The current imbalance between donor supply and patients on the waiting list for liver transplantation (LT) is significant. To resolve this situation, marginal organs, such as those from type 2 donation after cardiac death (DCD2), are being considered.
MethodsIn the present article, we present the first LT with a new protocol consisting in normothermic regional perfusion (NRP) and normothermic machine perfusion (NMP) for a type 2 DCD graft initially rejected for LT.
ResultsAfter a favorable evolution with NMP (improved macroscopic appearance of the graft, acid–base equilibrium control and bile production), the transplantation was performed without major incidents. The evolution of the graft and patient were favorable. After 3 months, cholangiography showed no signs of ischemic cholangiopathy.
ConclusionsThree-month patient and graft survival are encouraging, but more cases are needed to test the clinical efficacy of the new protocol.
Hoy en día, el desequilibrio entre el número de donantes y los pacientes en lista de espera para trasplante hepático (TH) hace necesaria la utilización de órganos con criterios expandidos, como los de donación en asistolia tipo 2.
MétodosPresentamos el primer TH realizado mediante un protocolo de perfusión regional normotérmica y máquina de perfusión normotérmica para un injerto de donación en asistolia tipo 2 inicialmente descartado para TH.
ResultadosTras una evolución favorable en máquina de perfusión normotérmica (mejoría del aspecto macroscópico del injerto, control del equilibrio ácido-base y producción de bilis), el TH se realizó sin incidencias. Después de 3 meses la evolución del paciente y del injerto son correctas, sin signos de colangiopatía isquémica en la colangiografía trans-Kehr.
ConclusionesLa supervivencia del injerto y del receptor invitan al optimismo. Sin embargo, son necesarios más casos con el objetivo de verificar la eficacia clínica del nuevo protocolo.
Liver transplantation (LT) is the most successful treatment for terminal liver disease and hepatocellular carcinoma. However, there is a significant imbalance between the number of organs available for LT and patients on the waiting list.1,2 The alternative of using livers from type 2 donation after cardiac death (DCD) presents an unexplored potential.3 Its use is generally associated with higher rates of post-transplant complications (especially primary graft failure and ischemic cholangiopathy) as a consequence of the warm ischemia time to which these organs are subjected.4–6 Our group has worked extensively in the field of LT with type 2 DCD grafts, developing a protocol based on normothermic regional perfusion (NRP).7 The results obtained with this protocol are comparable to those of LT in brain death donors, but their applicability is reduced due to the strict acceptance criteria.4 The use of ex vivo normothermic machine perfusion (NMP) after NRP could offer more physiological organ preservation than static cold, enabling the graft to be evaluated for viability before LT.8
Two years ago, we initiated a study protocol for livers from type 2 DCD that had been rejected for transplantation using NMP after NRP in order to establish criteria that would increase the number of organs valid for LT.9 After completing this study, new criteria for transplantation were established related with the evolution of NMP10: hemodynamic stability during perfusion (mean arterial flow>100mL/h, and portal>500mL/h), ASAT/ALAT<3000IU/L, pH>7.25 after 6h of NMP, start of bile production 2h after NMP, with a flow≥10mL/h after 6h and glucose consumption present. In this article, we describe the first case of LT of a type 2 DCD graft performed after the implementation of the new NRP+NMP protocol.
