Chylothorax is a rare complication in esophagectomies that is associated with increased postoperative mortality. Several factors have been described that may favor its appearance. Its treatment is controversial, and lymphography with percutaneous embolization of the thoracic duct is used by several groups.
Material and methodOur retrospective study included patients who underwent esophagectomy for cancer of the esophagus or the esophagogastric junction (Siewert I/II) between January 2010 and April 2019 and developed chylothorax as a complication. Epidemiological data, type of surgery, morbidity and treatment were analyzed.
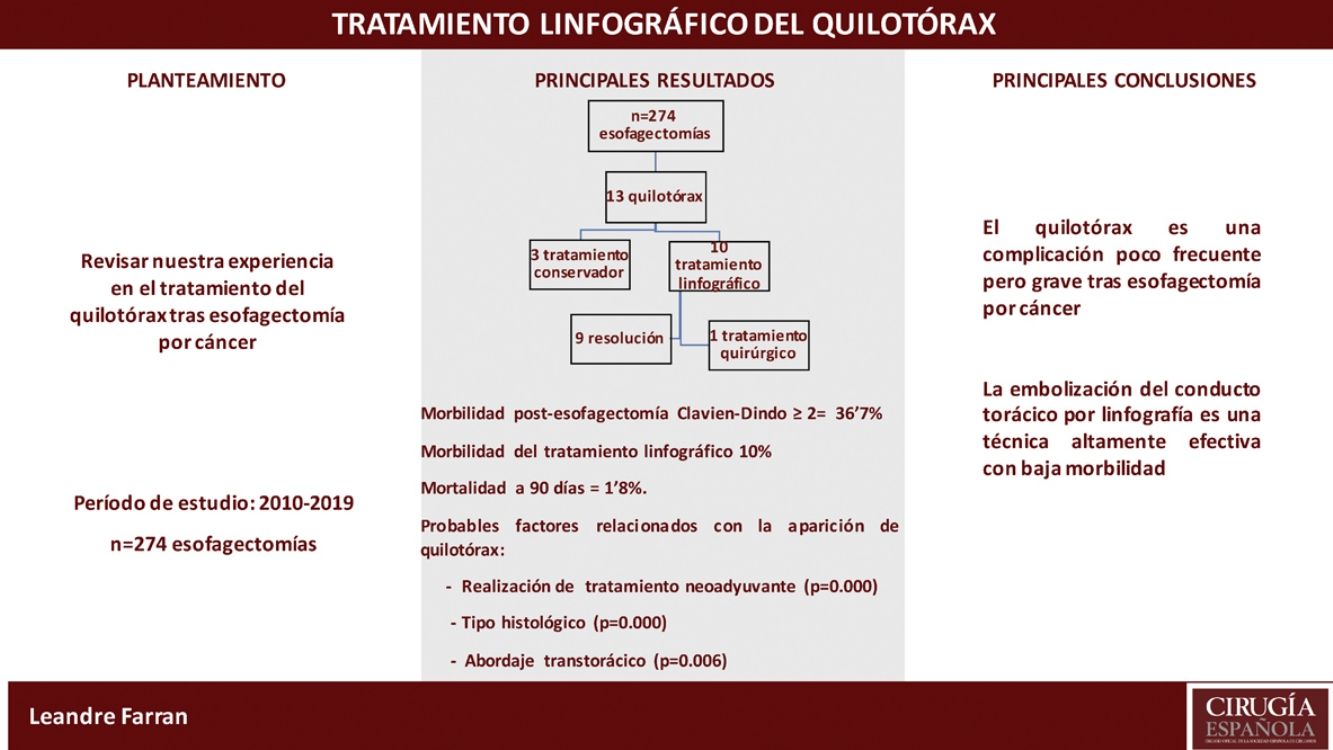
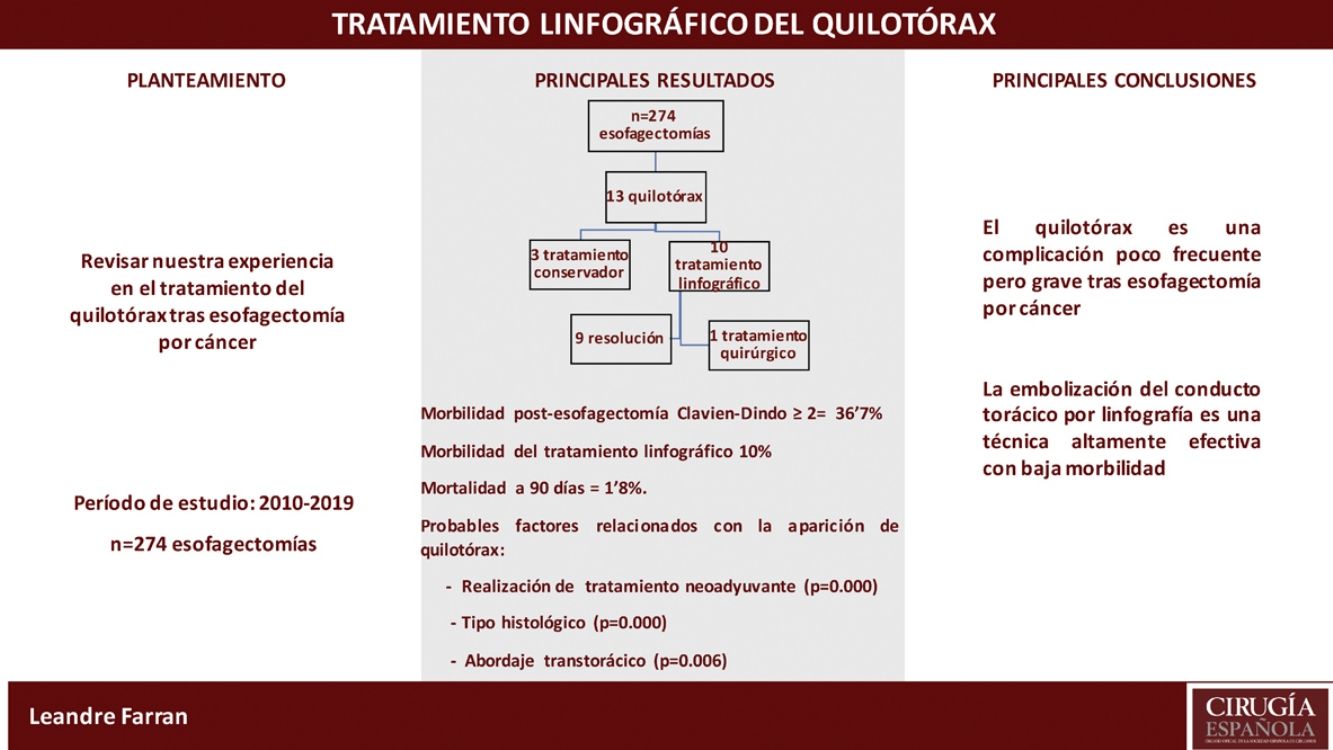
Results274 cancer-related esophagectomies were performed in the study period. Thirteen patients (4.7%) were diagnosed with chylothorax in the postoperative period; 3 were resolved with conservative treatment. In the remaining 10 patients, lymphography was performed with aspiration of the cisterna chyli and thoracic duct embolization, which resolved the chylothorax in 9. One patient (10%) presented a biliary fístula after the procedure.
ConclusionsLymphography with aspiration of the cisterna chyli and thoracic duct embolization is a technique with low morbidity that provides good results for the resolution of chylothorax after esophagectomy.
