The greater survival of transplanted patients is accompanied by an increase in the rate of de novo malignancies (NM), which are the most frequent late-onset complication. We can distinguish between non-melanoma skin cancers (NMSC), post-transplant lymphoproliferative disorders (PTLD) and solid organ cancers (SOC). Our objective is to determine the incidence of the different types of NM, the time elapsed until diagnosis and survival rates in our setting.
MethodsWe conducted a retrospective study of 1071 liver transplant patients from 1990 to 2015 at our center. We analyzed the demographic variables, incidence of NM and survival.
Results184 NM developed in 1071 transplant patients (17%), specifically 19% of the males and 13% of the females (P=.004). The most frequent NM were NMSC (29%), lung (18%), head and neck (16%), PTLD (10%) and gastrointestinal (8%). The median time of diagnosis was 7.9 years in NMSC, 3.9 years in PTLD and 9.8 years in SOC. Patients with NMSC had significantly better survival than those with PTLD or SOC. The incidence of de novo tumors (excluding NMSC) was 1889/100,000 transplants/year. By gender, lung cancer was the most common TOS in men and breast cancer in women.
ConclusionIn our setting, excluding NMSC, the incidence is 8.8 times greater than estimations for the general population, with a high rate of lung cancer, so we should implement preventive and diagnostic strategies.
La mayor supervivencia del paciente trasplantado viene acompañada del aumento en la tasa de tumores de novo (TN) que representan la complicación tardía más frecuente. Podemos distinguir entre tumores de piel no melanoma (TPNM), síndrome linfoproliferativo postrasplante (SLPT) y tumores de órgano sólido (TOS). Nuestro objetivo es determinar la incidencia de los distintos TN, el tiempo trascurrido hasta su diagnóstico y su supervivencia en nuestro medio.
Material y métodoRealizamos un estudio retrospectivo de 1.071 trasplantados hepáticos desde 1990 hasta 2015 en nuestro centro. Analizamos las variables demográficas, la incidencia de TN y la supervivencia.
ResultadosSe desarrollaron 184 TN en 1.071 pacientes trasplantados (17%), en el 19% de los varones y en el 13% de las mujeres (p=0,004). Los TN más frecuentes fueron los TPNM (29%), pulmón (18%), cabeza y cuello (16%), SLPT (10%) y gastrointestinales (8%). La mediana del tiempo de diagnóstico fue de 7,9 años en los TPNM, 3,9 años en SLPT y de 9,8 años en TOS. Los pacientes con TPNM tuvieron significativamente mejor supervivencia que aquellos con SLPT o TOS. La incidencia de los tumores de novo (excluidos TPNM) fue 1.889/100.000 trasplantados/año. Por género, el cáncer de pulmón fue el TOS más común en varones y el cáncer de mama en mujeres.
ConclusiónEn nuestro medio, excluidos los TPNM, la incidencia es 8,8 veces la estimada para la población general, con una alta tasa de cáncer de pulmón por lo que deberíamos implementar estrategias preventivas y diagnósticas.
Liver transplantation (LT) has been established as a standard treatment for liver failure, with more than 120,000 procedures to date. One-, 5- and 10-year survival rates have improved significantly in the last 25 years to 83%, 71% and 61%, respectively.1 The incidence of de novo malignant tumors in transplant recipients was first described by Penn and Starzl in 1972.2 In recent years, its incidence has varied from 2.2% to 26%.3,4 Studies of large registries5–8 indicate that transplant recipients are 2–7 times more likely to develop de novo malignancies than the general population, which are a frequent cause of mortality.9,10 Different factors have been involved in the development of these tumors: the immunosuppression used, the time elapsed since the transplant was performed and risk factors generally associated with carcinogenesis (viral infections, smoking, alcohol abuse, etc.).
In Spain, according to the Spanish Society of Medical Oncology (SEOM),11 in the last 20 years, the number of tumors diagnosed in the general population has experienced constant growth, due not only to the population increase, but also to early detection techniques and increased life expectancy. In 2015, the most frequently diagnosed tumors in men were prostate, colorectal and lung, while the most frequent in women were breast, colorectal and uterine. Currently, there is a significant number of published studies conducted in patients treated with different solid organ transplants. The aims of the present study were: 1) to analyze the cumulative incidence and characteristics of de novo tumors in patients who have undergone LT in our setting; and 2) to determine survival after diagnosis in order to assess the need for preventive strategies and specific early-diagnosis protocols for this population.
MethodsWe performed a retrospective analysis of 1071 adult patients who had received a liver transplant at our institution between 1990 and 2015. The variables analyzed included: recipient age, sex, primary indication, date of transplantation, tumor type, date of diagnosis and date of last follow-up. These data were obtained by reviewing patient medical records. The protocol for tumor screening prior to transplantation included: chest x-ray and abdominal ultrasound (thoracoabdominal computed tomography if alterations were found in previous tests), oral endoscopy and colonoscopy in patients over the age of 50 or at risk for colorectal carcinoma; in women, mammography and cervical cytology were performed.
In the post-transplant follow-up, the diagnosis of de novo tumor was established by histological examination of tumor biopsies or surgical sample; precancerous lesions have not been included in the analysis. The biopsy date was designated as the date of diagnosis of the de novo tumor. Immunosuppressive treatment at our hospital has varied over the years. Currently, patients follow an induction protocol with tacrolimus, mycophenolate mofetil and corticosteroids, the latter of which are withdrawn early. In patients at high risk for renal dysfunction, basiliximab is used with delayed introduction of calcineurin inhibitors. In transplant patients with hepatocellular carcinoma and criteria for poor explanation prognosis, the calcineurin inhibitor is replaced with an mTOR inhibitor.