Surgical TechniqueDonor Characteristics and Phases Prior to Normothermic Machine PerfusionThe donor was a 43-year-old male patient; warm ischemia time (from cardiac arrest to the beginning of NRP) was 2h and 37min (2h and 25min with advanced CPR maneuvers). Type 2 DCD criteria, the cannulation procedure and NRP have been previously described.7
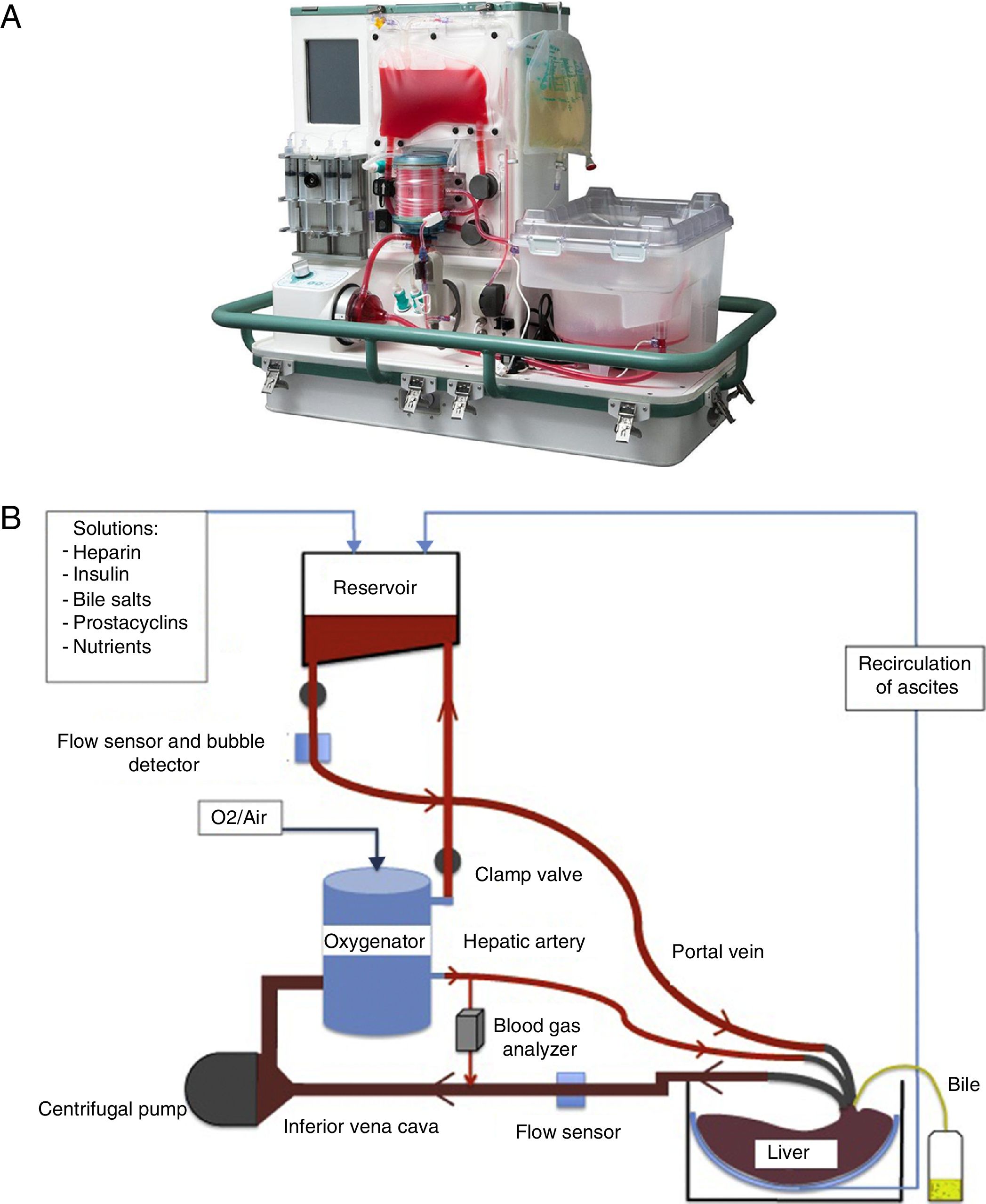
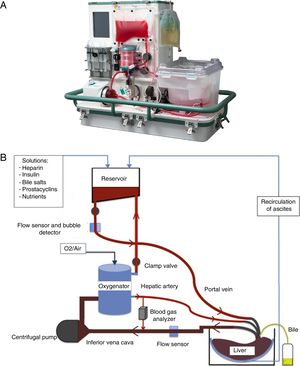
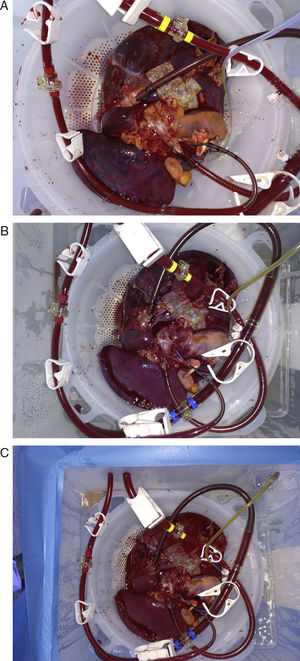
Placement of Graft in a Normothermic Perfusion MachineDonor and bench surgeries were performed in accordance with the habitual technique used at our hospital. During bench surgery, cannulae were placed in the hepatic artery, portal vein, the inferior vena cava and the bile duct. At