El quilotórax es una complicación poco frecuente en las esofagectomías pero que se asocia a un aumento de la mortalidad posquirúrgica. Se han descrito diversos factores que pueden incrementar su aparición y el tratamiento del mismo es controvertido, siendo la linfografía con embolización percutánea del conducto torácico uno de los usados por varios grupos.
Material y métodoEstudio retrospectivo de los pacientes a los que se les realizó una esofagectomía por cáncer de esófago o de la unión esofagogástrica a Siewert I/II entre enero del 2010 y abril del 2019, y desarrollaron un quilotórax como complicación. Se analizan datos epidemiológicos, el tipo de cirugía, la morbilidad y el tratamiento.
ResultadosSe realizaron 274 esofagectomías por cáncer en el período comprendido. Trece pacientes (4,7%) fueron diagnosticados de quilotórax en el postoperatorio; 3 se resolvieron con tratamiento conservador. En los 10 pacientes restantes se realizó linfografía con punción de la cisterna de Pécquet y embolización del conducto torácico, con resolución del quilotórax en 9. Un paciente (10%) presentó una fístula biliar después del procedimiento.
ConclusionesLa linfografía con punción de la cisterna de Pécquet y embolización del conducto torácico es una técnica con baja morbilidad y buenos resultados en la resolución del quilotórax postesofagectomía.
