Up to 40% of all initial operations for soft tissue sarcoma (STS) are unplanned, which would leave residual macroscopic tumor in more than 50% of the cases. The effect this has on local recurrence rate, metastases rate and survival has never been fully established, due to the lack of randomized studies.
MethodsRetrospective review of patients with STS treated in our unit between January 2001-January 2016. We classified them whether they had been treated by initial planned or unplanned operation. Outcomes were compared in both groups globally and stage-matched. Endpoints were local recurrence and distant metastases.
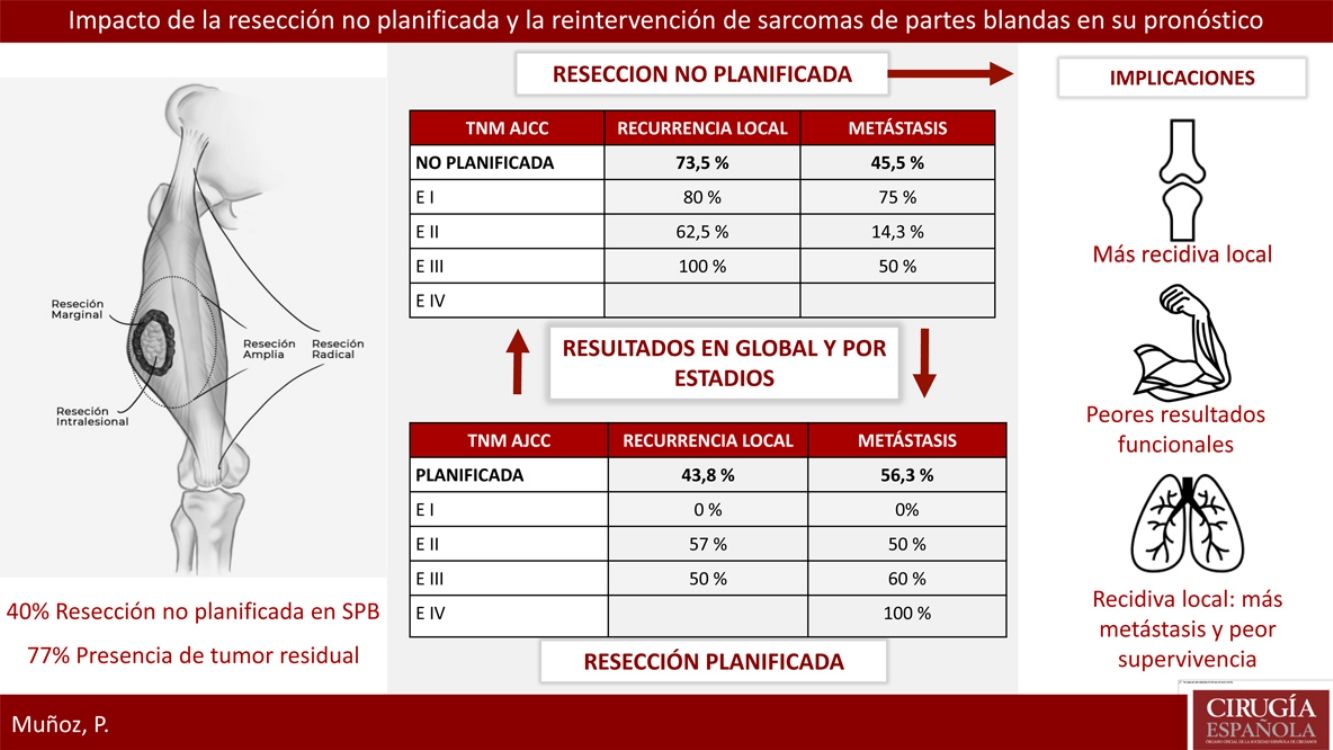
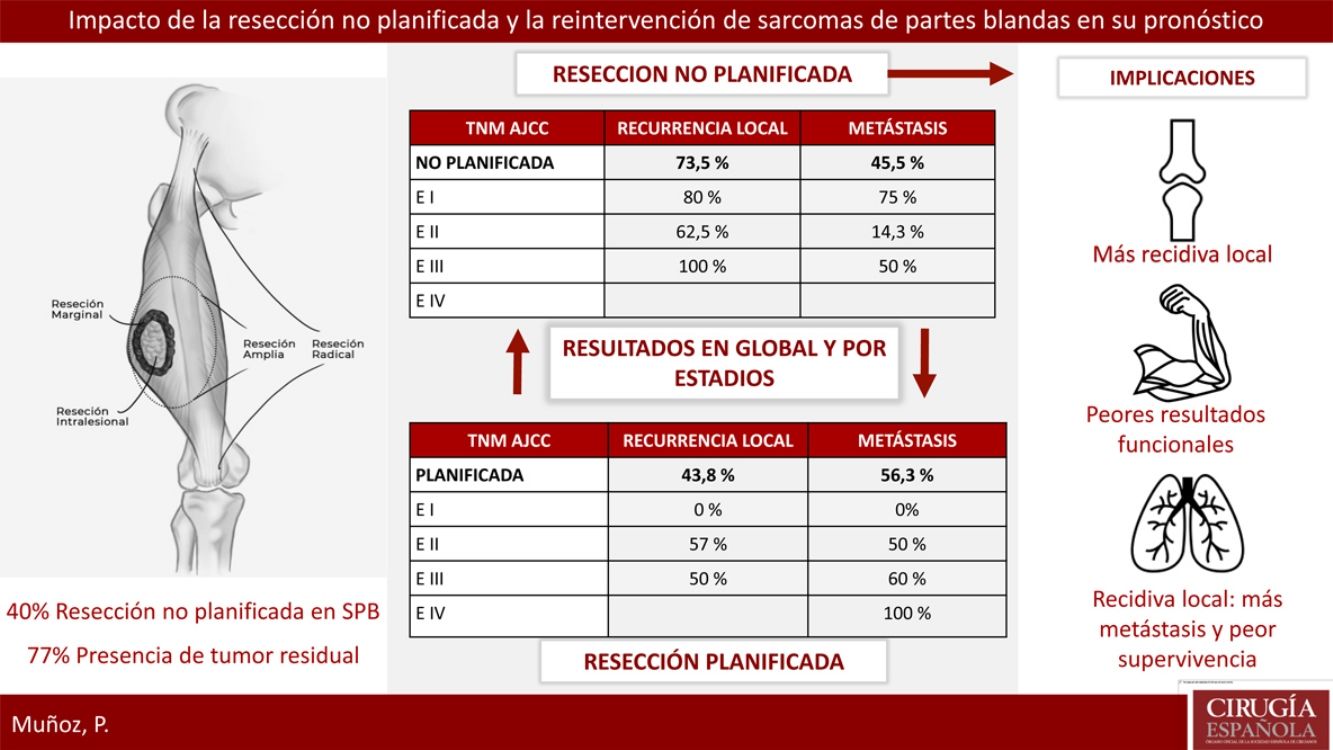
ResultsTwenty-three patients of STS underwent a planned excision and 16 an unplanned excision, 13 of them underwent further re-excision. 40% of patients with planned excision had an advanced stage in regard to the unplanned excision group which presented earlier stages. 77% of patients with unplanned excision had residual tumor identified after surgical re-excision. Local recurrence rate in the unplanned excision group was considerably higher 73.5% vs 43.8%. Metastases rate was lower in planned excision group, 45.5% vs 56.3% (P>.05). The recurrence pattern in the unplanned excision group was unstable, with worse outcomes in earlier stages.
ConclusionThe unplanned excision of a soft tissue sarcoma may compromise disease local control, with higher rates of local recurrence and metastases, and worse functional outcomes, despite further oncological treatment. We need to recognize the clinical features for malignancy risk in soft tissue lumps for a safe diagnosis to avoid inadequate resections.