the same time, preparations were made for the NMP OrganOx metra® (Fig. 1A): the preparation and priming of the circuit using 500mL of colloid and 3 units of packed red blood cells as well as the preparation of the perfusion solutions. A diagram of the machine function is shown in Fig. 1B. The graft was placed in the sterile compartment of the machine and connected to the device through the cannulae. From the beginning of the perfusion, the process is automatic. NMP shows real-time vascular and bile flows, pO2, pCO2, pH, glucose and the temperature of the perfusion fluid. The biochemical evolution of the graft was analyzed by studying serial samples of the perfusion fluid (after 5min, 1, 3, 6 and 9h of NMP) and of the bile produced.
Characteristics of the Recipient and Implant SurgeryThe recipient was the first patient on the waiting list at our hospital. He was a 43-year-old man with indication for transplantation due to alcohol-related cirrhosis of the liver and alpha-1-antitrypsin deficiency. The preoperative MELD was 28. The hepatectomy of the recipient was performed according to the standard technique with preservation of the inferior vena cava and portacaval shunt. At the end of NMP, the graft was disconnected from the machine and perfused with preservation fluid (University of Wisconsin). Before reperfusion, the graft was washed with 1000mL of physiological saline at body temperature. The rest of the implant was performed as usual. The study of the present case