Chylothorax is one of the major complications that can follow esophagectomy,1–3 with an incidence of between 0.4% and 4%.4 It causes hypoproteinemia, lipid loss, and respiratory failure5 and is associated with an increase in postoperative mortality and hospital stay.6
Different factors have been described that may increase the risk of its appearance: histology for squamous carcinoma,4 body mass index (BMI)<25kg/m2,7 neoadjuvant chemoradiotherapy, resection of the thoracic duct (TD) and balance≥6.55mL/kg/h in intraoperative fluids.6 As a prophylactic measure, some authors have advocated mass ligation of the TD during surgery.8,9
The treatment of chylothorax is controversial. Somatostatin has demonstrated clinical efficacy10 by decreasing the flow.11 Treatment with octreotide, a somatostatin analog, decreases the chylothorax volume, allowing for removal of the chest drain tube in 86.6% of diagnosed patients.12 Other groups have advocated early re-thoracotomy with ligation of the TD13 or pleurodesis with platelet-rich plasma and fibrin glue.14
The efficacy of percutaneous embolization of the TD as a treatment for chylothorax is well established,15 and some groups defend it as the first therapeutic strategy in the approach to chylothorax after esophagectomy.16
This study retrospectively analyzes chylothorax diagnosed in the last 10 years secondary to esophagectomies for cancer performed by the Esophagogastric Surgery Unit at the Hospital Universitari de Bellvitge, Spain. We have analyzed epidemiological data, risk factors and treatment.
MethodsRetrospective study of all patients who underwent esophagectomy for cancer of the esophagus or of the esophagogastric junction (Siewert I/II) between January 2010 and April 2019, and who developed post-operative chylothorax. We have analyzed epidemiological data, risk factors, type of surgery and morbidity.