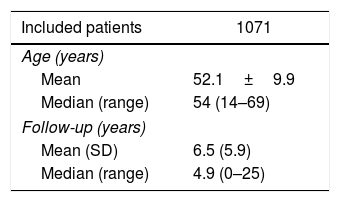
Statistical AnalysisThe statistical analysis was completed using the SPSS package, version 15.0 (SPSS, Chicago, IL, USA) and R v.3.1.3 (R Development Core Team 2015). The results of the categorical variables are presented as percentages, for the continuous variables as a mean (standard deviation) if they follow a normal distribution and a median (range) according to the asymmetry of the distribution. The categorical variables were analyzed with the chi-square test or Fisher's F, and for the difference between continuous variables, Student's t was used. The estimates of the incidence of de novo tumors have been calculated with software R using the “survival” and “cmprsk” libraries, considering patient death to be a competitive risk. We analyzed patient survival by age at the time of transplantation, using the median age of our series (54 years) as the cut-off point between both groups. The survival estimates were calculated using the Kaplan–Meier method and the comparison between the groups with the log-rank test. A P value < .05 was considered statistically significant.
ResultsDe novo tumors were diagnosed in 184 patients. Table 1 shows the clinical and demographic characteristics of the patients, and Table 2 shows the distribution of the 189 de novo tumors developed in 184 patients.
Clinical and Demographic Characteristics of Liver Transplant Patients.
| Included patients | 1071 |
|---|---|
| Age (years) | |
| Mean | 52.1±9.9 |
| Median (range) | 54 (14–69) |
| Follow-up (years) | |
| Mean (SD) | 6.5 (5.9) |
| Median (range) | 4.9 (0–25) |
| Patients (%) | De novo tumors (%) | |
|---|---|---|
| Sex | ||
| Male | 811 (75.7) | 150 (18.5) |
| Female | 260 (24.3) | 34 (13.1) |
| Age | ||
| <54 years | 524 (49) | 71 (13.5) |
| >54 years | 547 (51) | 113 (20.6) |
| Indication for transplant | ||
| Alcohol-related cirrhosis | 434 (40.5) | 87 (20) |
| Viral cirrhosis | 428 (39.9) | 65 (15.1) |
| HCV | 318 (29.7) | 40 (12.5) |
| HBV | 110 (10.2) | 25 (22.7) |
| Cholestatic diseases | 56 (5.2) | 10 (17.8) |
| NAFLD | 15 (1.4) | 2 (13.3) |
| ALD | 22 (2) | 2 (9) |
| Other | 117 (10.9) | 18 (15.4) |
| HCC | 237(22.1) | 14 (6.4) |
HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty-liver disease; ALD, acute liver failure; HBV, hepatitis B virus; HCV, hepatitis C virus.
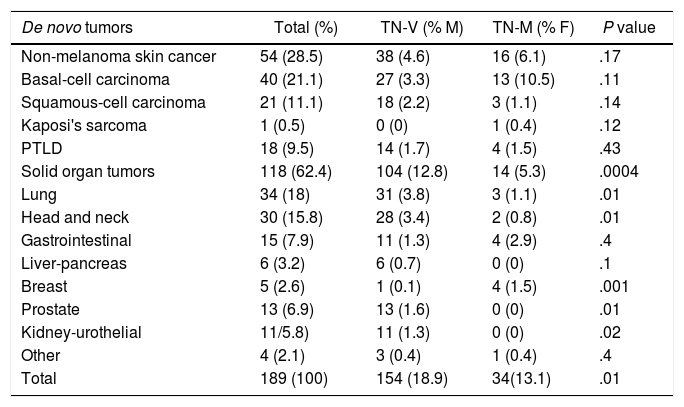
Distribution of de novo Tumors in Liver Transplant Patients.
| De novo tumors | Total (%) | TN-V (% M) | TN-M (% F) | P value |
|---|---|---|---|---|
| Non-melanoma skin cancer | 54 (28.5) | 38 (4.6) | 16 (6.1) | .17 |
| Basal-cell carcinoma | 40 (21.1) | 27 (3.3) | 13 (10.5) | .11 |
| Squamous-cell carcinoma | 21 (11.1) | 18 (2.2) | 3 (1.1) | .14 |
| Kaposi's sarcoma | 1 (0.5) | 0 (0) | 1 (0.4) | .12 |
| PTLD | 18 (9.5) | 14 (1.7) | 4 (1.5) | .43 |
| Solid organ tumors | 118 (62.4) | 104 (12.8) | 14 (5.3) | .0004 |
| Lung | 34 (18) | 31 (3.8) | 3 (1.1) | .01 |
| Head and neck | 30 (15.8) | 28 (3.4) | 2 (0.8) | .01 |
| Gastrointestinal | 15 (7.9) | 11 (1.3) | 4 (2.9) | .4 |
| Liver-pancreas | 6 (3.2) | 6 (0.7) | 0 (0) | .1 |
| Breast | 5 (2.6) | 1 (0.1) | 4 (1.5) | .001 |
| Prostate | 13 (6.9) | 13 (1.6) | 0 (0) | .01 |
| Kidney-urothelial | 11/5.8) | 11 (1.3) | 0 (0) | .02 |
| Other | 4 (2.1) | 3 (0.4) | 1 (0.4) | .4 |
| Total | 189 (100) | 154 (18.9) | 34(13.1) | .01 |
F, females; PTLD, post-transplant lymphoproliferative disorder; M, males.
In general, de novo tumors in transplant patients were more frequent in men than in women (18.5% vs 13.1%; P=.004) and in patients over the age of 54 (20.6% vs 13.5%; P=.002). With a median follow-up of 4.9 years, the detailed analysis of the different tumors showed that non-melanoma skin cancer (NMSC) was the most frequent neoplasm. In NMSC as well as post-transplant lymphoproliferative disorder (PTLD), there were no gender-related differences; however, such differences were observed in solid-organ cancers (SOC). Alcoholic cirrhosis was the most frequent primary indication for transplant in 434 patients (40.5%), and de novo tumors were detected in 87 patients (20%) in this group: 61 (14%) SOC (20 head-neck tumors, 19 lung and 6 prostate), 20 NMSC and 6 PTLD.