was accepted by the hospital ethics committee (2015/0884). Prior to extraction, specific informed consent was obtained from the donor's family and from the recipient.
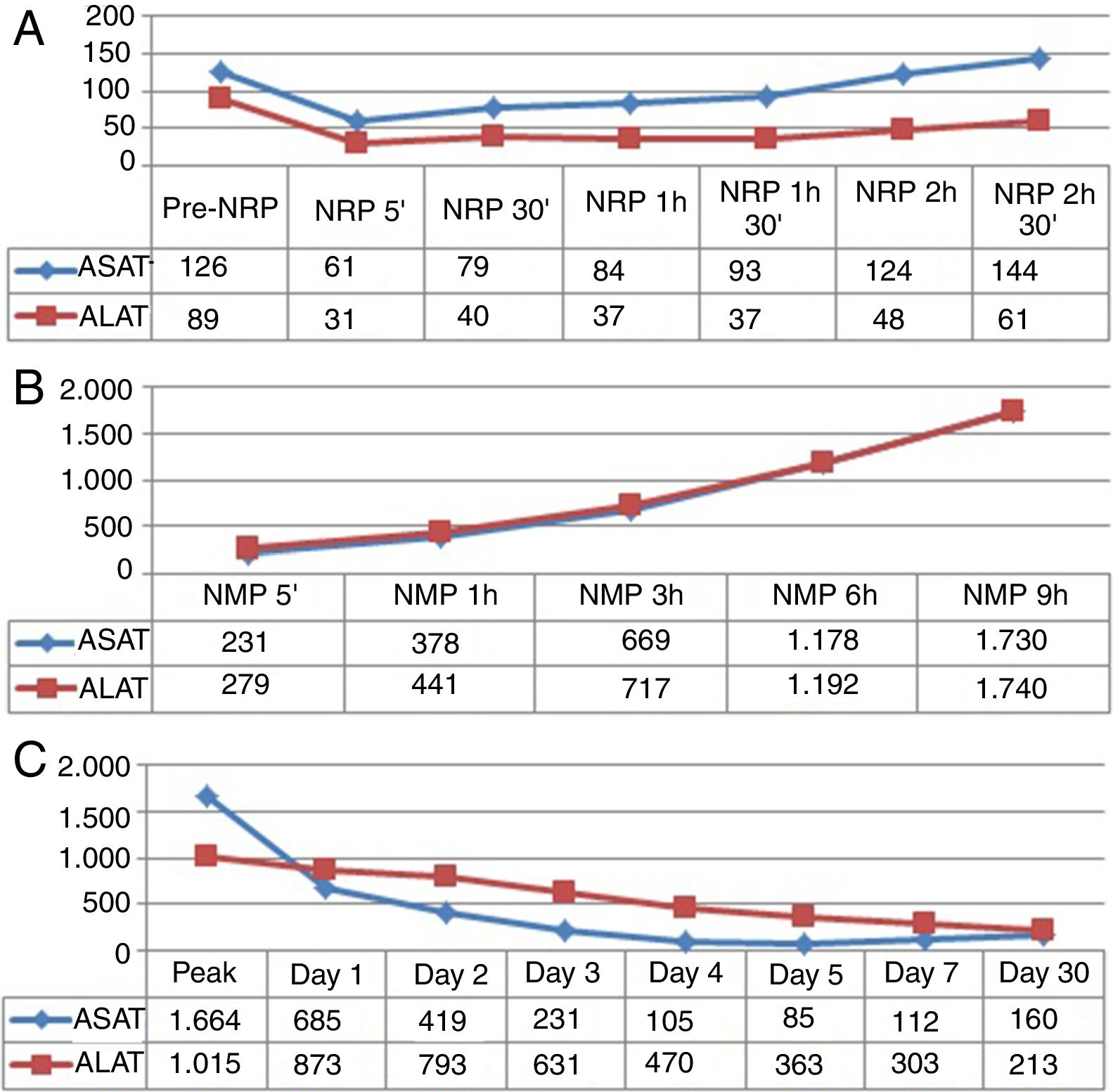
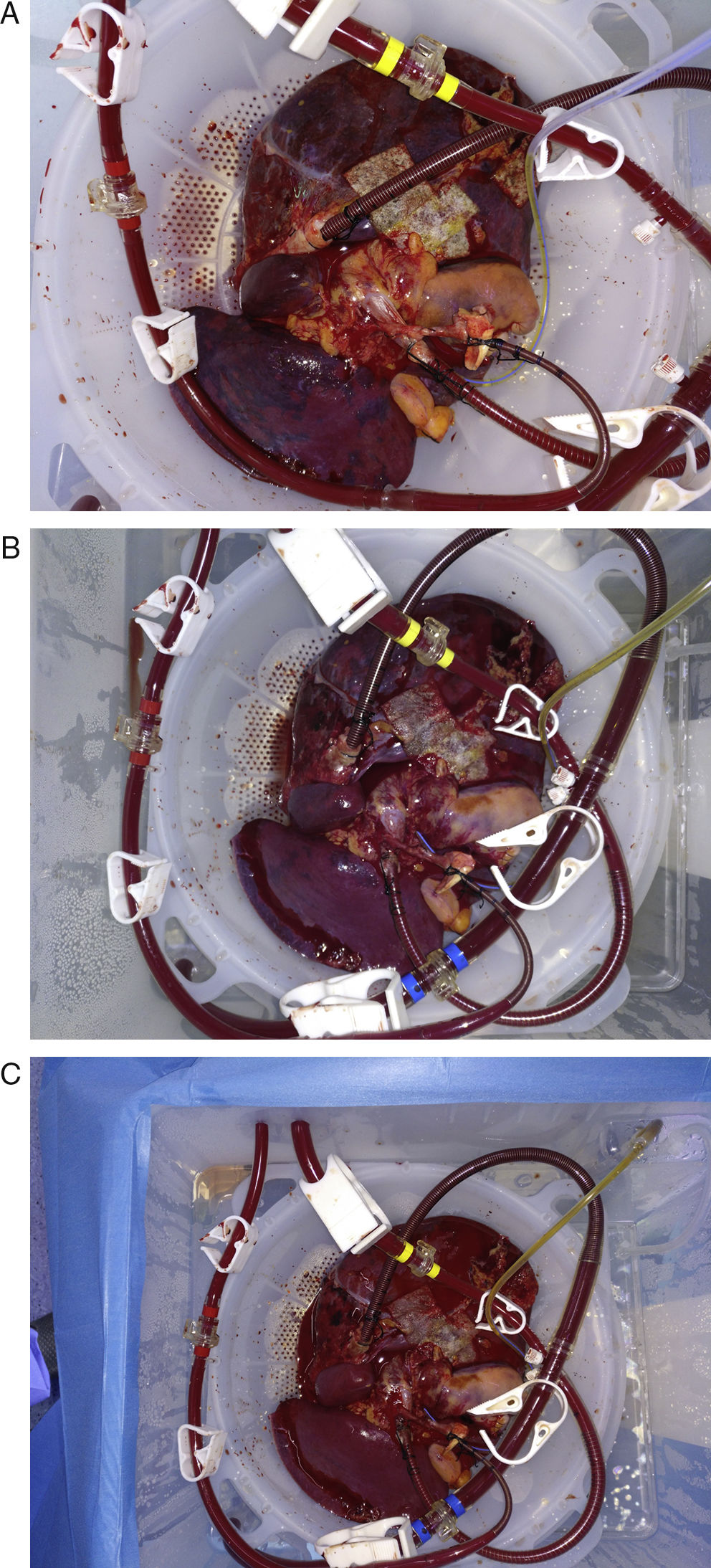
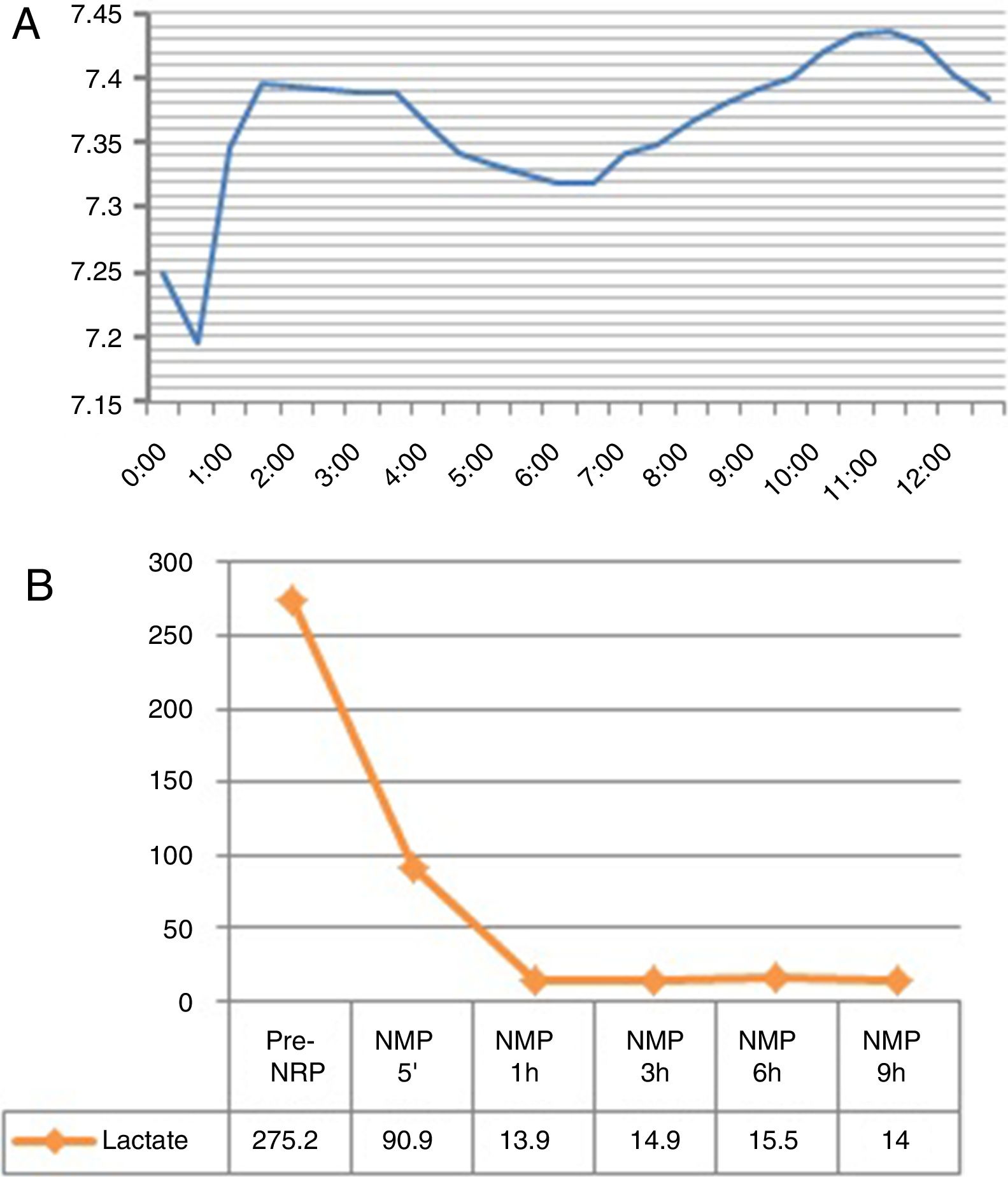
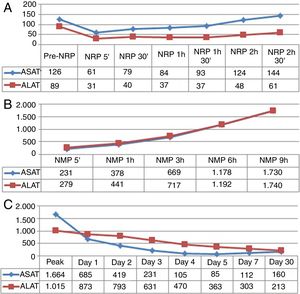
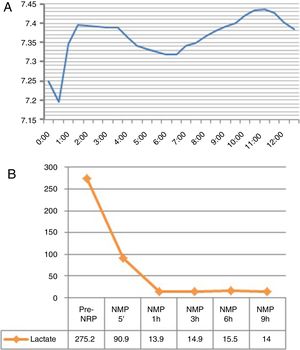
Evolution of the Graft in Normothermic Regional Perfusion and Normothermic Machine PerfusionNRP lasted 3h and 31min. Before the start of this phase, the ASAT was slightly above the accepted upper limit for transplantation7; the evolution of transaminases in NRP was favorable (Fig. 2A). NMP lasted 12h and 41min. During perfusion, improved macroscopic appearance of the organ was observed (Fig. 3). Vascular flows remained stable, with a mean of 130mL/min for the hepatic artery and 1120mL/min for the portal vein. The evolution of transaminases in NMP is shown in Fig. 2B. Six hours after NMP, the ASAT/ALAT level was 1178/1192IU/mL. The pH after 6h was 7.35, while the lactate levels decreased from 90mg/mL after 5min of perfusion to 15mg/mL 6h afterwards (Fig. 4). The production of bile started 2h after perfusion and reached a flow of 10mL/h 6h after NMP. Bile pH and bilirubin concentration improved during perfusion (pH 7.52 and bilirubin 6.1mg/dL after 3h; pH 8.1 and bilirubin 27.4mg/dL after 6h). The initial blood glucose level in NMP was 276mg/dL, which dropped to 115mg/dL 6h later, demonstrating consumption.
