et al. described the Saint-Antoine Score, which is a predictive score based on 5 factors independently associated with early hospital discharge (BMI <28kg/m2, leukocyte count <15000/μl, CRP <30mg/L), absence of radiological signs of perforation, and appendix diameter (≤10mm on imaging) when observing that 71% of the patients with 4 criteria and 92% with 5 criteria were day surgery patients.18
In hospitals and selected cases (without protocolization), uncomplicated acute appendicitis has been successfully managed as day surgery,19 with outpatient surgery rates of 35%. Several groups have developed day surgery protocols for laparoscopic appendicitis, increasing the outpatient rate without an increase in morbidity and mortality,20,21 achieving outpatient treatment rates of 85%, without a greater number of readmissions, and estimated cost savings.22 Several subsequent studies confirm the safety of this approach in adults, without higher rates of complications or readmissions.23,24 The absence of mortality and low morbidity (5%) observed in recent studies,20,23,25 demonstrate the safety and efficacy of this strategy.
Acute cholecystitisAcute lithiasic cholecystitis is diagnosed in 3%–10% of patients with acute abdominal pain and represents 1/3 of emergency admissions.26
Most of the measures do not differ from the usual ERAS recommendations listed above. Below, we show some specific features in the case at hand.
Preoperative measuresThere is some controversy regarding the timing of cholecystectomy in acute cholecystitis. A meta-analysis27 showed that morbidity and conversion to an open procedure is the same for early cholecystectomy (within 7 days of onset of symptoms) and that performed in a second stage. Early cholecystectomy is associated with a significantly shorter total hospital stay and is the treatment of choice according to the recommendations of the latest guidelines.26–28
According to the World Society of Emergency Surgery (WSES) guidelines, early laparoscopic cholecystectomy should be performed as soon as possible, but can be performed up to 10 days after the onset of symptoms (GR-IA). However, we should realize that surgery prior to this time period is associated with a shorter hospital stay and fewer complications (GR-IIB).
With regard to antibiotic prophylaxis, uncomplicated acute cholecystitis can be treated without routine postoperative antibiotics as long as the focus of the infection is controlled by cholecystectomy (GR-IB).29
Intraoperative measuresThe laparoscopic approach for acute cholecystitis is considered safe, feasible, with a low complication rate and associated with a shorter hospital stay (GR-IA). Initially, a laparoscopic approach should be attempted in all patients, except in the case of absolute contraindication due to anesthesia or septic shock (GR-IIB).29
There is no consensus (with retrospective and uncontrolled studies) regarding the value of abdominal drainage after early laparoscopic cholecystectomy for mild or moderate acute cholecystitis (I or II of the Tokyo classification).30 Its use and characteristics depend on the surgeon. It is often used in high-risk populations, although there is no measurable benefit in the postoperative period, and it may even compromise patient recovery in the context of early laparoscopic cholecystectomy for grades I or II acute cholecystitis.31
A Cochrane meta-analysis included a series of randomized studies that compared ‘no drain’ and ‘drain’ strategies after open cholecystectomy, demonstrating that routine use of surgical drains after open cholecystectomy does not provide any patient benefit.30
In contrast, drainage increased the incidence of wound infections, chest infections, and atelectasis, and yet did not affect the incidence of postoperative abdominal collections.32
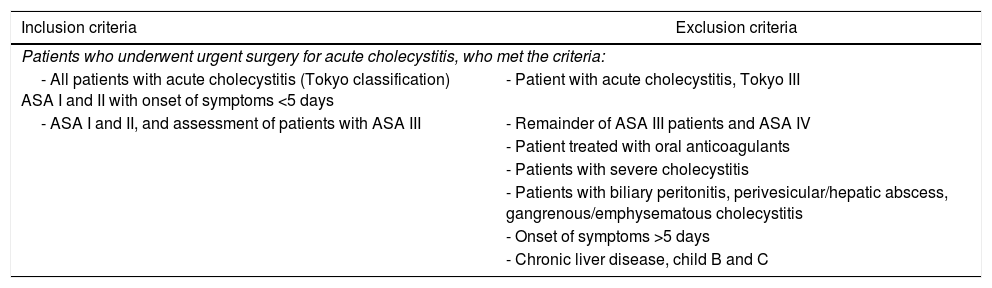
Postoperative measuresIn complicated cholecystitis, antimicrobial regimens will depend on the suspected pathogens involved and the risk factors for the main resistance patterns (GR-IIIB).29Table 1 shows the multimodal rehabilitation protocol in acute cholecystitis of the Spanish Multimodal Rehabilitation Group (Zaragoza, 2016).