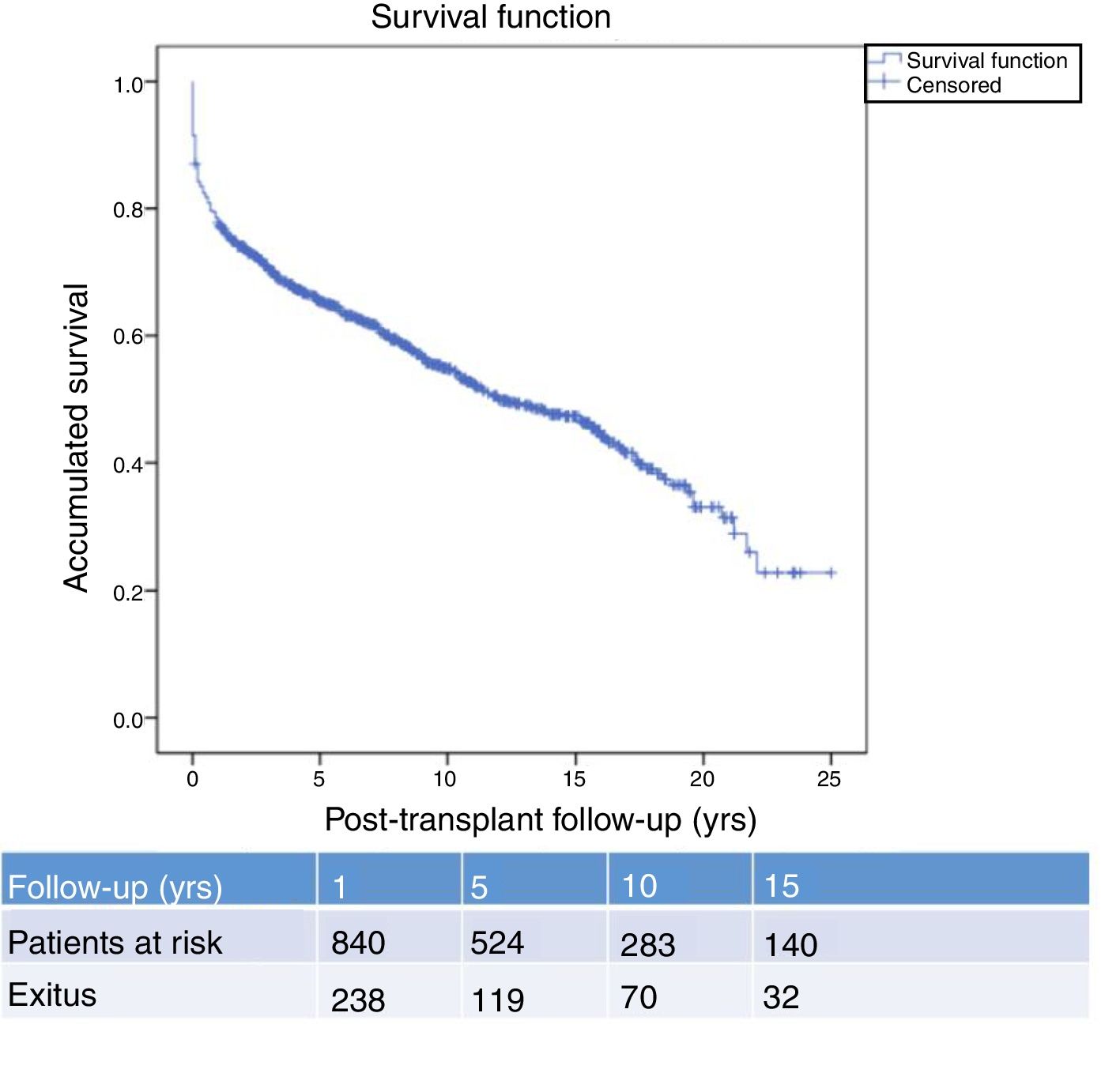
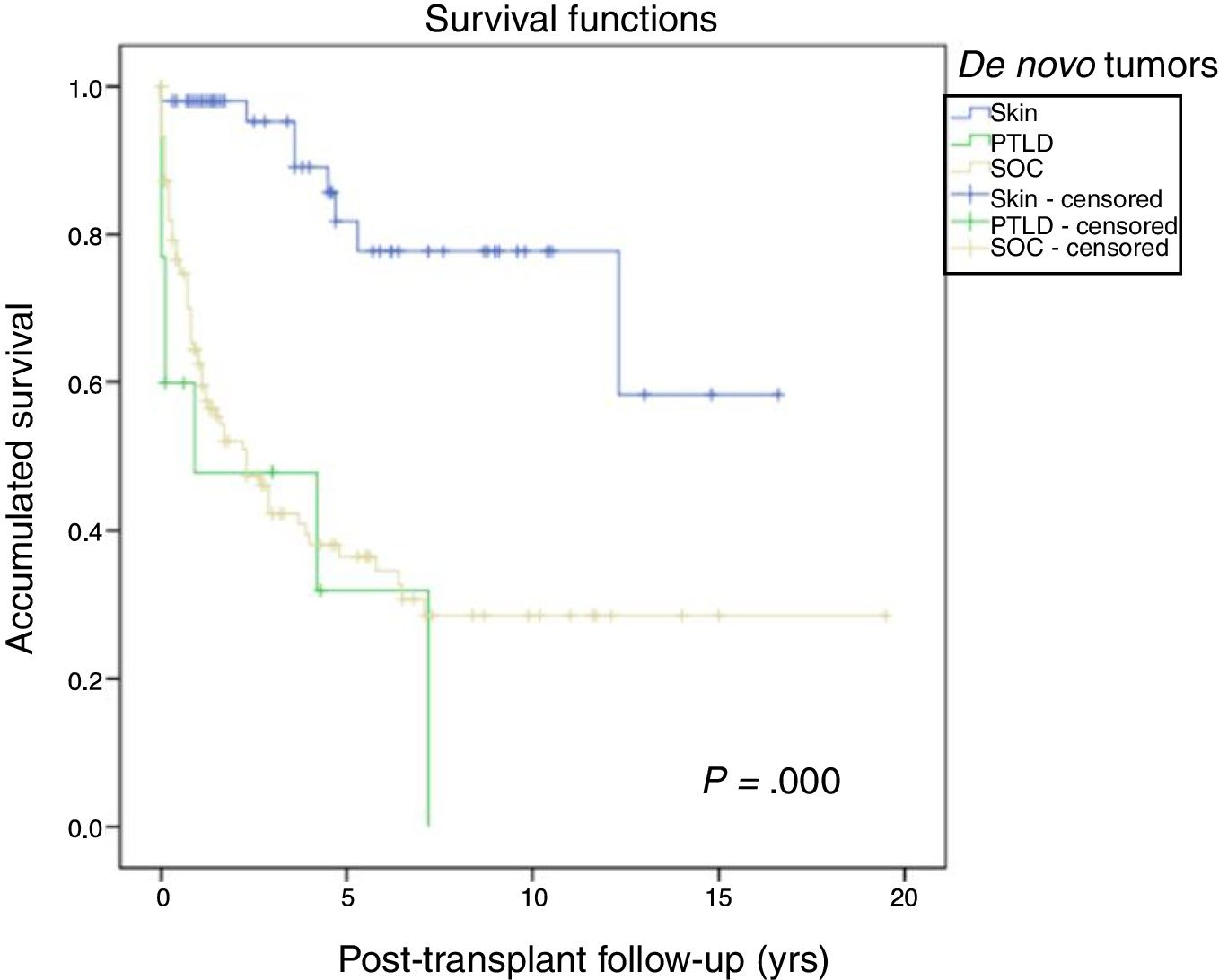
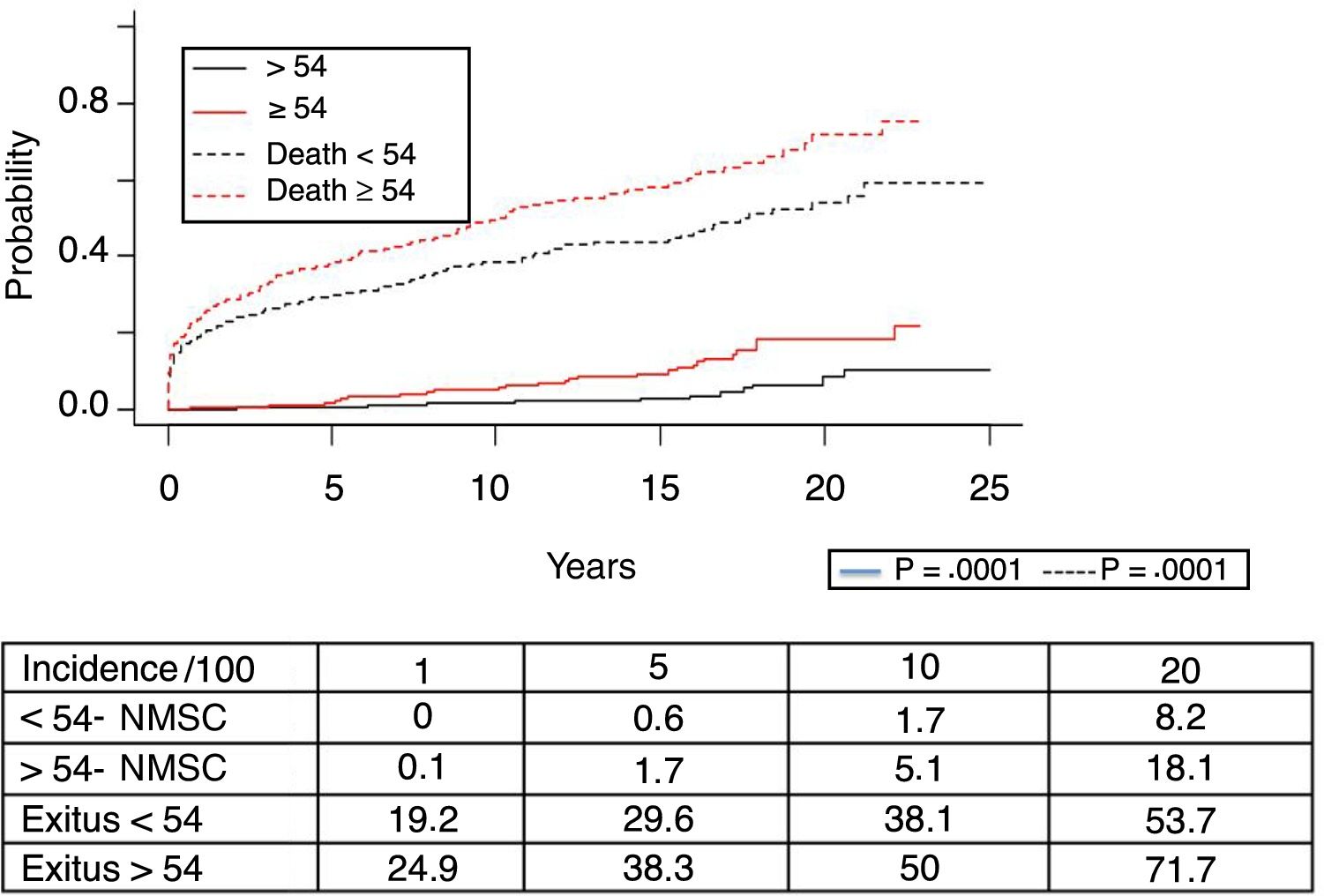
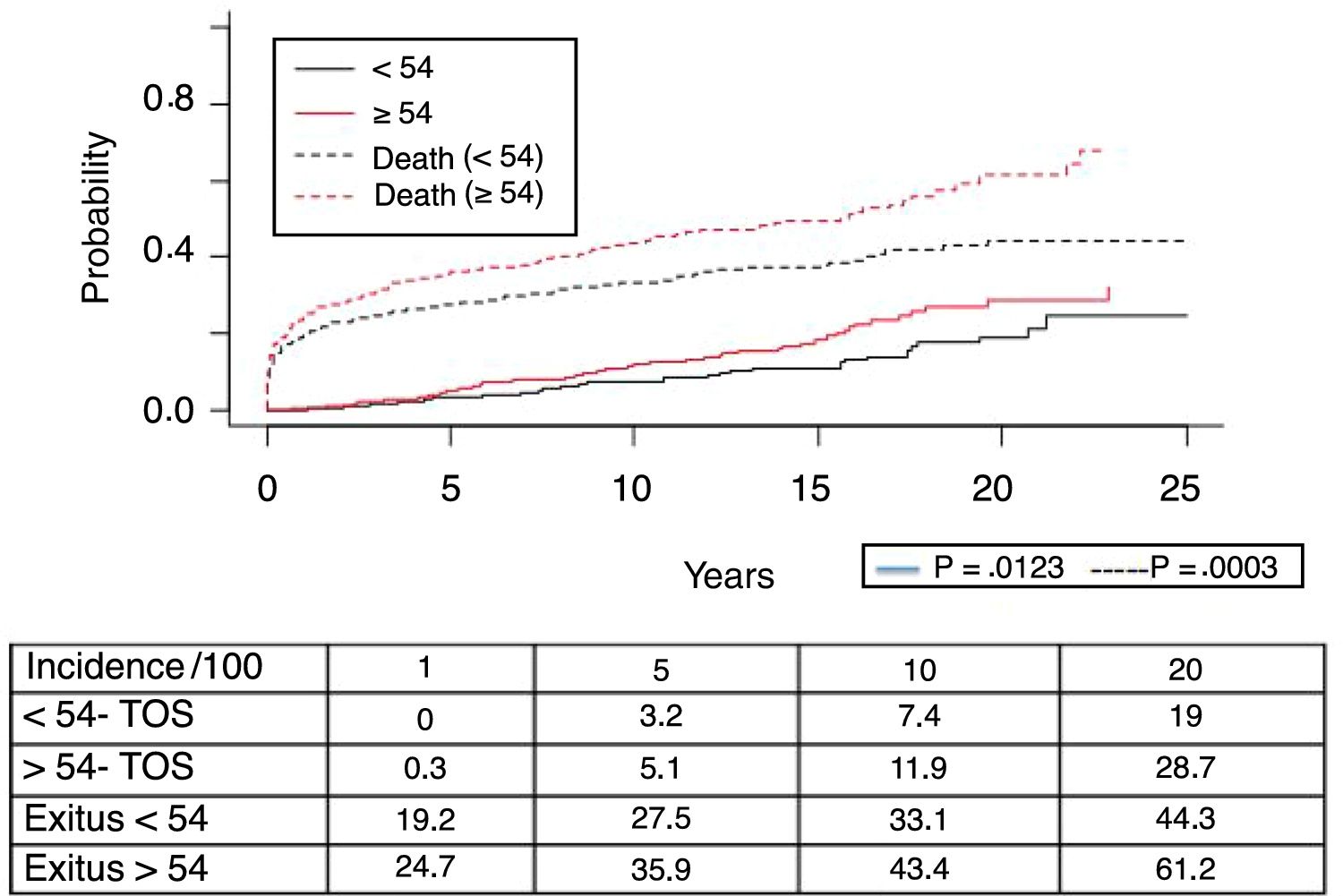
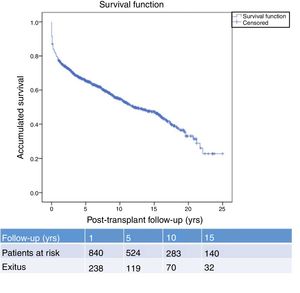
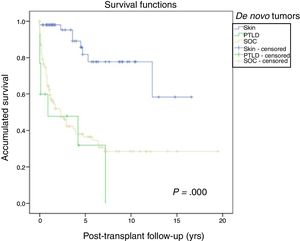
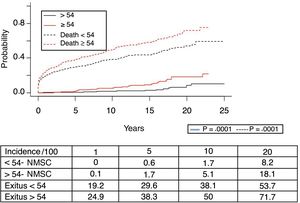
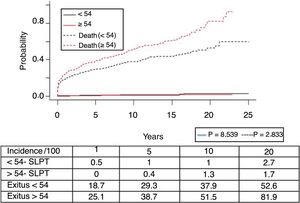
Fig. 1 shows the one-, 5- and 10-year post-transplant survival rates of our series, which stand at 77.8%, 65.4% and 54.8%, respectively. Survival was lower in the group of patients over the age of 54 (75%, 61%, 48% for one-, 5- and 10-year survival, respectively) with no statistically significant differences compared to the group of patients under 54 (81%, 71%, 62% for one-, 5- and 10-year survival, respectively). Survival after diagnosis varied according to the type of tumor (NMSC, PTLD and SOC) (P=.000). As seen in Fig. 2, patients with NMSC had significantly better survival than those with PTLD or SOC. Fig. 3 shows that the incidence of NMSC increased over the years of follow-up and that there were differences between the age groups (P=.0001). NMSC developed in 54 patients (5%). In 31 patients, the type was basal-cell carcinoma, in 12 squamous cell carcinoma, in 9 patents both types of tumors, and in one patient a Kaposi's tumor was identified. The median time before diagnosis was 7.9 years (0.3–15.4). The one-, 5- and 10-year survival rates after diagnosis were 100%, 83.1% and 79%, respectively.