Hasta un 40% de los sarcomas de partes blandas (SPB) son resecados de forma no planificada, dejando tumor residual en más del 50% de los casos. La implicación pronóstica de estas resecciones no está claramente definida, dado que existen escasos estudios comparativos que demuestren cómo afecta a la tasa de recurrencia local, de metástasis y de supervivencia.
MétodosRevisión retrospectiva de pacientes intervenidos de un SPB de enero de 2000 a enero de 2016 clasificándolos respecto a intervención planificada o no planificada. Se compararon las tasas de recurrencia y metástasis en global y por estadios.
ResultadosVeintitrés pacientes con SPB fueron tratados de forma planificada y 16 de forma no planificada, con 13 reintervenciones. El 40% del grupo planificado presentó un estadio avanzado respecto al 20% del grupo no planificado. El 77% de los pacientes con resección no planificada reintervenidos presentaron tumor residual en la pieza. La tasa de recidiva local en el grupo de no planificados fue considerablemente más alta (73,5% frente al 43,8%). La tasa de metástasis en no planificados fue del 45,5%, frente al 56,3% en planificados (p>0,05). En el grupo de no planificados el patrón de recidiva fue más errático con peores resultados en estadios precoces.
ConcusionesLa resección no planificada de los SPB asocia mayores tasas de recurrencia local y peores resultados funcionales a pesar del manejo oncológico posterior. En las lesiones de partes blandas es fundamental reconocer los signos de alarma que sugieren malignidad para llevar a cabo un estudio diagnóstico específico y evitar resecciones inadecuadas.
Soft tissue sarcomas (STS) are a heterogeneous group of tumors with more than 70 different histological types, and their prognosis is determined by the characteristics of the primary tumor.1 They are rare lesions, so it is common for them to be managed incorrectly, compromising the prognosis of the disease. These are aggressive tumors, and it is estimated that approximately 50% of patients will die from their sarcoma.