Multimodal rehabilitation protocol in acute cholecystitis of the Spanish Multimodal Rehabilitation Group.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients who underwent urgent surgery for acute cholecystitis, who met the criteria: | |
| - All patients with acute cholecystitis (Tokyo classification) ASA I and II with onset of symptoms <5 days | - Patient with acute cholecystitis, Tokyo III |
| - ASA I and II, and assessment of patients with ASA III | - Remainder of ASA III patients and ASA IV |
| - Patient treated with oral anticoagulants | |
| - Patients with severe cholecystitis | |
| - Patients with biliary peritonitis, perivesicular/hepatic abscess, gangrenous/emphysematous cholecystitis | |
| - Onset of symptoms >5 days | |
| - Chronic liver disease, child B and C | |
| Time | Protocol | Responsibility |
|---|---|---|
| Preoperative | Preoperative assessment and ER workup, including PCR | Surgeon+Anesthesiologist+Nurse |
| Antibiotic prophylaxis according to the hospital protocol (maintain until surgery and withdraw post-op) | ||
| All patients who meet the criterion to join the protocol with be well informed and give their written consent | ||
| Perioperative | Intraoperative | SurgeonNurse+Anesthesiologist |
| Induction of anesthesia | ||
| Oxygenation FiO2 0.6–0.8 | ||
| Hemodynamic optimization with goal-directed fluid therapy | ||
| Fluid therapy in continuous balanced perfusion (3.5mL/kg/h for laparoscopy; 7mL/kg/h for laparotomy) | ||
| Urinary catheter, if necessary | ||
| Minimally invasive surgery | ||
| Active heating with a thermal blanket and fluid warmer | ||
| Prophylaxis for postoperative nausea and vomiting, according to the Apfel scale | ||
| No drain tubes, when possible | ||
| Infiltration of laparoscopy ports or TAP block, according to procedure | ||
| Postoperative | Immediately after surgery | Nurse+Anesthesiologist |
| Active temperature maintenance | ||
| Maintenance of FiO2 0.5 2h after surgery | ||
| Analgesia administered according to procedure; minimal administration of morphine | ||
| Restrictive fluid therapy | ||
| Initiation of oral tolerance 6h after surgery (or the following morning if the surgery is evening) | ||
| Ambulation 8h after surgery | ||
| Prophylaxis for thromboembolism starting 12h after surgery | ||
| 1st postoperative day | Progressive diet; if correct oral tolerance, withdrawal of iv fluids | Nurse+Surgeon |
| Assess withdrawal of any surgical drains | ||
| Active movement (bed/chair/initiate walking) | ||
| Oral analgesia | ||
| Assess withdrawal of bladder catheter | ||
| Withdrawal epidural catheter | ||
| Follow-up lab work with CRP | ||
| Prophylaxis for thromboembolism | ||
| Assess discharge | ||
| 2nd postoperative day | Normal diet | Nurse+Surgeon |
| Oral analgesia | ||
| Active movement (walking) | ||
| Prophylaxis for thromboembolism | ||
| Assess discharge | ||
| Discharge | Telephone follow-up after discharge | Nurse+Surgeon+MAP |
| General criteria after discharge: No surgical complications, no fever, pain under control with oral analgesia, normal walking, patient willingness | ||
| Follow-up after discharge/continued care | ||
| Home support-Coordination with Primary Care | ||
The presence of a perforated peptic ulcer is a surgical emergency in which a delay of more than 12h significantly increases mortality. Associated comorbidity increases postoperative complications by nine-fold.33 Diabetic patients have a higher risk of 30-day mortality.34 Advanced age is an independent risk factor for higher mortality in ulcer perforations,35 and the onset of patients with hypotension, metabolic acidosis, kidney damage or hypoalbuminemia is associated with a worse prognosis.36,37 All of this means that there are very few preparatory measures that can be performed before urgent surgery, with 30-day mortality estimated to be around 24%.
Preoperative measuresThe use of a nasogastric tube is indicated to avoid the leakage of irritants.