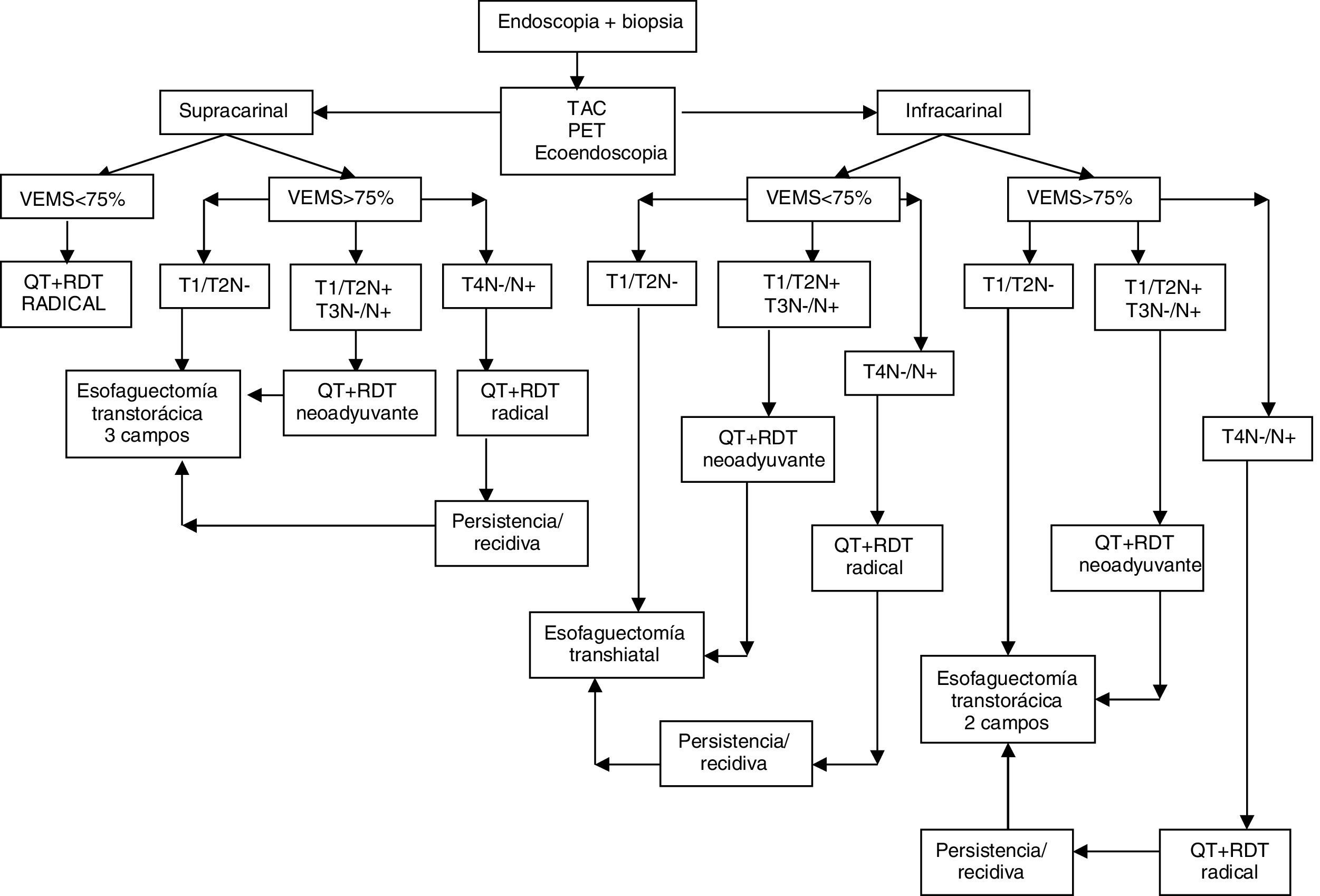
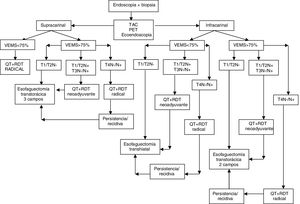
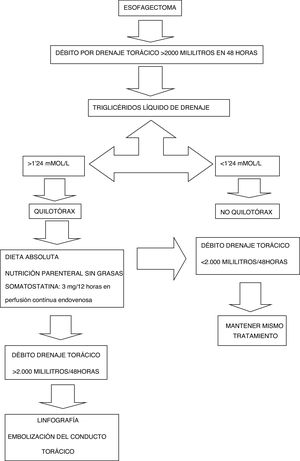
The treatment protocol for esophageal cancer in our unit is shown in Fig. 1.
Neoadjuvant treatment for esophageal neoplasia and Siewert I was performed with intravenous cisplatin 75mg/m2 (day 1) and 5-fluorouracil in continuous infusion at 1000mg/m2/day (days 1–4 or 5) or carboplatin and 5-fluorouracil at the same doses in patients with a history of oto- or nephrotoxicity. Two cycles of chemotherapy were administered in the first and fourth weeks of radiation therapy. The total dose of radiotherapy was 45Gy (1.8Gy/fraction in the tumor and prophylaxis in the lymphoid areas).
In Siewert II tumors, the FLOT regimen (fluorouracil, leucovorin, oxaliplatin, taxanes) was administered every 2–3 weeks.
The surgical approach was transthoracic as long as the patient presented a forced expiratory volume in one second (FEV1) >75% on pulmonary function tests; otherwise, we performed transhiatal esophagectomy.
In the transthoracic approach, we conducted standard or extended lymph node dissection (parabronchial, infracarinal and subcarinal paraesophageal lymphadenopathies) when the esophagectomy was 2 or 3 fields, respectively. Ligation and en bloc resection of the TD was included in all cases. In supracarinal squamous carcinomas, bilateral cervical lymph node dissection was also carried out. In the abdominal field, we added a compartment II lymphadenectomy (lymph node stations 7–11). A jejunostomy was routinely created for enteral feeding (Fresubin®, Fresenius Kabi Deutschland GmbH, 61346 Bad Homburg, Germany), starting 6h after surgery. A radiological follow-up with a computed tomography scan was done one week after the operation; when dehiscence was ruled out, oral diet was initiated.