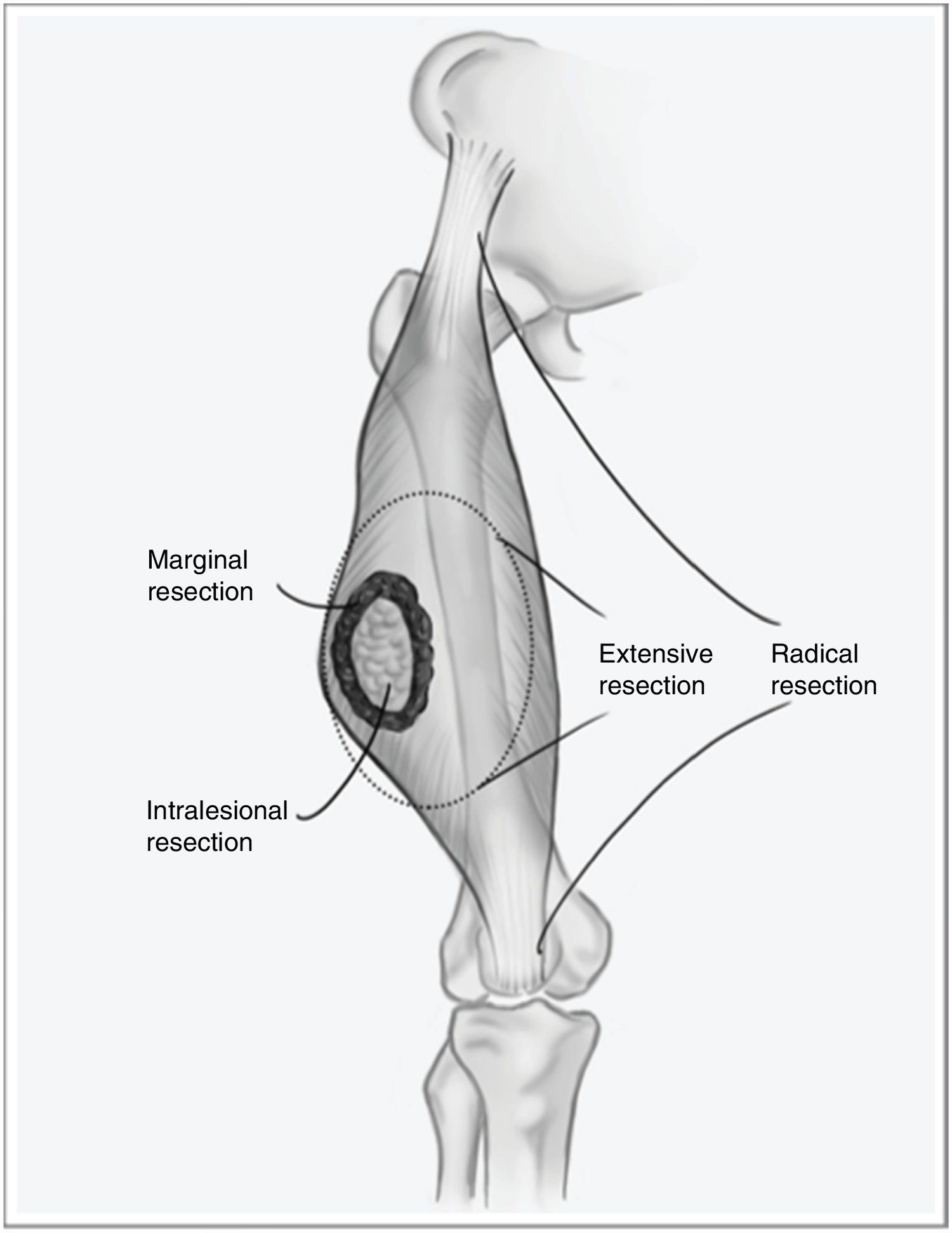
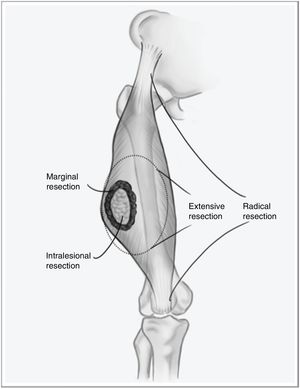
However, when the disease debuts locally, curative treatment is possible. The fundamental pillar of this treatment is surgery with wide resection margins using an extensive, and not marginal, excision, which would involve going through the tumor pseudocapsule (Fig. 1). Associated radiotherapy is administered in cases with high tumor risk – high histological grade, deep presentations, or larger than 5cm – and in cases where the tumor resection margin is less than 1cm.2,3 The benefit of adjuvant chemotherapy is limited, with little impact on survival compared to the side effects it causes. Recent studies have shown that neoadjuvant chemotherapy is able to improve survival in five types of high-risk sarcomas. Furthermore, it favors the possibility of less aggressive resections and improving the rate of complete resections, which are fundamental for local control of this disease, and to a lesser extent for overall survival.4
Given the low frequency and heterogeneity of these tumors, clinical guidelines consider it essential for them to be managed at hospitals specialized in sarcomas with extensive experience in their treatment.5
One of the main stumbling blocks in the management of STS is unplanned resection, or non-oncological resection, which affects approximately 40% of diagnosed cases.6,7 This type of surgery involves intralesional resection or enucleation through the tumor pseudocapsule, which is not a histological delineation of the tumor but instead a crown of healthy cells from the surrounding tissues infiltrated by the tumor. This phenomenon implies that there is no tissue ‘border’ for these tumor cells, and it is associated with a significant increase in local recurrence compared to standardized resections. Zagars et al.8 published a study whose objective was to document the influence of re-resection in patients with unplanned surgery on the prognosis of the disease, finding that more than 53% of the re-operated patients had residual tumor in the surgical specimen. Reoperation was shown to be a determining factor for the lower local recurrence rate (LR) (85% vs 78%), longer metastasis-free time and increased disease-specific survival in patients who had undergone previous incomplete surgery.