The infusion of high doses of inhibitors is recommended in digestive bleeding, where the cessation of bleeding and the healing of ulcers have been seen. Although its impact on perforations secondary to ulcers has not been documented, it is recommended to start high doses of proton pump inhibitors as soon as possible with a loading dose of 80mg and 8mg/h of the inhibitor as it is believed to favor fibrin formation and promote rapid sealing of perforations.38
Antibiotic therapy should cover a spectrum that includes enteric gram-negative colonies, anaerobes, and oral mucosal flora. Given the recent resistance of enterobacteria, mainly Escherichia coli, empirical antibiotic therapy should be based on local/regional sensitivity and will have to detect patients at risk of having extended-spectrum beta-lactamases, where antibiotic therapy should be based on ertapenem-type drugs.39
The importance of adequate empirical treatment was made apparent in a study of 425 patients with secondary peritonitis (including patients with perforated ulcer). In this study, 13% of the treatment was inappropriate, and in these patients the resolution of the condition occurred only in 53%, compared to 70% of those treated appropriately. This failure to resolve the symptoms was associated with a 6-day increase in patient hospitalization.40 The efficacy of intravenous treatment for Helicobacter pylori during the postoperative period has not been established, and it is recommended to start treatment after discharge once oral tolerance has been correctly established to avoid resistance if it is interrupted due to lack of tolerance.41
Both intra- and postoperative measures do not differ greatly from the usual ERAS recommendations.
Intraoperative measuresThere is currently no evidence that laparoscopy is superior to open surgery, but there is also no evidence that laparoscopy is harmful in patients with sepsis or generalized peritonitis. As no difference in mortality has been demonstrated by the open versus the laparoscopic technique, the choice of one or the other will be determined by the surgeon's experience and the characteristics of the patient.42
Drain tube placement is recommended in peritonitis, as it has shown fewer postoperative complications.43
Postoperative measuresThe nasogastric tube should not remain in place for more than 2 days, and early onset of tolerance is preferred, although there are few related studies.44
Mechanical obstruction due to colon cancerERAS programs in elective colon surgery have been used with favorable results,45–49 and the implementation in Spain of the IMPRICA program for adherence to the RICA guidelines (Recuperación Intensificada en Cirugía Abdominal or Intensified Recovery in Abdominal Surgery)1 is also relevant.
Their application in urgent colon surgery is rather scarce, and they focus on the obstruction. Shida et al.50 evaluated 122 urgent colectomies for colorectal obstructive neoplasm, 48 treated traditionally and 80 with a modified ERAS program, concluding that these programs reduce hospital stay without increasing morbidity.
Within the management of complete mechanical obstruction due to colon cancer, one of the most debated concepts is the role of stents. It is accepted that in patients with potentially curable colon cancer on the left side of the colon, with high surgical risk, ASA III or >70 years of age, stents could be considered a good alternative as a bridge to subsequently perform scheduled ERAS surgery.51
In urgent surgery for colon cancer with complete mechanical obstruction, various measures have been studied based on the recommendations of the ERAS group and their impact on morbidity and mortality.4
Preoperative measuresIt is recommended to estimate the surgical risk (ASA, CR-POSSUM). A risk greater than 10% implies a need for admission to the intensive care unit, postoperatively and even preoperatively for optimization prior to surgery.52
A central venous catheter is necessary for goal-directed fluid therapy (central venous pressure between 8 and 12cm H2O, measured arterial pressure of 65 or less, and urinary volume of at least 0.5mL/kg/h). Volume replacement is indicated with isotopic saline solutions or lactated Ringer's.53
In patients with severe colon distention and vomiting, a nasogastric tube is recommended.54
Early initiation of broad-spectrum antibiotic therapy is also recommended, and the empirical choice of antibiotics is determined according to the sensitivities in the area.
Intraoperative measuresIn patients who are scheduled to undergo open surgery, an epidural catheter should be considered together with general anesthesia for better control of postoperative pain. Its use should not be indicated in patients with coagulopathy, bleeding tendencies or hemodynamic instability.55
Goal-directed fluid therapy is recommended, although there are not many studies in emergency surgery, and the results do not seem to influence morbidity, mortality, or renal function.53
Perioperative hypothermia is associated with increased surgical site infection, more cardiac complications, and increased blood loss. Hypothermia prevention is recommended from the preoperative period, using active heating systems.
Laparoscopic surgery in urgent colectomy is difficult to perform. Rea et al. analyzed 67645 patients (multicenter data), and only 3.9% were treated laparoscopically, with a conversion rate of 55%.56 The laparoscopic approach is associated with a shorter hospital stay and a lower rate of postoperative complications, although the extreme technical difficulty of laparoscopic surgery in patients with complete mechanical obstruction due to colon cancer must be taken into account, which requires super-specialization that is not always available at the time of surgery, so the laparoscopic approach is only recommended in specialized units.57
The latest WSES 2017 guidelines do not recommend intraoperative colon lavage (GR-IB) as it does not influence anastomotic dehiscence or morbidity.