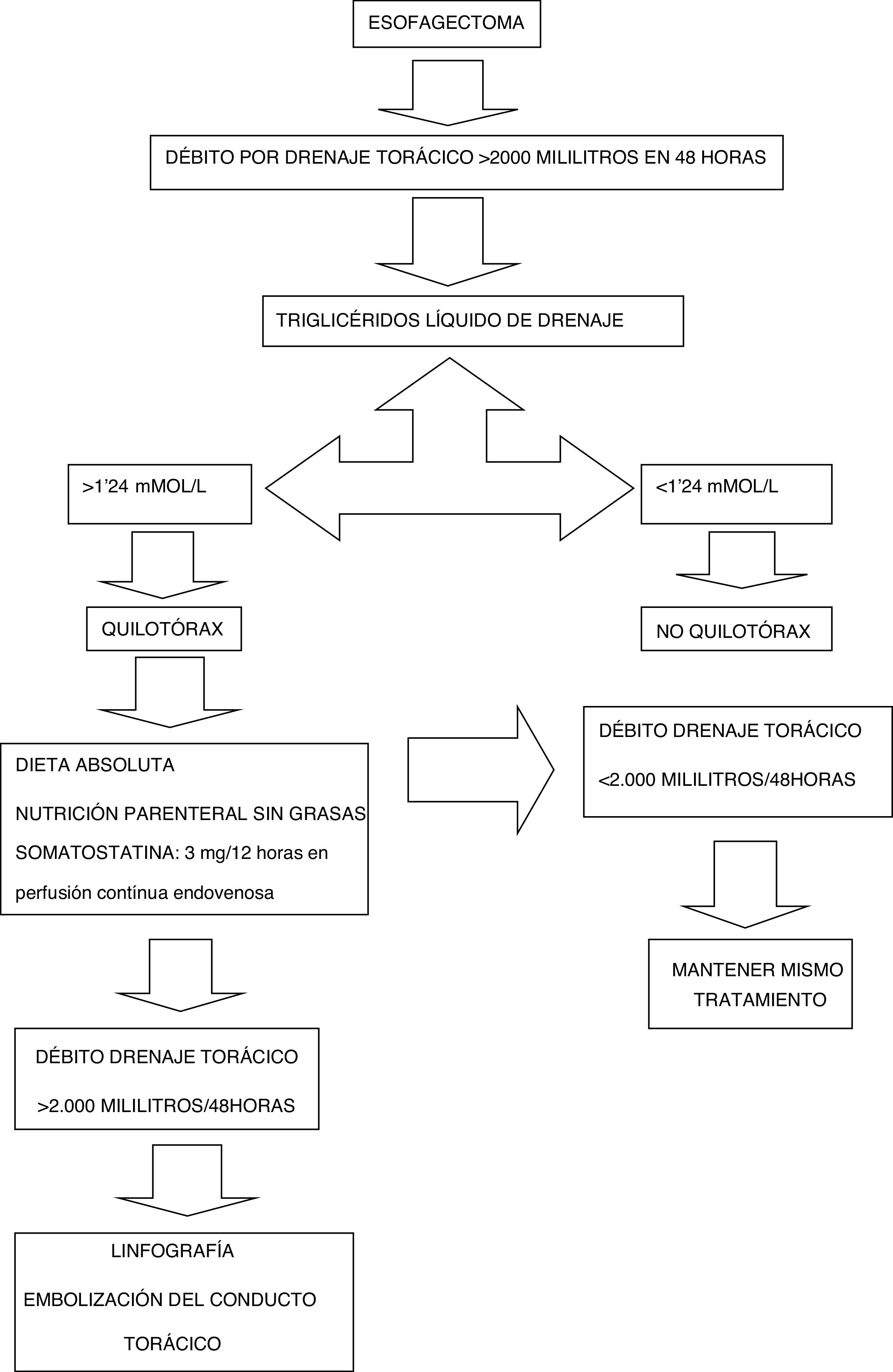
The diagnosis of suspected chylothorax was made when the discharge from the thoracic drains was greater than 2000mL accumulated within 48h after the intervention, and the triglyceride level of the drainage fluid is confirmed to be greater than 1.24mmol/L or 110mg/dL. At this time, conservative treatment began: NPO/enteral feeding, somatostatin at a dose of 3mg/12h in continuous infusion, and total parenteral nutrition. If the chylothorax discharge did not decrease to less than 2000mL in the 48h after diagnosis, we indicated lymphography and embolization (Fig. 2).