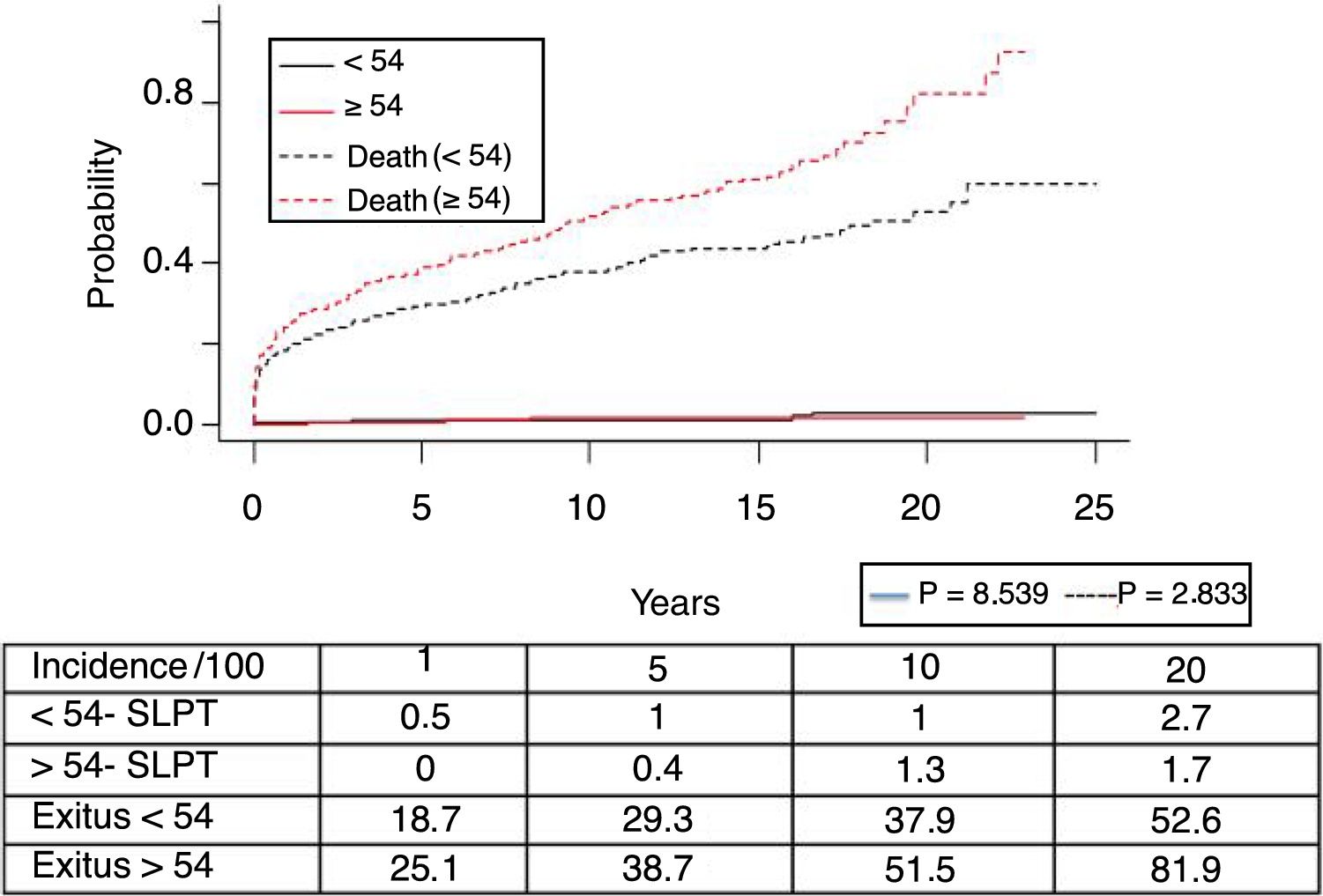
Post-transplant lymphoproliferative syndrome was diagnosed in 18 patients, with a mean recipient age of 52. As shown in Fig. 4, its incidence increased with follow-up time, especially in younger patients, but without reaching statistically significant differences. The median time for diagnosis was 3.9 years (0.1–12.3). Table 3 shows the characteristics of the patients diagnosed with PTLD. In 6 patients, we found an association with the Epstein–Barr virus (EBV), while in 4 patients PTLD developed in the first year after transplantation. The one-, 5- and 10-year survival rates after diagnosis were 63.8%, 33% and 16.8%, respectively.
Characteristics of Patients With PTLD.
| Patient | Sex | Age (yrs) | LT | EBV recipient | EBV donor | Immunosuppression | WHO categories | Associated with EBV |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | 1991 | Positive | Unknown | CSA | Early-stage lesion | Yes |
| 2 | M | 62 | 1995 | Positive | Unknown | CSA+AZA | Early-stage lesion | Yes |
| 3 | M | 47 | 1995 | Negative | Unknown | CSA | PTLD monomorphic-B Cell | NC |
| 4 | M | 49 | 1998 | Positive | Negative | CSA | PTLD monomorphic-B Cell | NC |
| 5 | M | 44 | 1999 | Positive | Negative | CSA | PTLD monomorphic-B Cell | NC |
| 6 | F | 50 | 1999 | Negative | Negative | CSA | PTLD monomorphic-B Cell | NC |
| 7 | M | 20 | 1999 | Positive | Unknown | CSA | Early-stage lesion | Yes |
| 8 | F | 57 | 2000 | Negative | Unknown | Tacrolimus | PTLD monomorphic-B Cell | NC |
| 9 | F | 67 | 2002 | Positive | Positive | TAC+MMF | PTLD classic Hodgkin lymphoma | Yes |
| 10 | F | 52 | 2004 | Positive | Unknown | TAC+MMF | PTLD monomorphic-B Cell | NC |
| 11 | M | 54 | 2006 | Positive | Unknown | TAC+MMF | PTLD monomorphic-B Cell | NC |
| 12 | M | 64 | 2008 | Negative | Negative | TAC+MMF | PTLD monomorphic-B Cell | Yes |
| 13 | M | 54 | 2009 | Positive | Positive | TAC+MMF | PTLD monomorphic-T Cell | NC |
| 14 | M | 61 | 2009 | Positive | Positive | TAC+MMF | PTLD monomorphic-B Cell | NC |
| 15 | M | 63 | 2009 | Positive | Unknown | CSA+MMF | PTLD monomorphic-B Cell | NO |
| 16 | M | 55 | 2011 | Positive | Positive | TAC+MMF | PTLD monomorphic-T Cell | NC |
| 17 | M | 51 | 2012 | Positive | Unknown | TAC+MMF | PTLD monomorphic-B Cell | NC |
| 18 | M | 35 | 2014 | Positive | Positive | CSA | PTLD monomorphic-B Cell | Yes |
AZA, azathioprine; CSA, cyclosporine; F, female; MMF, mycophenolate mofetil; WHO, World Health Organization; PTLD, post-transplant lymphoproliferative disorder; TAC, tacrolimus; LT, liver transplant; M, male; EBV, virus de Epstein–Barr.