Surgical drains are not generally recommended, except in cases associated with significant bleeding, purulent or fecal peritonitis, and high-risk anastomosis.58
Postoperative measuresMultimodal analgesia adapted to the emergency is recommended, where prior standardization is not possible.54
Several studies recommend early removal of the nasogastric tube, even after surgery, since its early removal is not related to a worse outcome.54
No obvious improvement in the recovery of lung function or in the decrease in respiratory complications59 has been demonstrated with the use of an incentive spirometer.
Early initiation of oral tolerance is different from scheduled surgery, and the presence of ileus should make us consider slower initiation.58
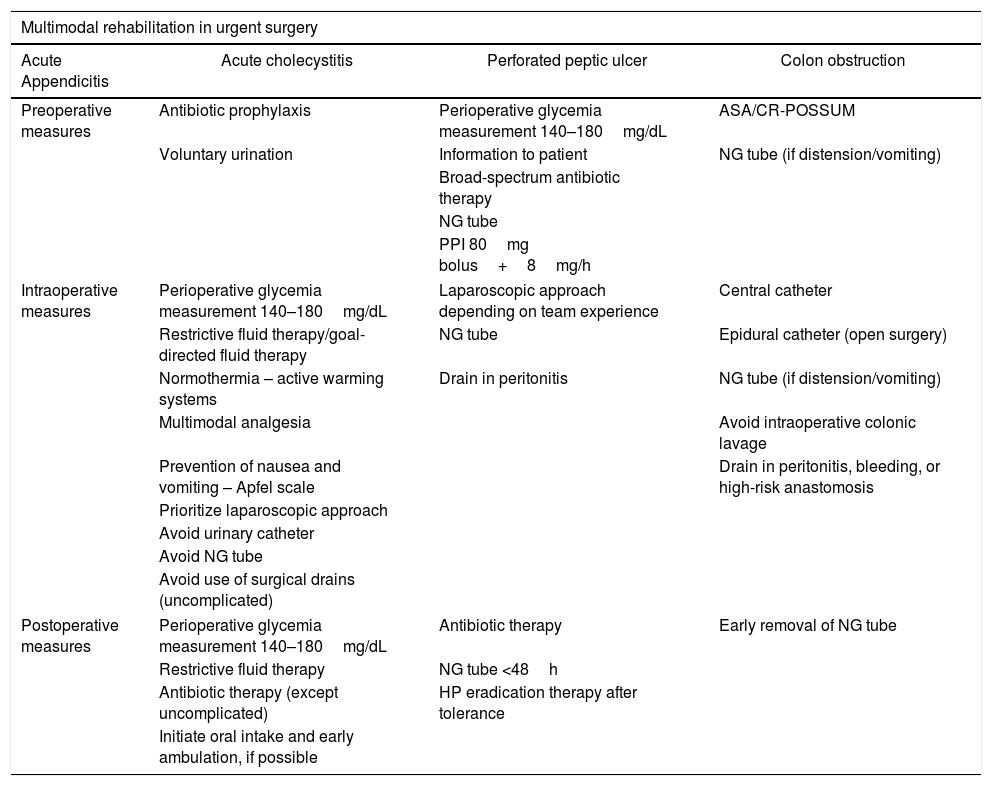
And lastly, although there is no evidence on the benefits, early mobilization is recommended because chronic bedrest is associated with a higher risk of thromboembolism, muscle weakness, pneumonia, and insulin resistance.60Table 2 summarizes the current recommendations for the different pathologies set out in the text.
Summary of current recommendations for different pathologies discussed in the text.