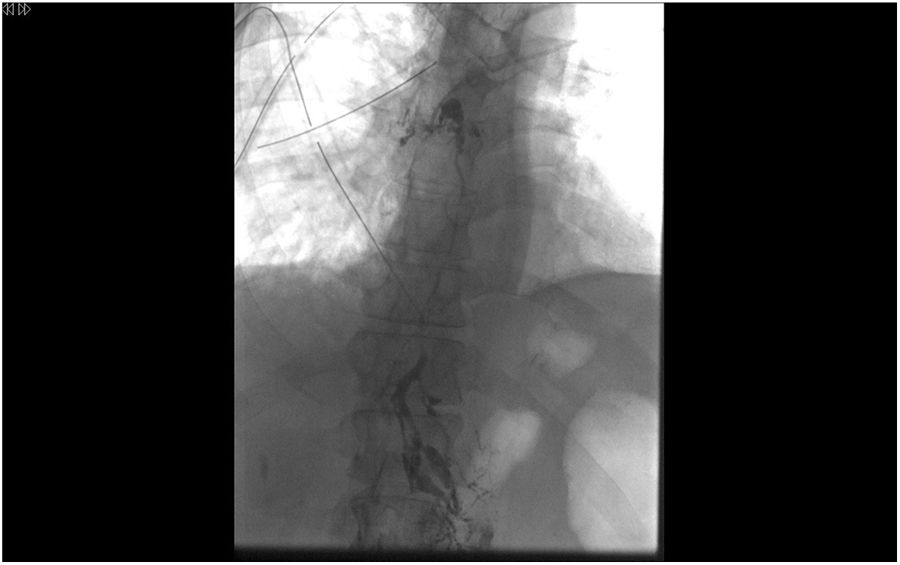
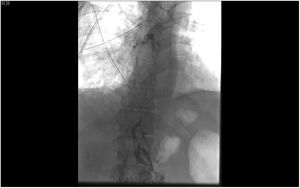
Lymphography and embolization techniqueThe diagnostic lymphographic study was performed by ultrasound-guided needle aspiration of the inguinal lymph nodes or dissection of interdigital lymph vessels. A solution of iodinated fatty acid ethyl esters (Lipiodol® Ultra Fluid, Guerbet, Aulnay-sous-Bois, France) was injected and the opacification of the cisterna chyli (Pécquet's cistern) was confirmed. Needle aspiration was performed with a 21G needle from the coaxial percutaneous set (AccuStick™, Boston Scientific, France) and after insertion of a 0.018″ nitinol guidewire (Nitrex™, ev3, USA), which was advanced to exchange for the coaxial percutaneous set (Fig. 3).
Once the dilator has been removed from the set and after verifying the endoluminal situation with an injection of about 2mL of iso-osmolar radiopaque contrast medium, the TD is navigated with a microcatheter (Progreat®2’7 Frx130cm, Terumo, Tokyo, Japan), to try to come as close as possible to the leak point.
Once the desired position is reached, the entire catheterized TD segment, as well as the extraluminal access tract, undergoes retrograde embolization with the agent of choice. At our hospital, the current embolizing agent of choice is the mixture of n-Butyl-2-Cyanoacrylate (Glubran® 2, GEM Srl, Viareggio (LU), Italy) with ethyl esters of iodized fatty acids (Lipiodol®), which can also be done with microcoils.
The IBM SPSS version 18.0 statistical program (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
Quantitative data were expressed as mean and standard deviation, or as median and interquartile range when the data did not follow a normal distribution. Qualitative data were expressed as frequencies and percentages. The Student's t test was used to compare quantitative variables. In the case of quantitative variables that did not follow normal distribution, the Mann–Whitney U was applied. For the comparison of qualitative variables, the chi-square test was used. In all cases, P<.05 was considered statistically significant.
ResultsBetween January 2010 and April 2019, 274 esophagectomies were performed for cancer of the esophagus or of the gastroesophageal junction (Siewert I/II) by the Esophagogastric Surgery Unit of the Hospital Universitari de Bellvitge. The study included 239 men (86.9%) and 35 women (13.1%), with a mean age of 60.4 years (30–85); 72.7% of the tumors were located at the infracarinal level, 61.7% were adenocarcinomas, and 69% of patients received neoadjuvant treatment.
Among the surgical procedures, 77.1% were transthoracic esophagectomies (45.5% in 2 fields and 31.6% 3 fields) and 22.9% were transhiatal; 4.7% of the latter were coloplasty.
Out of the 211 patients who underwent a transthoracic approach, 12.8% (27 cases) were performed by thoracoscopy.
36.7% of the patients presented complications ≥2 according to the Clavien-Dindo classification. Hospitalization time was 16 days, and 90-day mortality was 1.8%.