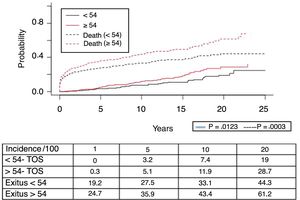
A total of 118 de novo solid organ tumors developed in 115 patients (12%): 97 men (13.3%), and 18 women (7.7%) (P=.0086). The median time before diagnosis was 9.8 years (0.1–21). Fig. 5 demonstrates how the incidence increased with the follow-up time and was greater in the group of patients over the age of 54 (P=.0001), as 28% of patients>54 years had 20 years of follow-up. One-, 5- and 10-year survival rates after diagnosis were 64.7%, 34.9% and 25.4%, respectively, with differences in the different diagnosed tumor types (P=.000).
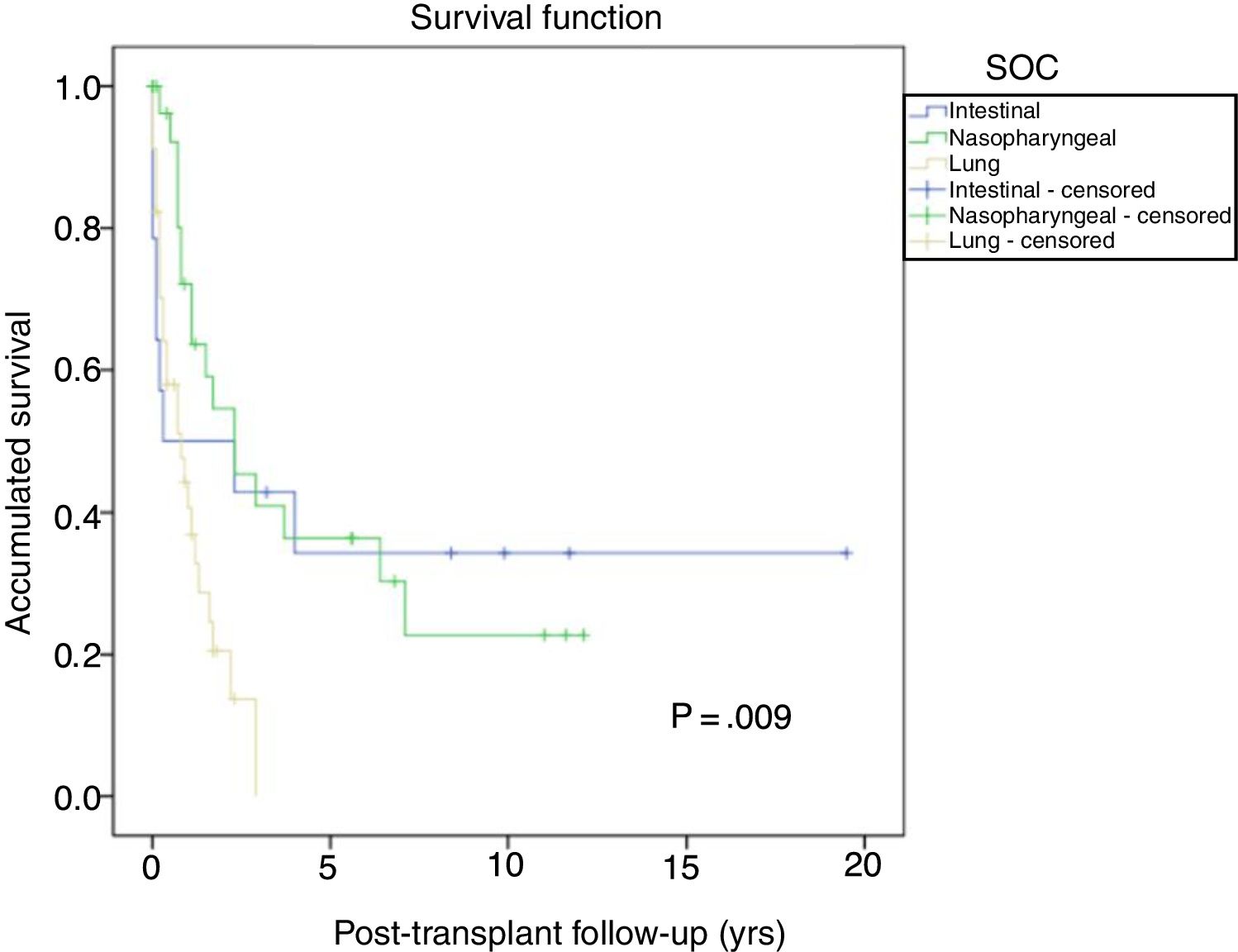
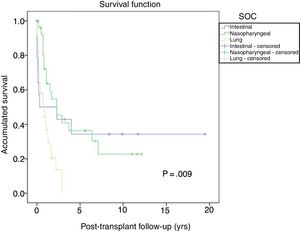
The most frequent solid organ tumors were lung tumors (29%), followed by tumors of the head and neck (25.6%) and gastrointestinal tumors (12%). Fig. 6 shows the survival after tumor diagnosis of the most frequent SOC: - Lung tumors: the overall incidence was 3.1% and was higher in men than in women (3.8% vs 1.1%; P=.01), with a median time before diagnosis of 6.3 years (0.7–21). The 5-year survival after transplant was 64.7%. The one-, 3- and 5-year survival rates after diagnosis were 44%, 13.5% and 0%, respectively.