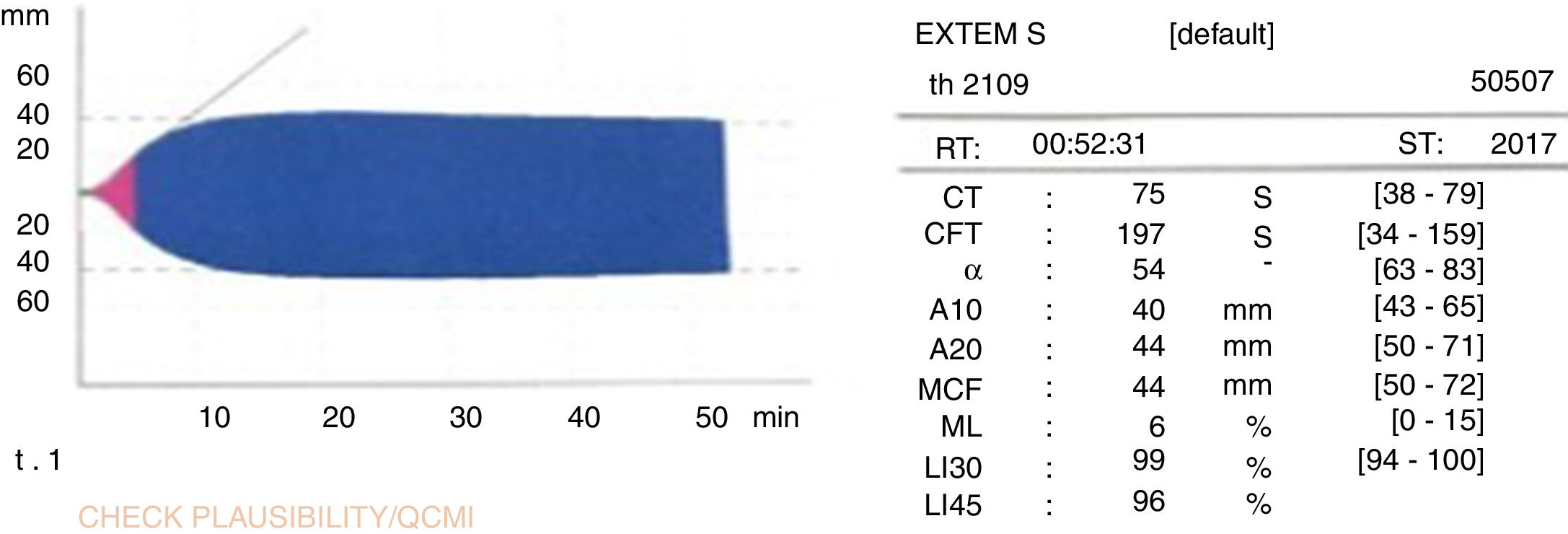
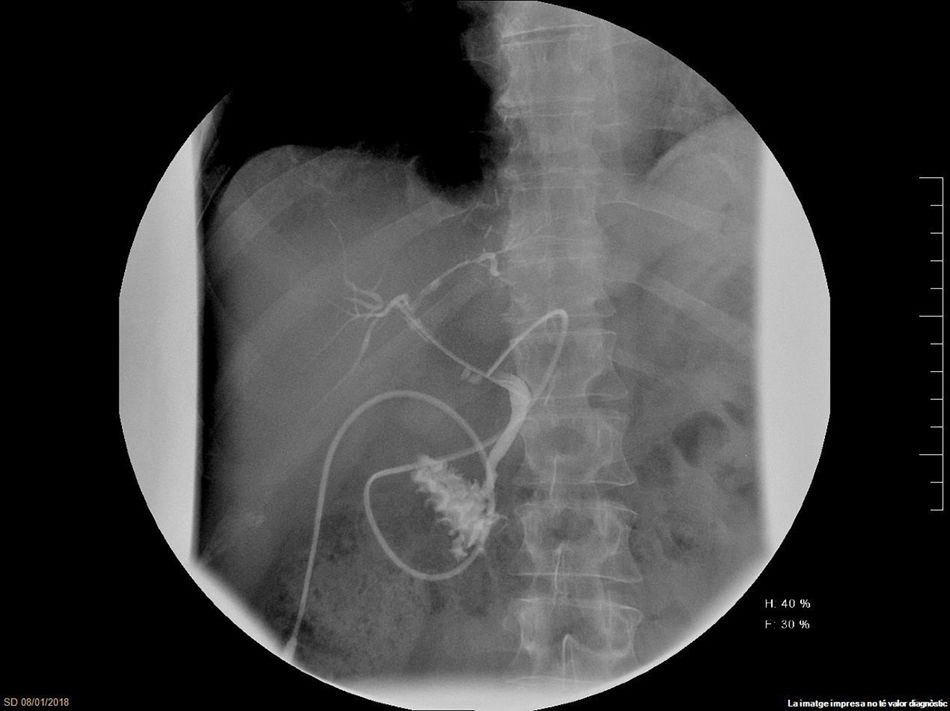
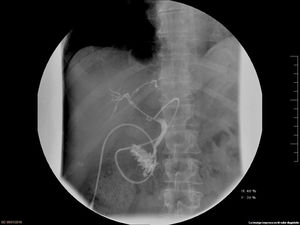
The graft was implanted without incident. The reperfusion of the graft was homogeneous and there was no evidence of post-reperfusion syndrome or need for transfusions during surgery. The thromboelastogram performed 1h after portal reperfusion showed characteristics similar to those of a conventional graft transplant (Fig. 5). The postoperative evolution was correct, with an ASAT/ALAT peak of 1664/1015IU/mL 12h post-LT, with rapid subsequent normalization (Fig. 2C). The prothrombin time on the third postoperative day was 75%, and bilirubin was 2.9mg/dL. The only postoperative complication was acute cellular rejection that required recycling with methylprednisolone. Total hospital stay was 20 days. A trans-Kehr cholangiogram performed 2 months after surgery demonstrated the bile duct was normal (Fig. 6).
DiscussionToday, type 2 DCD is a way to increase the number of liver grafts available for transplantation. However, the use of these organs remains low due to the higher complication rates associated with this type of donation.11 The implementation of a protocol capable of evaluating the behavior of hepatic grafts from type 2 DCD before LT is of great interest. Currently, the standard method for preservation prior to transplantation is still static cold. However, the combination of hot and cold ischemia makes the behavior of type 2 DCD liver grafts suboptimal.12 For this reason, until now, the good results of LT with type 2 DCD were sustained with very strict donor selection.4
Compared to static cold, NMP has several advantages. By reproducing an environment that is nearly physiological, NMP allows the viability of the graft to be evaluated before LT.8 Several authors have cited various parameters to evaluate the viability of grafts in NMP.13–16 After applying the NRP+NMP protocol in type 2 DCD grafts rejected for LT, our group identified transaminase levels, ability to regulate the acid–base balance, production of bile and glucose consumption as key factors to evaluate this type of grafts.10 In the present case, the macroscopic aspect of the liver continuously improved during normothermic perfusion, and all these parameters registered favorable evolution.