When a sarcoma is resected in an unplanned manner, the patient should be sent to a referral hospital for specific management. Once there, the pathology study should be reviewed, the risk of the tumor stratified, an imaging study carried out to determine the feasibility and extent of the new resection, and an extension study, especially if it has been a long time since the first surgery. Clinical guidelines recommend that these cases should always be treated with a second resection providing free margins and adjuvant radiotherapy, whether or not there is evidence of a residual lesion, as it is not possible to predict which patients will have residual tumor.9 Several studies have analyzed the impact of these resections on the rate of LR, distant recurrence and overall survival, demonstrating a clear detriment to local control and functional results. However, the results are less clear in terms of overall survival.10
This study retrospectively analyzes the prognostic differences in terms of LR, distant recurrence and survival of patients diagnosed with STS who were treated with unplanned resection at our hospital compared to those who underwent planned surgery, with or without adjuvant treatment.
MethodsA retrospective descriptive study was carried out of all the patients treated surgically for STS since 2000 in our service. Patient data was collected from our hospital's database and medical records archive.
Initially, patients were classified as to whether the first intervention was planned or unplanned. Unplanned resection was defined as one not performed according to established oncological standards, either due to not having a preoperative histological diagnosis, or due to erroneous surgical management – enucleation or intralesional surgery – when a benign lesion was suspected as the initial diagnosis. Patients who were referred to our hospital after an unplanned resection were also included.
Age at diagnosis, sex, location, histology, tumor stage, and tumor grade were recorded. Tumor grade is a histological characteristic of aggressiveness related with the risk of metastasis. In our series, we used the grading system of the French Federation of Cancer Centers Sarcoma Group (FFCCSG).11 Staging was done in accordance with the 7th edition of the American Joint Commission on Cancer for STS and not the 8th, since this latest update had not been published at the time of the review.12
In the ‘unplanned’ group, we recorded whether reoperation had been carried out and whether there was residual tumor in the new surgical specimen. Finally, the rates of local recurrence and metastasis in both groups were compared, both globally and by stages.
Statistical AnalysisStatistical analysis was conducted with version 24.0 of the SPSS statistical software. The chi squared test was used for the analysis of the variables of this study, and the log rank test for the survival analysis. A P value of <.05 was considered statistically significant.
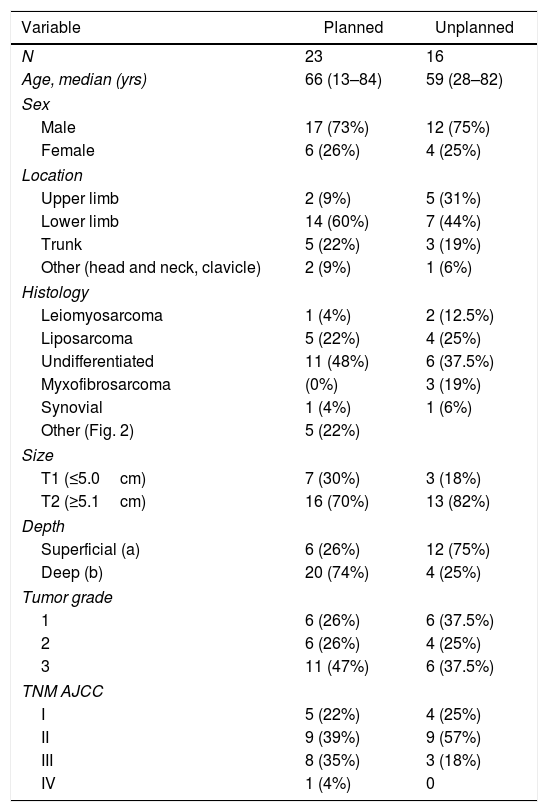
ResultsFrom January 2000 to January 2016, 23 patients with STS were treated with planned resection, and 16 with unplanned resection, 13 of which were reoperated.