- Head and neck tumors: the incidence of these tumors was higher in men than in women (3.4% vs 0.8%; p=0.01), with a median time before diagnosis of 3.6 years (0.7–12.7). Five-year survival after transplantation was 56.6%. One-, 3- and 5-year survival rates after diagnosis were 73.2, 43.4% and 34.7%, respectively. - Gastrointestinal tumors: diagnosed in 15 patients with no differences in terms of sex. The most frequent histological type was colon adenocarcinoma in 5 patients (in none was PSC disease the primary indication for LT), followed by 4 gastric tumors, 3 esophageal tumors and one duodenal adenocarcinoma, with a median time before diagnosis of 5.3 years (1.3–19.6). Five-year survival of these patients after transplantation was 80%. One-, 3- and 5-year survival rates after tumor diagnosis were 53.3%, 40% and 32%, respectively. Other tumors, such as prostate adenocarcinoma or breast cancer, had 5-year survivals after diagnosis of 85.5% and 60%, respectively.
DiscussionThe cancer data in Spain from 2015 published by the SEOM11 exclude non-melanoma skin cancer and have an incidence of 215.5 tumors per 100,000 inhabitants. In our series, 132 patients were identified with de novo tumors (12.3%) (excluding non-melanoma skin cancer), representing an incidence of 1889.1/100,000 transplanted patients/year, which is 8.8 times greater than in the general population.
This incidence rate is among the highest reported in the literature (2.2%–26%).3,4 The explanations for the discrepancies include differences in the size of the population studies and duration of follow-up, since the probability of developing these malignant tumors increases after 5 years of follow-up; therefore, any study with less than 5 years of follow-up underestimates the incidence.12 The median duration of the follow-up in our cohort is comparable with other reports, so it is likely that our results are influenced by other factors involved (geographical variation, immunosuppressant drugs used and the different methods for identifying and reporting de novo malignant tumors).13,14
Although the risk factors for the development of malignant neoplasms after LT have not been fully defined, in our setting as well as other studies15,16 the male gender is significantly associated with an increased risk of cancer.
We agree with the majority of authors that non-melanoma skin cancer is the most frequent de novo tumor and that survival after diagnosis does not differ from transplant patients without neoplasms.4,17,18 Included in this group are squamous-cell cancer (SCC), basal-cell cancer (BCC) and Kaposi's sarcoma. Although it has been reported that the 4/1 ratio of BCC/SCC in the general population seems to be inverted in transplant patients,19 in our study there is a predominance of BCC with a 2/1 ratio, similar to other national series4; nevertheless, we found a median in the time of diagnosis after major transplant (4.1 vs 7.9 years). This difference may be due to the decrease in the incidence of skin tumors, especially SCC, in transplant patients in recent decades.20 In our setting, these tumors are still the most frequent and are not exempt from aggressive behavior. Their main known risk factors (UV radiation, chronic immunosuppression and advanced age) are common in most patients, so the strategies to avoid its appearance are aimed at increasing awareness and the use of sun protection, as well as periodic dermatology revisions of those patients with suspected lesions or a personal history of epithelial cancer.
De novo tumors, excluding non-melanoma skin lesions, are the major cause of mortality in patients transplanted for alcohol-related liver disease.21 In our setting, this was the most frequent primary indication. De novo tumors, excluding skin tumors, developed in 15% of these patients, and more than 50% were aerodigestive tumors. Alcohol and its relationship with a history of smoking have previously been described as the main risk factors.22–24
Recipient seronegativity for EBV and incompatibility with donor serology is the main risk factor for PTLD, which includes a broad spectrum of lymphoproliferative disorders. In our setting, Govantes et al.25 identified 60 PTLD in 5775 kidney transplants from the Andalusian SICATA registry (1990–2009), with a shorter median time until diagnosis of 5.9 years. In our setting, 18 PTLD were identified in 1071 patients, with a shorter median time until diagnosis. This contrasts with series where the rate of PTLD in liver transplant recipients is lower than in other solid organ recipients,26 while concurring with recent data indicating that liver transplant patients have a higher risk of PTLD compared with renal transplant recipients.6,27 Hypothetically, the presence of lymphoid tissue in the liver graft could be the contributing factor.28 We had few cases of PTLD associated with EBV, but the sensitivity of the diagnosis of EBV has changed during the time of the study, so we may have underestimated the actual incidence.
Within the SOC, the incidence of lung cancer varies according to the series (0%–19%).3,4 In our series, lung cancer was identified in 34 patients and was the most frequent SOC (29%). This incidence is higher than recent publications8 of multicenter registries that establish the increase of this type of tumor in recent years and differences according to the countries included (it is worth mentioning that 30 lung tumors were identified in 4246 liver transplant recipients). This datum is important because the survival of transplant patients diagnosed with lung tumors is limited, so strategies to reduce the risk of these neoplasms and facilitate their early detection are of utmost importance.
Lastly, we have not found a higher incidence of colon tumors in liver recipients transplanted due to PSC, as has been reported.8,17 Our pre-transplant screening protocol did not change during the study period and included colonoscopy for patients over 50 years of age or with a history of colorectal cancer risk. Our data do not support considering more frequent colon cancer screening after transplantation than what is already recommended for the general population. In conclusion, this study confirms that transplant patients with de novo solid organ tumors have lower survival rates after diagnosis than patients with non-melanoma skin tumors or those with no post-transplant tumors. Our results differ from other published reports, finding a high incidence of lung neoplasms associated with poor prognosis and poor survival. Therefore, we believe that preventive strategies and early detection protocols are justified to detect de novo tumors while still in an early and potentially curative stage. The limitations of this study include its retrospective, single-center design, where data on risk factors and the incidence of cancer in our general population have not been validated. As a reference, we have used national data provided by the SEOM.11
AuthorshipCarmen Bernal Bellido: study design, data collection, analysis and interpretation of the results, article composition and approval of the final version.
José María Álamo Martínez: data collection, analysis and interpretation of the results, critical review.
Gonzalo Suárez Artacho, Luis Miguel Marín Gómez, Carmen Cepeda Franco and Lydia Barrera Pulido: data collection, critical review.
Javier Padillo Ruiz and Miguel Ángel Gómez Bravo: critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Bernal Bellido C, Suárez Artacho G, Álamo Martínez JM, Marin Gómez LM, Cepeda Franco C, Barrera Pulido L, et al. Incidencia y supervivencia de los tumores de novo en trasplante hepático. Cir Esp. 2018;96:501–507.