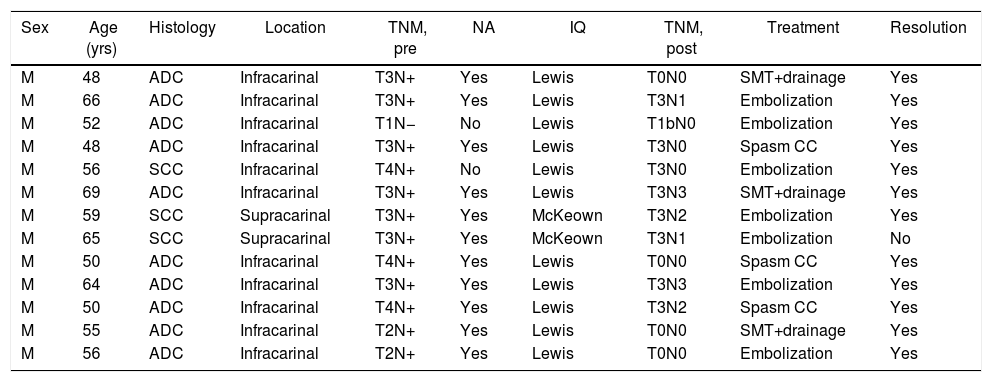
Thirteen patients (4.7%) were diagnosed with postoperative CT scan, all in the first 48h. All 13 underwent thoracotomy. The characteristics and the treatment used are shown in Table 1. In all cases, conservative treatment was started with somatostatin, parenteral nutrition and thoracic drainage. In 3 patients, chylothorax was resolved in 5, 6, and 9 days, respectively.
Characteristics of the patients diagnosed with chylothorax and treatment.
| Sex | Age (yrs) | Histology | Location | TNM, pre | NA | IQ | TNM, post | Treatment | Resolution |
|---|---|---|---|---|---|---|---|---|---|
| M | 48 | ADC | Infracarinal | T3N+ | Yes | Lewis | T0N0 | SMT+drainage | Yes |
| M | 66 | ADC | Infracarinal | T3N+ | Yes | Lewis | T3N1 | Embolization | Yes |
| M | 52 | ADC | Infracarinal | T1N− | No | Lewis | T1bN0 | Embolization | Yes |
| M | 48 | ADC | Infracarinal | T3N+ | Yes | Lewis | T3N0 | Spasm CC | Yes |
| M | 56 | SCC | Infracarinal | T4N+ | No | Lewis | T3N0 | Embolization | Yes |
| M | 69 | ADC | Infracarinal | T3N+ | Yes | Lewis | T3N3 | SMT+drainage | Yes |
| M | 59 | SCC | Supracarinal | T3N+ | Yes | McKeown | T3N2 | Embolization | Yes |
| M | 65 | SCC | Supracarinal | T3N+ | Yes | McKeown | T3N1 | Embolization | No |
| M | 50 | ADC | Infracarinal | T4N+ | Yes | Lewis | T0N0 | Spasm CC | Yes |
| M | 64 | ADC | Infracarinal | T3N+ | Yes | Lewis | T3N3 | Embolization | Yes |
| M | 50 | ADC | Infracarinal | T4N+ | Yes | Lewis | T3N2 | Spasm CC | Yes |
| M | 55 | ADC | Infracarinal | T2N+ | Yes | Lewis | T0N0 | SMT+drainage | Yes |
| M | 56 | ADC | Infracarinal | T2N+ | Yes | Lewis | T0N0 | Embolization | Yes |
ADC: adenocarcinoma; SCC: squamous carcinoma; NA: neoadjuvant; SMT: somatostatin.
Lymphography was indicated in the remaining 10 cases. In 7, the leak was identified in the TD and embolization was achieved, with resolution of the chylothorax in 6. In 3 patients, the cistern chyli was punctured, although the TD was not able to be catheterized or embolized. In spite of this, the chylothorax was resolved in all patients.
One patient had a low-output biliary fistula after transhepatic puncture of the cisterna chyli, which resolved spontaneously.
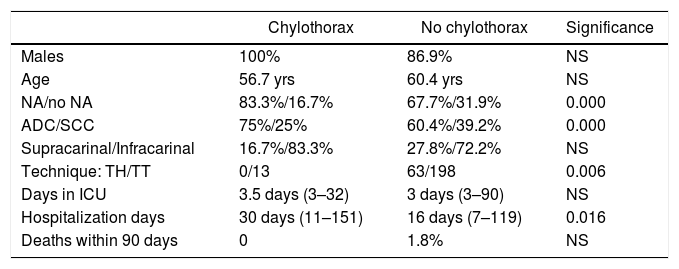
Table 2 compares the characteristics of patients who developed chylothorax versus those who did not.