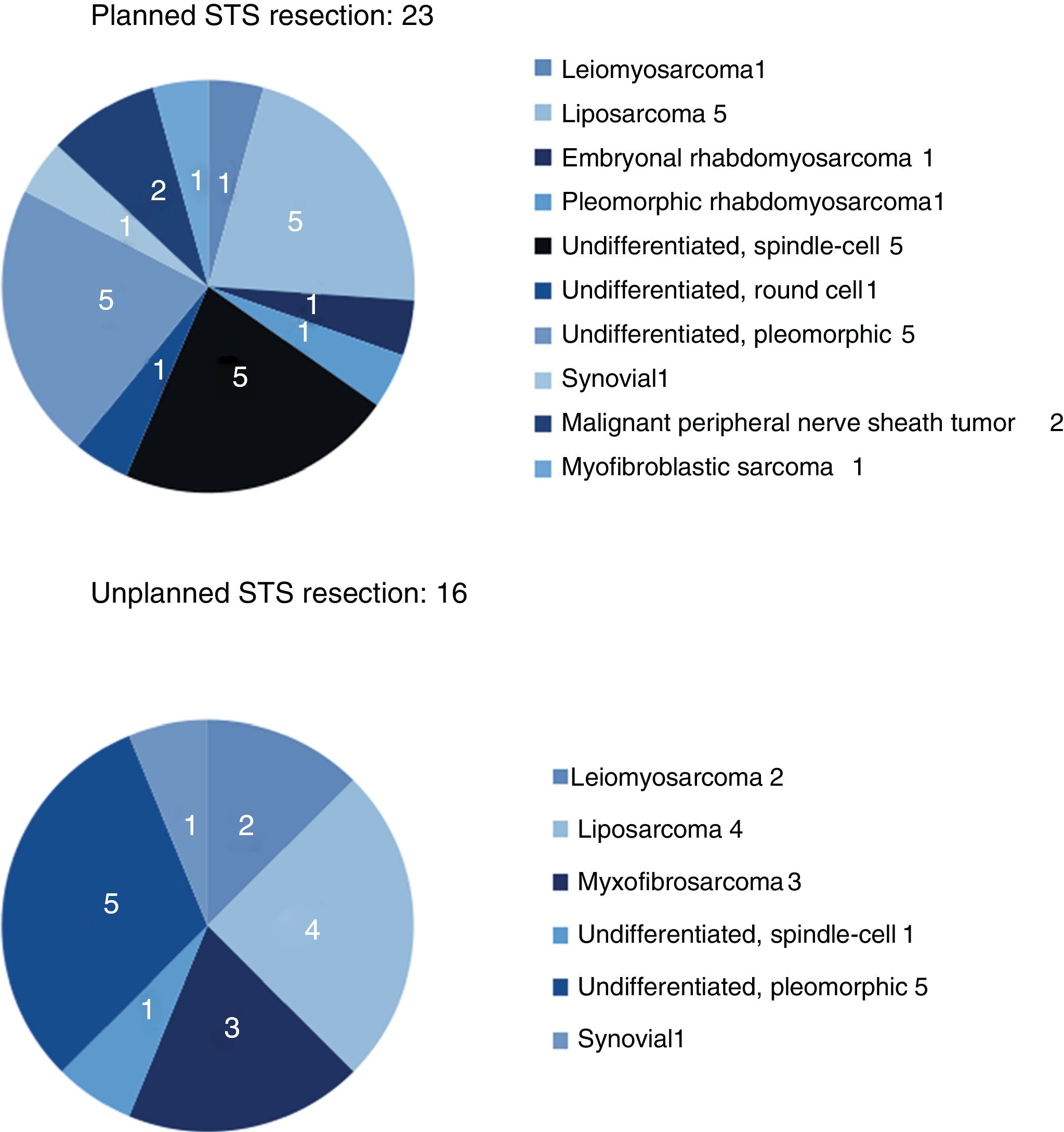
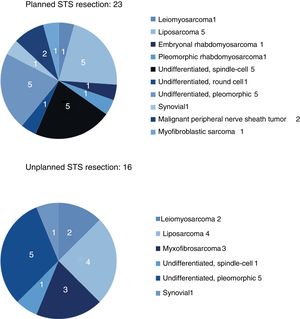
The results for sex and location were similar in both groups, and the most frequent location was the lower limbs. The most frequent histology in the unplanned resection group was liposarcoma (25%), and undifferentiated sarcoma in the planned resection group, reaching 50% when the different subtypes were grouped. Between both groups, more than 11 different histologies were identified (Fig. 2).
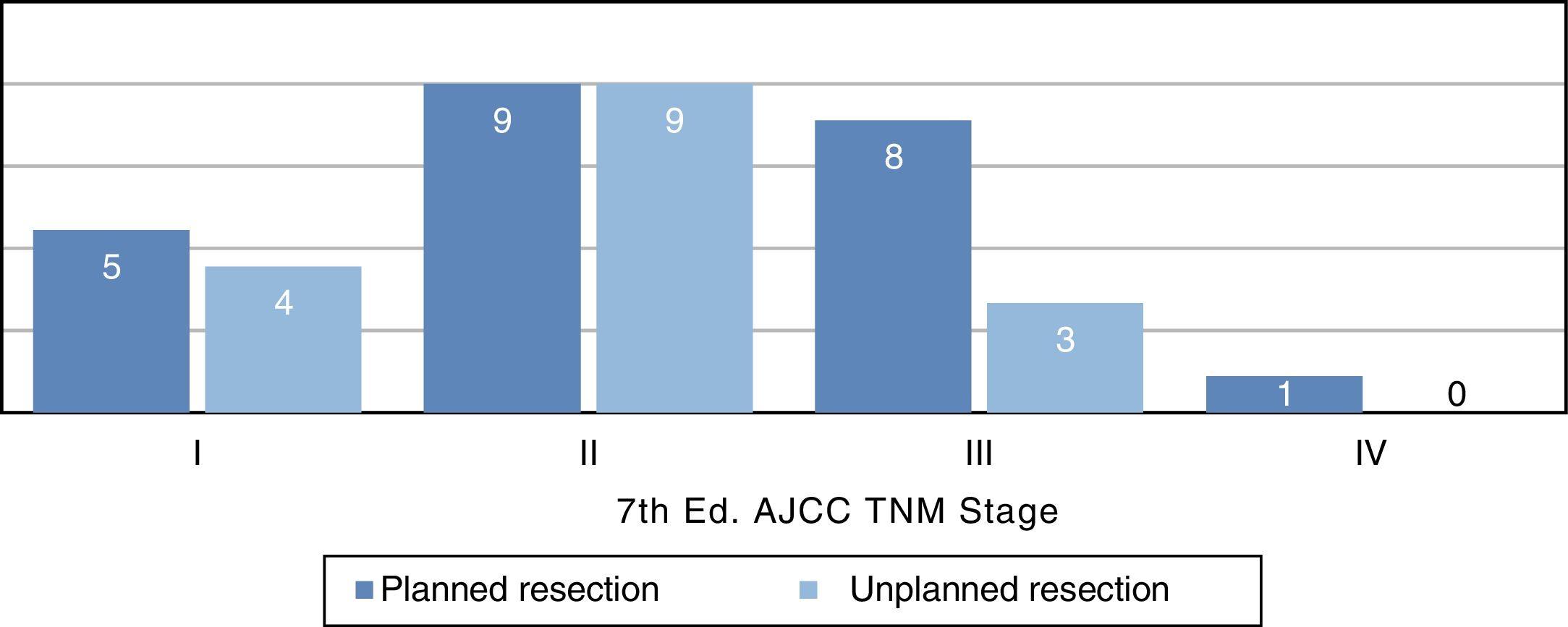
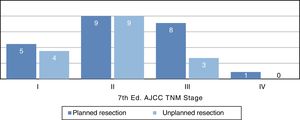
Distribution by stages (Fig. 3) was not statistically significant, probably due to a type 2 error as a result of the small sample size; however, the majority of tumors in the unplanned resection group belonged to stages I and II. The demographic and pathological characteristics of both groups are summarized in Table 1.
Demographics and Pathological Characteristics.
| Variable | Planned | Unplanned |
|---|---|---|
| N | 23 | 16 |
| Age, median (yrs) | 66 (13–84) | 59 (28–82) |
| Sex | ||
| Male | 17 (73%) | 12 (75%) |
| Female | 6 (26%) | 4 (25%) |
| Location | ||
| Upper limb | 2 (9%) | 5 (31%) |
| Lower limb | 14 (60%) | 7 (44%) |
| Trunk | 5 (22%) | 3 (19%) |
| Other (head and neck, clavicle) | 2 (9%) | 1 (6%) |
| Histology | ||
| Leiomyosarcoma | 1 (4%) | 2 (12.5%) |
| Liposarcoma | 5 (22%) | 4 (25%) |
| Undifferentiated | 11 (48%) | 6 (37.5%) |
| Myxofibrosarcoma | (0%) | 3 (19%) |
| Synovial | 1 (4%) | 1 (6%) |
| Other (Fig. 2) | 5 (22%) | |
| Size | ||
| T1 (≤5.0cm) | 7 (30%) | 3 (18%) |
| T2 (≥5.1cm) | 16 (70%) | 13 (82%) |