One of the main problems of type 2 DCD recipients is marked coagulopathy and hemodynamic instability during reperfusion.17 In the current case, there was no evidence of post-reperfusion syndrome and the thromboelastogram did not show the signs of early fibrinolysis that are characteristic of this type of grafts. Postoperative progress was favorable, with no primary graft dysfunction. Cholangiography performed 2 months after the procedure showed no evidence of ischemic cholangiopathy.
Other authors report the use of NMP to perform the transplantation of grafts with expanded criteria.14,16 In the COPE-WP2 study, type 3 DCD grafts treated with NMP presented better results in terms of peak transaminases and primary graft dysfunction than those preserved in cold.18 However, the case we present is the first type 2 graft transplanted after NRP and NMP. In conclusion, despite its complexity, the potential of the NRP+NMP protocol in type 2 DCD is evident, as it offers not only increased quality of the grafts that are currently transplanted, but also a greater number of organs available for transplantation. However, a more extensive study of more cases would be needed to confirm its efficacy in the clinical setting.
FundingThis study received support from the Fundación Mutua Madrileña (project FUNDMM_16_01), Fundación “La Caixa” and OrganOx.
Conflicts of InterestsThere are no conflicts of interests to declare.
The authors would like to thank the Fundación Mutua Madrileña, Fundación “La Caixa” and the OrganOx Company for their support of this study.
Please cite this article as: Pavel M-C, Reyner E, Fuster J, Garcia-Valdecasas JC. Trasplante hepático con injerto de donante en asistolia tipo 2 con perfusión regional normotérmica y máquina de perfusión normotérmica. Primer caso descrito a nivel mundial. Cir Esp. 2018;96:508–513.