Comparison of patients who developed post-esophagectoy chylothorax versus the total of patients with resection.
| Chylothorax | No chylothorax | Significance | |
|---|---|---|---|
| Males | 100% | 86.9% | NS |
| Age | 56.7 yrs | 60.4 yrs | NS |
| NA/no NA | 83.3%/16.7% | 67.7%/31.9% | 0.000 |
| ADC/SCC | 75%/25% | 60.4%/39.2% | 0.000 |
| Supracarinal/Infracarinal | 16.7%/83.3% | 27.8%/72.2% | NS |
| Technique: TH/TT | 0/13 | 63/198 | 0.006 |
| Days in ICU | 3.5 days (3–32) | 3 days (3–90) | NS |
| Hospitalization days | 30 days (11–151) | 16 days (7–119) | 0.016 |
| Deaths within 90 days | 0 | 1.8% | NS |
ADC: adenocarcinoma; SCC: squamous carcinoma; NA: neoadjuvant; NS: not significant; TH: transhiatal; TT: transthoracic; ICU: Intensive Care Unit.
Injury to the thoracic duct during esophagectomy is a complication with a low incidence (0.6% to 4%),1–3 although some groups report up to 12%.6 Our series had a rate of 4.7%. Among the risk factors studied, some authors have considered BMI<25 a predisposing factor.7 In our series, we have not been able to corroborate the histology of squamous carcinoma, which was described by Shah et al.4 as another risk factor for the appearance of chylothorax in our series. On the contrary, we found a statistically significant difference in favor of adenocarcinoma in patients diagnosed with chylothorax. The proportion in our setting between adenocarcinomas and squamous cell carcinomas would not explain this difference, nor would the neoadjuvant treatment, which is the same in both tumors.
Neoadjuvant treatment with chemoradiotherapy6,17 is another risk factor in the appearance of post-esophagectomy chylothorax due to fibrosis that results in more difficult mediastinal dissection.18 In our case, 11 of the patients who presented chylothorax had received neoadjuvant treatment with chemo- and radiotherapy, and only 2 were directly indicated for surgery. Although there was statistical significance and concurrence with reports by various authors regarding radiotherapy as a risk factor, the small number of patients in our series does not allow us to differentiate it from the association with chemotherapy. In any case, no patient with chemotherapy as the sole neoadjuvant treatment developed chylothorax.
Even though some authors have shown an increased risk of chylothorax in TD resection compared to its preservation,6 our group performs systematic resection of TD distal to the arch of the azygos vein with the en bloc mediastinal lymph node dissection, with ligation of both ends using absorbable suture. For its identification, we do not use an auxiliary technique. Other studies argue that mass ligation of the TD can prevent chylothorax after esophagectomy.8,9
Our therapeutic approach to chylothorax (Fig. 3) always started with conservative treatment, including total parenteral nutrition and somatostatin.10 We believe that this may lead to resolution of chylothorax with a volume below 1000cc/24h. In other cases, our chosen option was embolization of the TD.15,16 Out of the 10 patients in whom embolization was attempted, the entire procedure was performed in 7, with chylothorax resolution in 6. In 3 cases, TD catheterization and embolization were not possible, so the decision was made to disrupt the Pécquet cistern. This phenomenon was already described by Cope and Kaiser,19 in whom the disruption of the Pécquet cistern causes a spasm and chylothorax resolution. In a study by Boffa et al.,20 out of 22 lymphographies performed, it achieved TD embolization in 12 and in the remaining 10 it caused disruption. However, the authors stated that embolization is preferable to disruption. The angioradiologists in our group opted for disruption when they were unable to catheterize TD.
We had one case of failure to resolve the chylothorax, despite the fact that the TD was correctly embolized. It was resolved when it became necessary to perform urgent left thoracotomy due to iatrogenic hemothorax, and during surgery we performed talc pleurodesis.14 In this case, we believe that the failure to resolve the chylothorax could be due to a second injury at the level of the left cervical lymph node dissection, which we systematically perform in esophagectomy for supracarinal neoplasms.
In conclusion, the patients in our series who presented chylothorax as a complication of esophagectomy were men diagnosed with adenocarcinoma, who had undergone neoadjuvant treatment, a transthoracic approach, and TD resection.
Although the low number of cases makes it impossible to generalize conclusions, we believe that the treatment of post-esophagectomy chylothorax should be initiated with conservative measures (NPO, somatostatin and parenteral nutrition). In cases of non-resolution, the treatment of choice should be a lymphographic study with embolization of the TD lesion. It is a procedure that, in the hands of expert angioradiologists, has low morbidity and is effective.
Conflict of interestsThe authors have no conflict of interests to declare.
Declaration of competing interestThe authors report no declarations of interest.
Please cite this article as: Farran L, Miró M, Alba E, Barrios O, Joudanin J, Estremiana F, et al. Linfografía y embolización del conducto torácico como tratamiento del quilotórax tras esofagectomía por cáncer de esófago. Cir Esp. 2021;99:208–214.