| Depth | ||
| Superficial (a) | 6 (26%) | 12 (75%) |
| Deep (b) | 20 (74%) | 4 (25%) |
| Tumor grade | ||
| 1 | 6 (26%) | 6 (37.5%) |
| 2 | 6 (26%) | 4 (25%) |
| 3 | 11 (47%) | 6 (37.5%) |
| TNM AJCC | ||
| I | 5 (22%) | 4 (25%) |
| II | 9 (39%) | 9 (57%) |
| III | 8 (35%) | 3 (18%) |
| IV | 1 (4%) | 0 |
Reoperation was performed in 13 of the 16 patients who underwent unplanned resection; 100% of these patients presented margin involvement in the first intervention, while 77% of the reoperated patients presented macroscopic residual tumor in the reoperation specimen.
The unplanned resection group underwent a greater number of reoperations (median of 4) and presented worse functional results, with at least three registered limb amputations and one hemipelvectomy. In the unplanned group, 71.4% received some type of radiotherapy treatment (intraoperative radiotherapy vs adjuvant radiotherapy), versus 85% of the planned group.
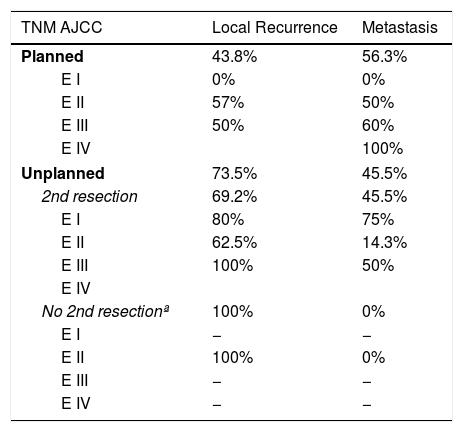
Regarding the presence of local or distant recurrence, global and stage analyses were carried out to eliminate the bias conferred by a worse prognosis in tumors with planned resection because they involved more advanced stages. The overall LR rate in the unplanned group was 73.5%, compared to 43.8% in the planned group. All tumors (100%) that had been resected in an unplanned manner and were not reoperated presented LR. The overall metastasis rate in the unplanned group was 45.5%, compared to 56.3% in the planned group. None of these differences was statistically significant (P>.05).