| Multimodal rehabilitation in urgent surgery | |||
|---|---|---|---|
| Acute Appendicitis | Acute cholecystitis | Perforated peptic ulcer | Colon obstruction |
| Preoperative measures | Antibiotic prophylaxis | Perioperative glycemia measurement 140–180mg/dL | ASA/CR-POSSUM |
| Voluntary urination | Information to patient | NG tube (if distension/vomiting) | |
| Broad-spectrum antibiotic therapy | |||
| NG tube | |||
| PPI 80mg bolus+8mg/h | |||
| Intraoperative measures | Perioperative glycemia measurement 140–180mg/dL | Laparoscopic approach depending on team experience | Central catheter |
| Restrictive fluid therapy/goal-directed fluid therapy | NG tube | Epidural catheter (open surgery) | |
| Normothermia – active warming systems | Drain in peritonitis | NG tube (if distension/vomiting) | |
| Multimodal analgesia | Avoid intraoperative colonic lavage | ||
| Prevention of nausea and vomiting – Apfel scale | Drain in peritonitis, bleeding, or high-risk anastomosis | ||
| Prioritize laparoscopic approach | |||
| Avoid urinary catheter | |||
| Avoid NG tube | |||
| Avoid use of surgical drains (uncomplicated) | |||
| Postoperative measures | Perioperative glycemia measurement 140–180mg/dL | Antibiotic therapy | Early removal of NG tube |
| Restrictive fluid therapy | NG tube <48h | ||
| Antibiotic therapy (except uncomplicated) | HP eradication therapy after tolerance | ||
| Initiate oral intake and early ambulation, if possible | |||
Although some groups have suggested global inclusion of patients and pathologies in ERAS programs, they often define exceptions and exclude patients with multiple comorbidities, significant chronic disease (including mental illness), high anesthetic risks (ASA>III), alcohol abuse, diseases that make epidural analgesia impossible, and language difficulties, while the exclusion factor par excellence was urgent clinical presentation.
Despite the large volume of existing literature on ERAS in elective surgery, there are few studies that have investigated the effectiveness of ERAS programs in urgent surgery patients, although some guidelines indicate that their use may be appropriate.5
Gonenc et al.44 demonstrated safety and viability in selected upper gastrointestinal tract emergencies and Lohsiriwat61 in colorectal emergencies. Wiseley and Barclay5 retrospectively studied all types of urgent surgery in 370 patients. The most frequent etiology was obstruction, but the etiology was not recorded by subgroup of surgical pathology, 169 patients in the study were pre-ERAS compared to 201 ERAS, showing a significant decrease in morbidity for this group and concluding that the application of ERAS programs in urgent patients is not harmful to them. Likewise, Le Guen et al.62 support many ERAS measures, although they acknowledge a limited level of evidence.
In 2009, within the English guidelines for the implementation of enhanced recovery protocols63 it was recommended that “everything should be done to implement as many measures as possible” in the context of enhanced recovery protocols in the emergency setting.
The current situation of our healthcare system forces us to identify areas for improvement where we can be more efficient without affecting the quality of care. Recent publications in the urgent field look for methods to reduce hospital costs and stays, maintaining a high quality of care and patient satisfaction.64
ConclusionIt is necessary to establish multidisciplinary working groups interested in developing ERAS protocols for patients with urgent pathology and implementing multicenter projects that guarantee their viability.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to acknowledge Carlo Brugiotti, Xavier Viñas, María José Fas, Alberto Felipe Bravo, Antonio Pérez, José Antonio López, Irene Ortega, Gianchandani Moorjani, Rajesh Haresh, Luis Tallón, Carlos David Albendea, Maria Infantes, Ruben Gonzalez, Ricardo Ortega, Ana Palomares, Antonio Dámaso, Luis Vega, Marina Molinete, Aitor Landaluce, Enric Macarulla y Bakarne Ugarte, who participated in the 2016 Zaragoza Conference for the development of the initial protocols for multimodal rehabilitation in urgent surgery of the Spanish Multimodal Rehabilitation Group (currently being revised by the workgroup*), from which we showed an example in this article.
*Emergency Surgery Workgroup of the Spanish Multimodal Workgroup: Bakarne Ugarte, Aitor Landaluce, Isaac Cabrera, José Antonio López, Susana Postigo, Manuel Artiles, Jaume Tur, María Dolores Pérez, Fernando Turégano, Alexander Forero, Andrea Craus, Francisca García-Moreno, Luis Tallón, Ricardo González, Pablo Muriel, Providencia García, Nicolás Macías, Jennifer García, Isidro Martínez, Luis Alberto Martínez, Diéter Morales, Andrea Campos, David Costa, Ignacio Rey, Francisco Blanco, Francisco Jiménez, Marta González, Rula Nasimi, Marta Gutiérrez.
Please cite this article as: Ugarte-Sierra B, Landaluce-Olavarria A, Cabrera-Serna I, Viñas-Trullen X, Brugiotti C, Ramírez-Rodríguez JM, et al. Rehabilitación multimodal en cirugía de urgencias: ¿utopía o realidad? Cir Esp. 2021;99:258–266.