Table 2 shows how local and distant recurrence patterns followed a progressive tumor stage distribution in tumors with planned resection. However, in unplanned cases, the distribution of recurrence was more erratic, and the poor prognosis of tumors at earlier stages in this group is striking.
Results by Stages in Both Groups.
| TNM AJCC | Local Recurrence | Metastasis |
|---|---|---|
| Planned | 43.8% | 56.3% |
| E I | 0% | 0% |
| E II | 57% | 50% |
| E III | 50% | 60% |
| E IV | 100% | |
| Unplanned | 73.5% | 45.5% |
| 2nd resection | 69.2% | 45.5% |
| E I | 80% | 75% |
| E II | 62.5% | 14.3% |
| E III | 100% | 50% |
| E IV | ||
| No 2nd resectionª | 100% | 0% |
| E I | − | − |
| E II | 100% | 0% |
| E III | − | − |
| E IV | − | − |
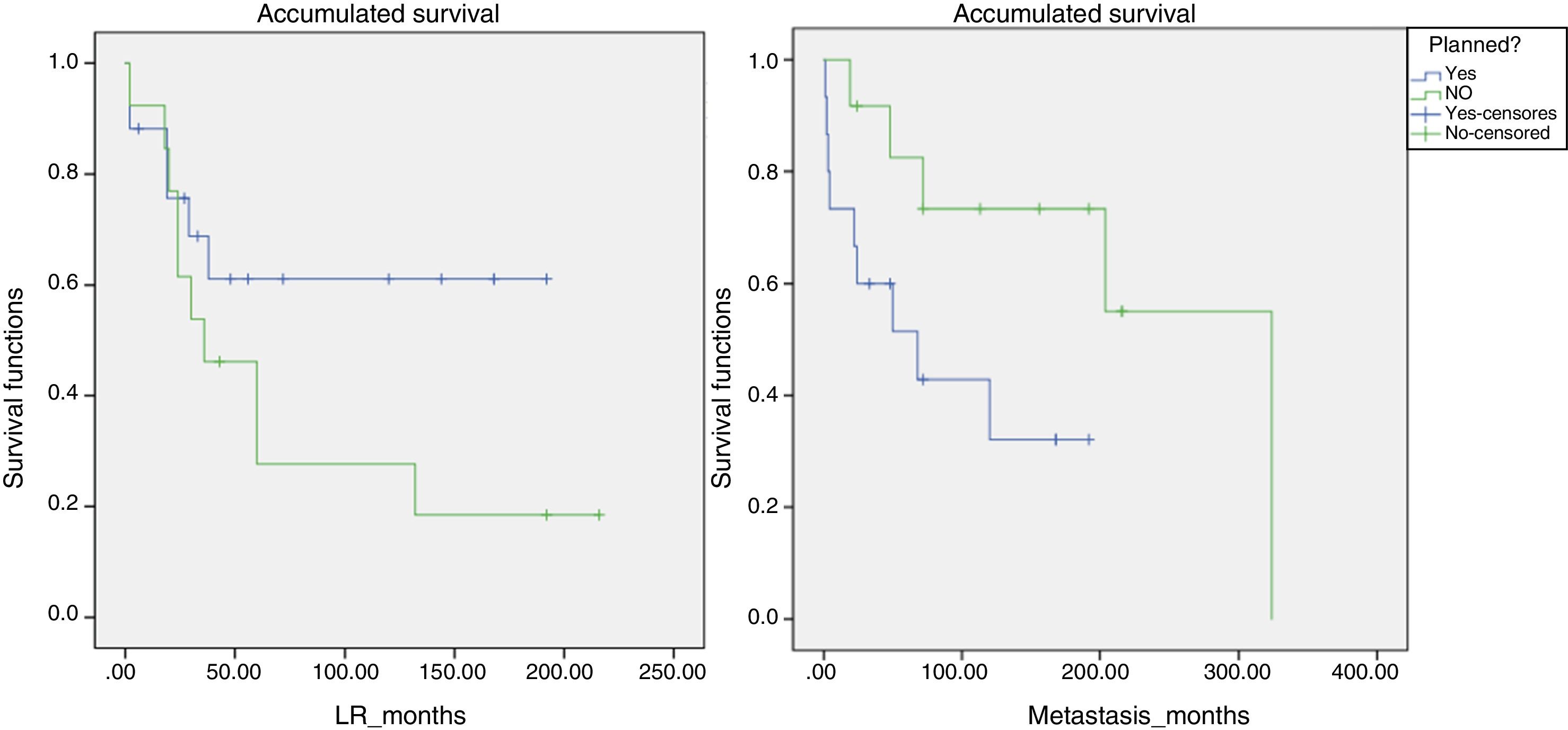
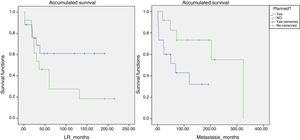
Likewise, a survival analysis was carried out for LR, metastasis and overall survival, comparing both groups. The median time to LR in the unplanned group was 6 years and in the planned group over 10 years (P>.05). However, the results were more favorable for the presence of metastases and survival in the group of unplanned patients, and the log-rank comparison for the development of metastases was statistically significant (P=.048) (Fig. 4). We also analyzed whether the LR rate was related to the use of adjuvant or intraoperative radiotherapy, although statistical significance was not reached. However, the median time until LR was 116 months in the group that received radiotherapy and 65 months in the non-treatment group.
We analyzed the correlation between LR and presence of metastasis and survival. Some 60% of patients with LR presented metastasis at some point, while only 25% of those who did not present LR developed metastasis (in all cases it was an early distant recurrence, within 4 months of diagnosis). In terms of mortality, 46% of the patients with LR died from the disease, compared to 18.2% of patients who did not have LR (P>.05).
DiscussionUnplanned resection of STS often compromises the oncological management of these tumors, which require extensive surgery with a margin of at least 1–3cm.13 This concept was first introduced by Giuliano and Eilber14 in 1985 as surgery in which an excisional biopsy is performed, or in which the lesion is resected without having completed a proper diagnostic process and without the intention of achieving an adequate margin.15 This situation is frequent because benign soft tissue tumors are much more frequent than sarcomas, and it is the lack of suspicion of a malignant lesion that leads to this error in management. As the literature shows, the majority of sarcomas managed with unplanned resection are small and superficial lesions.16 In our cohort, more than 80% of the unplanned resected tumors measured less than 5cm, and 75% were superficial to the fascia.
If we refer to the distribution by stages, we see a trend in which most unplanned tumors are stage IIb (Fig. 3), which corresponds with small tumors of high histological grade. The distribution by stages in the planned group is more homogeneous and also includes a higher percentage of advanced stages, which shows that large lesions are studied more frequently.
Sarcoma histology would also be a risk factor for unplanned resection, since very fatty homogeneous-looking lesions are most often confused with a lipoma or another benign lesion. The Fiore and Lewis series agree that liposarcoma is the most frequently found tumor in the group of unplanned resections, which coincides with our series, where liposarcoma was found in 25% of these cases.17
Unplanned sarcoma surgery implies the existence of residual tumor cells in the surgical site that would have lost their anatomical limit.18 This phenomenon has been associated with higher local recurrence rates and worse functional results due to the need for extensive reoperations for local control. In our series, 77% of the reoperated tumors presented residual tumor, and in all of them free margins were achieved during reoperation. However, reoperation could not compensate for the negative effect of unplanned resection, and this group of patients presented an LR rate of 73.5%, compared to 44% in the group that underwent a planned procedure. There is no clear negative impact of unplanned resection on metastasis or survival rates, and, in fact, the survival analysis of our series showed that the unplanned resection group presented better results than the control group. This controversy also appears in the majority of studies in this regard, where the detriment to local control and worse functional results is clear, but where, paradoxically, a higher rate of metastasis is evident in the control group.19 This phenomenon could be explained by the fact that there is no homogeneity in the tumor characteristics of both groups in terms of tumor stage. In a brilliant paper, Hayes et al. addressed this problem using a prognostic analysis stratified by tumor stage between the two groups, demonstrating that in stage III tumors the difference between the metastasis rate and disease-specific survival was significant, with worse results in the unplanned resection group.20 In addition to the different distribution by stages, there are also important differences in location and histology between the two groups, with pathological characteristics for a better prognosis in the unplanned group. In our series, 75% were located superficially and were mainly liposarcomas and pleomorphic sarcomas. Many superficial sarcomas – dermal pleomorphic sarcoma, atypical lipomatous tumor/well-differentiated liposarcoma, leiomyosarcoma – are known to be less aggressive than their deep counterparts, especially because of their limited metastatic capacity.21
Our review does not demonstrate the influence of LR on the development of metastases or mortality, although their percentages are much higher in patients who presented LR (60% metastasis and 46% mortality). Larger series have confirmed the hypothesis of the negative repercussions of LR on the prognosis of STS, suggesting that failed local control, with multiple recurrences and reoperations, would ultimately be a determining factor for worse survival due to the direct effect on metastatic spread or the detrimental effect of an LR on a critical anatomical location.22
The best strategy to prevent mishandling of STS is to disseminate the concept of how to deal with a soft tissue injury that could potentially be a sarcoma. While it is difficult to clarify the clinical characteristics of this disease, five warning signs have been described that should make us suspect malignancy: rapid growth, size greater than 5cm, painful injury, deep lesion to the fascia and recurrence after excision.23 If any of these characteristics exists, an ultrasound should be performed within 2 weeks. If the ultrasound scan cannot confirm that it is a benign lesion, or if suspicion persists, the patient should be referred to a specialized hospital for an MRI extension study and core needle biopsy, preferably radiology-guided to locate the most heterogeneous areas of the lesion.24,25 The preoperative pathology study is essential to identify the histology and tumor grade in order to guide the treatment plan, which may include neoadjuvant chemotherapy treatment in certain cases.
Conflict of InterestsAll authors declare that there is no conflict of interests.
Please cite this article as: Muñoz Muñoz P, Bajawi Carretero M, González Barranquero A, Mena Mateos A, Corral Moreno S, Sanjuanbenito Dehesa A, et al. Impacto de la resección no planificada y la reintervención de sarcomas de partes blandas en su pronóstico. Cir Esp. 2020;98:281–287.